Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From anthraquinone to heterocoronene as stable red chromophore†
Ruomeng
Duan
a,
Dieter
Schollmeyer
b,
Klaus
Müllen
cd and
Chen
Li
 *ac
*ac
aSchool of Environment and Civil Engineering, Dongguan University of Technology, No. 1, Daxue Rd., Songshan Lake, Dongguan, Guangdong Province, P. R. China. E-mail: lichen@mpip-mainz.mpg.de
bInstitute of Organic Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10-14, 55128 Mainz, Germany
cMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
dInstitute of Physical Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10-14, 55128 Mainz, Germany
First published on 19th January 2018
Abstract
Using the commercially available 1,4,5,8-tetrachloroanthraquinone (1) as starting material, a N-heterocoronene derivative 3 was synthesized via a straightforward two-step reaction. Compound 3 shows a similar photostability as perylene dyes, qualifying it as a promising colorant.
Most organic colorants exhibit three characteristic features: (1) a conjugated carbon-rich framework, often aromatic units such as naphthalene,1 (2) conjugated double bonds as occurring in azo dyes,2 and (3) additional substituents, so-called auxochromic groups, for example carboximides.3 Organic colorants have thus been categorized according to their chemical structures. From a commercial point of view, azo dyes dominate the markets with more than 60% due to their low production costs. The second most important organic chromophores are carbonyl based compounds, particularly anthraquinones.4 Using functionalized anthraquinones as starting materials, even larger polycyclic pigments have been developed, such as quinacridone,5 indanthrone,6 flavanthrone,7 and pyranthrone.8 Another approach uses polycyclic aromatic hydrocarbons (PAHs) as core that is substituted by multiple carbonyls and groups containing lone pairs of electrons.9 Typical examples for such chromophores are perylenecarboximide dyes,10 which serve not only as conventional colorants but also as active components of organic photonics and electronics.11 Alternative colorant structures are still in high demand.
Herein we combine coronene12 (an even larger PAH than perylene) and four carboximides to achieve a new N-heterocoronene chromophore, namely 1,4,7,10-tetra(4-alkylphenyl)-1,4,7,10-tetraazacoronene-2,3,8,9-tetraone (alkyl is: t-butyl for 3a, n-dodecyl for 3b). Starting from a commercially available anthraquinone (1)13i.e. 1,4,5,8-tetrachloro-9,10-anthracenedione, 3 was synthesized by a simple two-step protocol (Scheme 1). A four-fold Buchwald-Hartwig amination between 1 and 4-alkylaniline, first, furnished the intermediate 1,4,5,8-tetrakis((4-alkylphenyl)amino)anthracene-9,10-dione (2). Then, a microwave-assisted Knoevenagel reaction of 2 with diethyl malonate yielded target compound 3 (in 47% for 3a and 61% for 3b after purification). Generally, depending on the alkyl-substituent, 3 is well soluble in common organic solvents such as dichloromethane (DCM), tetrahydrofuran (THF) and toluene. The structures of 2 and 3 were proven with the help of high-resolution mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy. These results are presented in the ESI.†
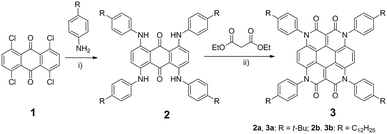 | ||
| Scheme 1 Synthesis of 3: (i) Pd2(dba)3, BINAP, Cs2CO3, toluene, 105 °C, 24 h, 2a: 58%, 2b: 44%; (ii) CH3COOK, DMF, microwave, 170 °C, 30 min, for 3a: 47%, 3b: 61%. | ||
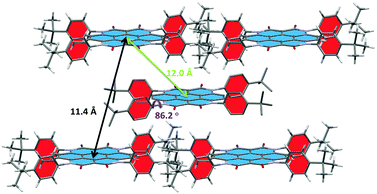
Single-crystals of 3a were grown from a chloroform-methanol mixture via slow evaporation of the solvents. Not surprisingly, 3b failed to yield crystals suitable for X-ray analysis due to the long and flexible alkyl substituents. In the crystal (Fig. 1), the molecules of 3a are arranged in a brick-wall fashion with π–π distances between neighbouring molecules of 11.4 Å and 12.0 Å, respectively. The dihedral angle between the heterocoronene core (highlighted in blue) and the N-alkylphenyl substituent (highlighted in red) is 86°, which obviously hinders the π–π overlap between the heterocoronene cores. The four adjacent phenyl moieties of 3a create empty space around the heterocoronene core. This allows the alkylphenyl substituents from neighbouring molecules to penetrate into the resulting voids. The torsion of the phenyl rings is not in the same direction, i.e. clockwise or counterclockwise, which is not favourable for molecular self-assembly via helical packing with regular intermolecular torsion angles (the random twisting of the alkylphenyls prevents the close packing of heterocoronene cores with consistent angle of rotation).
