Effective modulation of the photoluminescence properties of 2,1,3-benzothiadiazoles and 2,1,3-benzoselenadiazoles by Pd-catalyzed C–H bond arylations†
Abstract
A one step procedure towards the synthesis of 4-aryl-2,1,3-benzothiadiazoles, 4,7-diaryl-2,1,3-benzothiadiazoles and 4-aryl-2,1,3-benzoselenadiazoles using palladium-catalyzed regioselective C–H bond arylations of 2,1,3-benzothiadiazole and 2,1,3-benzoselenadiazole was developed. A donor–acceptor compound was also synthesized via two successive C–H bond arylations at C4 and C7 positions of the 2,1,3-benzothiadiazole unit. One of the major achivements of this methodology arises from the fine modulation of the fluorescence wavelength with emission colors covering blue to red regions of the visible spectrum by the simple introduction of the suitable aryl group on the 2,1,3-benzothiadiazole unit.
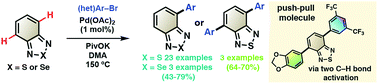


 Please wait while we load your content...
Please wait while we load your content...