Quantum-dot-encapsulated core–shell barcode particles from droplet microfluidics†
Abstract
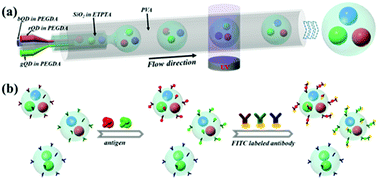
The development of robust quantum dot (QD) barcode particles with specific compositions and simple identification is important to meet the demand for high-throughput assays. Here, we present a multiple-inner phase channel capillary microfluidic approach to generate novel QD-encapsulated core–shell barcode particles with distinctive features for multiplexing analysis. By using different QD dispersed polyethylene glycol diacrylate (PEGDA) solutions as the inner phases, the particles were endowed with hydrogel locked QD cores, which could maintain the dispersed status and provide distinctive identification for the particles. The shells of the barcode particles were silica nanoparticle-dispersed ethoxylated trimethylolpropane triacrylate (ETPTA) resin, which could not only improve the stability and biocompatibility of QDs, but also provide functional groups for immobilization of biomolecules due to the assembling of the silica nanoparticles on their surfaces. Due to the advanced emulsification capability of the capillary microfluidic device, double emulsion templates with multiple inner droplet phases and their resultant multicomponent QD-encapsulated core–shell barcode particles could be continually generated. These particles showed remarkable spectral coding capacity in practice, which make them ideal for biomedical applications.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...