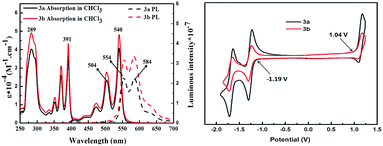
The optical and electrochemical properties of 3 are presented in Fig. 2. 3a and 3b reveal almost identical absorption spectra containing three bands (α, β, p) with peaks at maxima of 282 nm (β band), 391 nm (p band) and 540 nm (α band), with an absorption onset at 570 nm. The emission spectra contain two sub-peaks at 554 and 584 nm, respectively, which reflect a mirror relationship with the α band of the absorption with Stokes shifts of 14 nm (540–554 nm). The fluorescence of 3 in DCM solution is very weak with fluorescence quantum yields (Φfl) of ∼2% and fluorescence lifetime of 0.56 ns. Under the same conditions, perylene orange, i.e. N,N′-bis(2,6-diisopropylphenyl)perylenetetracarboxdiimide (PDI), presents a Φfl of 98% and fluorescence lifetime of 4.1 ns (data given in ESI†). Unlike most of perylene dyes which have no fluorescence in solid-state, the powders of 3a, however, show red fluorescence with emission maximum at 610 nm (see ESI†). In this regard, 3 can be considered as a member of aggregation-induced emissive (AIE) materials.14 Similar to tetraphenylethene (TPE), the central heterocoronene stator of the 3a molecule is surrounded by four phenyl ring-rotors, which dynamically rotate and scatter the exciton energy non-radiatively. Therefore, the photoluminescence of 3 is very weak in solution but not in solid. The HOMO and LUMO energy levels of 3 were estimated by cyclic voltammetry in DCM solution (Fig. 2). The (identical) oxidation and reduction onsets of 3a and 3bvs. ferrocene/ferrocene+ (Fc+/Fc) are 1.04 V and −1.19 V, respectively. The HOMO and LUMO values were thus calculated as −5.8 eV and −3.6 eV, respectively (Table 1). Compared with PDI, heterocoronene 3 is more easily oxidized owing to its higher HOMO (−6.1 eV for PDI).15 Additionally, the LUMO energy of 3 is more positive than those of the fullerene PC71BM (−4.00 eV)16a and PDI (−3.8 eV).15 When used as acceptor in solar cells, 3 is supposed to furnish a larger gap between HOMO (donor) and LUMO (acceptor), thereby possibly increasing the Voc value that depends on the energy gap between the HOMO level of the donor and the LUMO level of the acceptor of organic photovoltaics.16
| λ max (nm) | Experimental | DFT calculationb | |||||
|---|---|---|---|---|---|---|---|
| E g | HOMOb | LUMOc | E g | HOMO | LUMO | ||
| a E g = 1240/λonset. b Estimated vs. vacuum level from EHOMO = −4.80 eV − Eox. c Estimated vs. vacuum level from ELUMO = −4.80 eV – Ered. d E g = EcvLUMO − EcvHOMO. The Eox and Ered are calibrated with Fc/Fc+ as the reference. | |||||||
| 3a | 282; 391; 540 | 2.18 | −5.84 | −3.61 | 2.23 | −5.82 | −3.08 |
| 3b | 282; 391; 540 | 2.18 | −5.84 | −3.61 | 2.23 | −5.82 | −3.08 |
The thermal stability of 3 was evaluated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) (TAG and DSC results shown in ESI†). 3a and 3b demonstrate the 5% weight loss at 433 and 515 °C, respectively. The t-butyl groups appear to be more stable than the respective n-dodecyl groups. Furthermore, the weight loss can be attributed to the decomposition of the alkyl substituents, demonstrating an even higher thermal stability of the aromatic core. The DSC curve of 3a displays a small endothermic peak at 294 °C, whereas 3b exhibits a strong endothermic peak at 90 °C. The lower endothermic peak in 3b can be ascribed to the dodecyl-substituents, which bring about a lower melting point.
