Effects of varying the lengths of the donor units in π-extended thienothiophene isoindigo-based polymer semiconductors†
Abstract
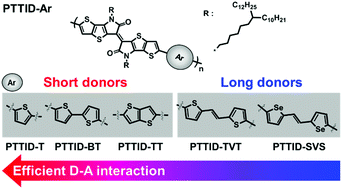
A series of donor–acceptor (D–A) type polymers based on a thieno[3,2-b]thiophene isoindigo (TTID) acceptor and five different donors was synthesized to investigate the versatility of the TTID acceptor moiety. To enhance intermolecular interactions, the branching site of the branched alkyl side chains was adjusted far from the polymer backbone. All of these polymers have planar backbone structures and exhibit highly ordered crystallinity. The TTID acceptor unit contains bulky electron-rich thieno[3,2-b]thiophene (TT), so the electronic structure and electrical properties of the TTID-based polymers are sensitive to the length of the donor units. The polymers conjugated with short donors exhibit effective D–A push–pull interactions and therefore have efficient intramolecular charge transfer characteristics. In particular, the polymer that was copolymerized with the short thiophene donor exhibits the highest hole mobility (0.54 cm2 V−1 s−1) of the TTID-based polymers, because it has a desirable crystalline structure and a smooth thin film morphology as well as efficient D–A interactions. Copolymerization with the longer donors containing vinylene linkages results in larger band gaps and moderate device performance (0.04 cm2 V−1 s−1), which probably result because the D–A interactions of the resulting polymers are weaker than those in the polymers produced by copolymerization of TTID with short donors.


 Please wait while we load your content...
Please wait while we load your content...