Bioelectronics goes 3D: new trends in cell–chip interface engineering
Abstract
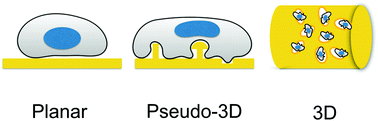
Bioelectronic platforms can be used for electrophysiology, monitoring and stimulating specific cellular functions. While planar electroactive materials have been extensively used, in the past decade new approaches have focused on engineering the interface with pseudo-3D micro and nanostructures and, more recently, on 3D geometries (i.e. scaffold-like). Here, we present an overview of this transition from 2D to 3D bioelectronic platforms and our recent achievements of characterizing the interface between the cells and the device.
- This article is part of the themed collections: Recent Review Articles and Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...