A comparison of frontier orbitals of 2, 3, coronene (4), and 1,4,7,10-tetraazacoronene (5)17 is depicted in Fig. 3. Obviously, 3, 4, and 5 possess similar molecular orbitals, but not 2. This indicates that the coronene character of 3 is maintained. Compounds 4 and 5 are yellow whereas 3 is dark red. Thus, the four amide groups of 3 lead to a strong change in colour without affecting the molecular orbitals.
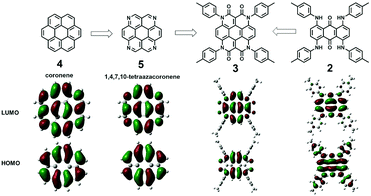 | ||
| Fig. 3 Representation of the molecular orbitals of 3, 4 and 5 (DFT calculations at the B3LYP/6-31G(d) level). | ||
The light stability of 3a was tested by using its DCM solution under sunlight. For comparison, the solution of PDI was prepared at the same concentration. When recording the UV-vis absorption spectra of 3a and PDI daily (Fig. 4), heterocoronene 3a shows the same stability as that of PDI. After 9 days of outdoor exposure to sunlight, the absorption spectrum of 3a remained nearly identical to the initial one. Light fastness is, of course, a key feature of a new chromophore.
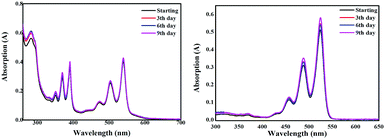 | ||
| Fig. 4 The photostability (absorption spectra were recorded after the solution was placed in the open air under sunlight) of 3a (left) and PDI (right) in DCM solution. | ||
Conclusions
In conclusion, two disc-like heterocoronenes 3a and 3b were synthesized from commercially available 1,4,5,8-tetrachloro-9,10-anthraquinone via Buchwald-Hartwig coupling and a subsequent Knoevenagel condensation. 3 exhibits similar optical properties to perylenetetracaboxdiimides but shows higher LUMO and HOMO levels than those of PDI. Although 3 has nearly no fluorescence in solution, it reveals a thermal stability up to 515 °C and strong photoluminescence in solid. Additionally, 3 possesses the same photostability as that of PDI dyes, qualifying this new heterocoronene-based chromophore as a promising colorant even for outdoor applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. M. is grateful for funding by the Johannes Gutenberg-Universität Mainz (JGU) through the Gutenberg Forschungskolleg Fellowship (GFK). Open Access funding provided by the Max Planck Society.References
- (a) S. L. Suraru and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 7428 CrossRef CAS PubMed; (b) A. Herrmann and K. Müllen, Chem. Lett., 2006, 35, 978 CrossRef CAS; (c) X. W. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268 CrossRef CAS PubMed; (d) W. Jiang, Y. Li and Z. H. Wang, Acc. Chem. Res., 2014, 47, 3135 CrossRef CAS PubMed.
- C. Sourisseau, Chem. Rev., 2004, 104, 3851 CrossRef CAS PubMed.
- (a) A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973 CrossRef CAS PubMed; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (c) F. Würthner, C. R. Saha-Moller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962 CrossRef PubMed.
