High-throughput roll-to-roll fabrication of flexible thermochromic coatings for smart windows with VO2 nanoparticles†
Abstract
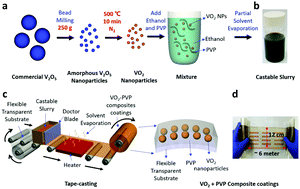
VO2-based ‘nanothermochromics’ that utilize the dispersion of VO2 nanoparticles in a passive host matrix have been evaluated as an economic strategy of “smart” windows to reduce energy consumption for heating and air conditioning in buildings. Here, we demonstrate a high-throughput roll-to-roll fabrication of thermochromic coatings for smart windows that can adapt their optical properties in accordance with external temperature. A large quantity (250 g) of VO2 nanoparticles (NPs) was synthesized at one time by controlled thermal treatment of bead-milled V2O5 NPs as a fast and inexpensive method. The amorphous nature of bead-milled V2O5 NPs combined with their nanometer size kinetically facilitates uniform synthesis of high-quality VO2 NPs even under less-reducing conditions than those used to obtain bulk VO2. This mass production of VO2 NPs could be used to fabricate the largest thermochromic coatings with VO2/PVP composites (12 cm × 600 cm) yet produced with excellent infrared modulation ability (∼45%). This scalable and continuous production of large coatings with thermochromic NPs will accelerate the commercialization of thermochromic coatings for smart windows, which will contribute to a large reduction in energy consumption to heat or cool buildings.
- This article is part of the themed collection: Industry R&D collection


 Please wait while we load your content...
Please wait while we load your content...