Active quinine-based films able to release antimicrobial compounds via melt quaternization at low temperature†
Alejandro
Latorre-Sánchez
ab,
Mats
Johansson
 *c,
Yuning
Zhang
c,
Michael
Malkoch
*c,
Yuning
Zhang
c,
Michael
Malkoch
 c and
José A.
Pomposo
c and
José A.
Pomposo
 *abd
*abd
aCentro de Física de Materiales (CSIC, UPV/EHU) and Materials Physics Center MPC, Paseo Manuel de Lardizabal 5, E-20018 San Sebastián, Spain. E-mail: josetxo.pomposo@ehu.eus
bDepartamento de Física de Materiales, Universidad del País Vasco (UPV/EHU), Apartado 1072, E-20800 San Sebastián, Spain
cDepartment of Fibre & Polymer Technology, School of Chemical Science and Engineering, KTH-Royal Institute of Technology, 10044 Stockholm, Sweden. E-mail: matskg@kth.se
dIKERBASQUE-Basque Foundation for Science, María Díaz de Haro 3, E-48013 Bilbao, Spain
First published on 27th November 2017
Abstract
The fabrication of antibacterial films based on renewable materials (e.g., chitosan) has attracted significant interest in fields such as food packaging, health care and medicine. However, exploiting the antibacterial properties of cinchona alkaloids to design active nanostructured films able to release quinine-based antimicrobial compounds has not been considered previously. Herein, we develop two different routes to produce active quinine-based nanostructured cross-linked films by exploiting the multiple reactive sites of quinine and, specifically, both the nitrogen atom and the vinyl group of the quinuclidine portion of the molecule, as well as their corresponding orthogonal quaternization and thiol–ene coupling reactions. The first synthetic strategy produces stiff and brittle nanostructured quinine-based films of limited utility for practical applications. Conversely, the second approach produces active, flexible and nanostructured quinine-based films (Tg = −14 °C, Young's modulus = 1.3 GPa), which are able to release antimicrobial compounds against E. coli that, remarkably, are noncytotoxic against mouse macrophage and human dermal fibroblast cells. These kinds of active cinchona alkaloid-based coatings are easy to prepare by means of simple, solvent-free, melt quaternization/spreading procedures at a relatively low temperature (120 °C), making this second approach one of the most facile reported procedures to date to produce active nanostructured bio-based films.
1. Introduction
Replacement of inert synthetic films by active bio-based coatings offers many opportunities to develop innovative products in the fields of food packaging, health care and medicine. Avoiding bacterial biofilm infections is one of the most active aims in the design of modern medical devices and prostheses such as artificial heart valves,1 intravenous catheters,2 hip prostheses,3 and dental implants.4 Also antibacterial fabric bandages have been developed based on the use of benzalkonium chloride in the wound pad, or silver nanoparticles to prevent infections.5 Two mechanisms can provide antibacterial protection: (i) passive protection in which the surface itself avoids protein adsorption and fouling processes (e.g., by using self-assembled monolayers6 to modify the surface energy or by growing poly(ethylene glycol) (PEG) brushes7 to give PEGylated surfaces that resist the attachment of bacteria) and (ii) active protection in which often an antibacterial compound is progressively released from the coating.8 In the latter case, a stringent requirement for the antibacterial compound is to demonstrate noncytotoxic activity during use.Quinine, 1 (see Fig. 1), is a natural compound extracted from the bark of cinchona and remijia trees, which for centuries constituted the only effective remedy for malaria.9 This cinchona alkaloid has been recognized as “the drug to have relieved more human suffering than any other in