- (a) M. Phillips, Chem. Rev., 1929, 6, 157 CrossRef CAS; (b) K. C. Nicolaou, Y. P. Wang, M. Lu, D. Mandal, M. R. Pattanayak, R. C. Yu, A. A. Shah, J. S. Chen, H. J. Zhang, J. J. Crawford, L. Pasunoori, Y. B. Poudel, N. S. Chowdari, C. Pan, A. Nazeer, S. Gangwar, G. Vite and E. N. Pitsinos, J. Am. Chem. Soc., 2016, 138, 8235 CrossRef CAS PubMed.
- Y. Liu, X. X. Hu, L. L. Wang, X. J. Liu, T. Bing, W. H. Tan and D. H. Shangguan, Dyes Pigments, 2017, 145, 168 CrossRef CAS.
- K. Kotwica, P. Bujak, D. Wamil, M. Materna, L. Skorka, P. A. Gunka, R. Nowakowski, B. Golec, B. Luszczynska, M. Zagorska and A. Pron, Chem. Commun., 2014, 50, 11543 RSC.
- K. Kotwica, P. Bujak, P. Data, W. Krzywiec, D. Wamil, P. A. Gunka, L. Skorka, T. Jaroch, R. Nowakowski, A. Pron and A. Monkman, Chem. – Eur. J., 2016, 22, 7978 CrossRef CAS PubMed.
- R. C. Ahuja, K. Hauffe, R. Schumacher and R. N. Schindler, Ber. Bunsen Phys. Chem., 1982, 86, 167 CrossRef CAS.
- (a) D. Q. Wu, W. Pisula, M. C. Haberecht, X. L. Feng and K. Müllen, Org. Lett., 2009, 11, 5686 CrossRef CAS PubMed; (b) J. Luo, M. F. Xu, R. Z. Li, K. W. Huang, C. Y. Jiang, Q. B. Qi, W. D. Zeng, J. Zhang, C. Y. Chi, P. Wang and J. S. Wu, J. Am. Chem. Soc., 2014, 136, 265 CrossRef CAS PubMed; (c) Z. B. Zeng, S. Lee, M. Son, K. Fukuda, P. M. Burrezo, X. J. Zhu, Q. B. Qi, R. W. Li, J. T. L. Navarrete, J. Ding, J. Casado, M. Nakano, D. Kim and J. S. Wu, J. Am. Chem. Soc., 2015, 137, 8572 CrossRef CAS PubMed; (d) J. Li, S. Chen, Z. Wang and Q. Zhang, Chem. Rec., 2016, 16, 1518 CrossRef CAS PubMed; (e) J. Li and Q. Zhang, Synlett, 2013, 686 Search PubMed; (f) J. Li and Q. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 28049 CrossRef CAS PubMed.
- (a) P. E. Hartnett, C. M. Mauck, M. A. Harris, R. M. Young, Y. L. Wu, T. J. Marks and M. R. Wasielewski, J. Am. Chem. Soc., 2017, 139, 749 CrossRef CAS PubMed; (b) R. J. Lindquist, K. M. Lefler, K. E. Brown, S. M. Dyar, E. A. Margulies, R. M. Young and M. R. Wasielewski, J. Am. Chem. Soc., 2014, 136, 14912 CrossRef CAS PubMed; (c) S. T. J. Ryan, R. M. Young, J. J. Henkelis, N. Hafezi, N. A. Vermeulen, A. Hennig, E. J. Dale, Y. L. Wu, M. D. Krzyaniak, A. Fox, W. M. Nau, M. R. Wasielewski, J. F. Stoddart and O. A. Scherman, J. Am. Chem. Soc., 2015, 137, 15299 CrossRef CAS PubMed.
- (a) H. L. Dong, X. L. Fu, J. Liu, Z. R. Wang and W. P. Hu, Adv. Mater., 2013, 25, 6158 CrossRef CAS PubMed; (b) L. Flamigni, B. Ventura, M. Tasior, T. Becherer, H. Langhals and D. T. Gryko, Chem. – Eur. J., 2008, 14, 169 CrossRef CAS PubMed; (c) H. Langhals, Helv. Chim. Acta, 2005, 88, 1309–1343 CrossRef CAS; (d) Y. Zagranyarski, L. Chen, D. Jansch, T. Gessner, C. Li and K. Müllen, Org. Lett., 2014, 16, 2814 CrossRef CAS PubMed; (e) G. Li, Y. Zhao, J. Li, J. Cao, J. Zhu, X. W. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 196 CrossRef CAS PubMed.
- (a) R. Rieger, M. Kastler, V. Enkelmann and K. Müllen, Chem. – Eur. J., 2008, 14, 6322 CrossRef CAS PubMed; (b) C. L. Eversloh, C. Li and K. Müllen, Org. Lett., 2011, 13, 4148 CrossRef CAS PubMed; (c) Q. Zhang, H. Q. Peng, G. S. Zhang, Q. Q. Lu, J. Chang, Y. Y. Dong, X. Y. Shi and J. F. Wei, J. Am. Chem. Soc., 2014, 136, 5057 CrossRef CAS PubMed; (d) Q. Yan, K. Cai, C. Zhang and D. Zhao, Org. Lett., 2012, 14, 4654 CrossRef CAS PubMed; (e) W. Mao, J. Zhang, X. Li, C. Li and H. Tian, Chem. Commun., 2017, 53, 5052 RSC; (f) G. Li, W.-W. Xiong, P.-Y. Gu, J. Cao, J. Zhu, R. Ganguly, Y. Li, A. C. Grimsdale and Q. Zhang, Org. Lett., 2015, 17, 560 CrossRef CAS PubMed.
- S. Hahn, F. L. Geyer, S. Koser, O. Tverskoy, F. Rominger and U. H. F. Bunz, J. Org. Chem., 2016, 81, 8485 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- S. K. Lee, Y. B. Zu, A. Herrmann, Y. Geerts, K. Müllen and A. J. Bard, J. Am. Chem. Soc., 1999, 121, 3513 CrossRef CAS.
- (a) W. C. Tsoi, D. T. James, J. S. Kim, P. G. Nicholson, C. E. Murphy, D. D. C. Bradley, J. Nelson and J. S. Kim, J. Am. Chem. Soc., 2011, 133, 9834 CrossRef CAS PubMed; (b) D. Sun, D. Meng, Y. H. Cai, B. B. Fan, Y. Li, W. Jiang, L. J. Huo, Y. M. Sun and Z. H. Wang, J. Am. Chem. Soc., 2015, 137, 11156 CrossRef CAS PubMed; (c) D. Meng, D. Sun, C. M. Zhong, T. Liu, B. B. Fan, L. J. Huo, Y. Li, W. S. Jiang, H. S. Choi, T. Kim, J. Y. Kim, Y. M. Sun, Z. H. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375 CrossRef CAS PubMed; (d) S. S. Chen, Y. H. Liu, L. Zhang, P. C. Y. Chow, Z. Wang, G. Y. Zhang, W. Ma and H. Yan, J. Am. Chem. Soc., 2017, 139, 6298 CrossRef CAS PubMed; (e) Y. Zhong, M. T. Trinh, R. S. Chen, W. Wang, P. P. Khlyabich, B. Kumar, Q. Z. Xu, C. Y. Nam, M. Y. Sfeir, C. Black, M. L. Steigerwald, Y. L. Loo, S. X. Xiao, F. Ng, X. Y. Zhu and C. Nuckolls, J. Am. Chem. Soc., 2014, 136, 15215 CrossRef CAS PubMed.
- (a) M. Takase, T. Narita, W. Fujita, M. S. Asano, T. Nishinaga, H. Benten, K. Yoza and K. Müllen, J. Am. Chem. Soc., 2013, 135, 8031 CrossRef CAS PubMed; (b) S. A. Tucker, W. E. Acree, M. J. Tanga, M. Zander, J. C. Fetzer, S. Tokita, K. Hiruta, K. Kitahara and H. Nishi, Appl. Spectrosc., 1991, 45, 1188 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization section, TGA and DSC curves of 3, fluorescence quantum yield measurement for 3a and perylenetetracaboxdiimide (PDI), and single crystal data for 3a. CCDC 1581941. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7tc05073a |
| This journal is © The Royal Society of Chemistry 2018 |