history”.10 In addition to the use of quinine as a treatment of systemic lupus erythematosus and rheumatoid arthritis, quinine salts have also been investigated as an effective treatment against Herpes simplex virus,11 and different Gram-positive and Gram-negative pathogenic organisms.12 Remarkably, quinine sulfate was reported to inhibit invasion of some bacterial skin pathogens.13 Moreover, significant antibacterial activity of amphiphilic cationic quinine-derived compounds against most pathogenic bacteria, including methicillin-resistant S. aureus, has been reported.14
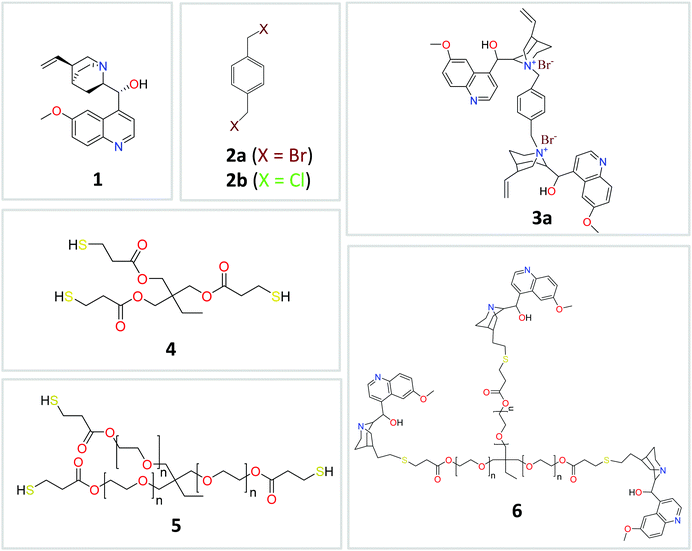 | ||
| Fig. 1 Chemical compounds utilised in this work to design active nanostructured cinchona alkaloid-based coatings. | ||
Despite the large number of proposed passive and active coatings based on renewable materials (e.g., chitosan),15 exploiting the antibacterial properties of cinchona alkaloids to design active nanostructured antibacterial films has not been considered previously. Herein we introduce, for the first time, a versatile and solvent-free synthetic strategy toward active nanostructured cinchona alkaloid-based coatings by means of simple melt quaternization/spreading procedures at relatively low temperature (120 °C). By combining this ground-breaking concept with the multiple reactive sites of 1 and, specifically, both the nitrogen atom and the vinyl group of the quinuclidine portion of the molecule, as well as their corresponding orthogonal quaternization16 and thiol–ene coupling17 reactions, we produce a unique type of active quinine-based nanostructured cross-linked film that is able to release quinine-based antimicrobial compounds that, remarkably, are noncytotoxic against mouse macrophage and human dermal fibroblast cells.
2. Results and discussion
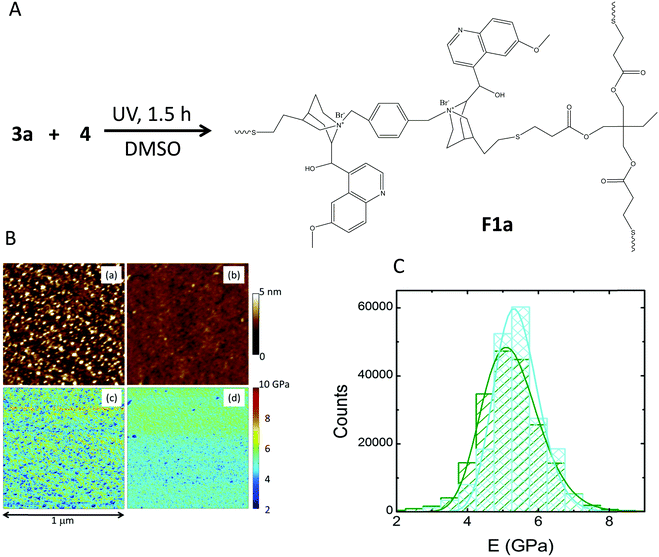
The different precursors of the quinine-based films investigated in this work are illustrated in Fig. 1, whereas their source/synthetic details are reported in the ESI.† Two different routes to produce the quinine-based films were investigated. When cross-linking of compounds 3 and 4 (Fig. 1) was performed in DMSO solution through UV-activated thiol–ene coupling, stiff and brittle quinine-based films of limited utility for practical applications were the result. In contrast, when cross-linking of precursors 2 and 6 (Fig. 1) was carried out via melt quaternization, uniquely active quinine-based nanostructured cross-linked films able to release antimicrobial compounds against E. coli were obtained showing remarkably noncytotoxic activity against mouse macrophage and human dermal fibroblast cells. The first synthetic strategy followed to produce quinine-based films involved the synthesis of compound 3 (3a, X = Br; 3b, X = Cl) from 1 and 2via quaternization. The reaction was carried out in DMSO at 80 °C for 24 h and 3 was isolated by simple precipitation in tetrahydrofuran and further drying under dynamic vacuum (yield = 58%, 3a; 65%, 3b). The 1H NMR spectrum of 3a is shown in Fig. 2A. The assignment of peaks was assisted by 1H COSY and 1H–13C HSQC NMR measurements (see the ESI†). Elemental analysis results of 3a and 3b were found to be in excellent agreement with the expected chemical composition of each compound. Fig. 2B shows the MALDI-TOF spectra of 3a and 3b. In both cases, two peaks were observed corresponding to molecules that have lost at least one (3a-1Br and 3b-1Cl) or two (3a-2Br and 3b-2Cl) counter-ion halogen atoms during the laser desorption/ionization experiments. Overall, all the characterization techniques support the synthesis of 3a and 3b in good yield with excellent purity. In the next step, 3 was reacted with the trifunctional thiolated cross-linker 4 (Fig. 1) in DMSO via UV-activated thiol–ene coupling reaction for 1.5 h to give – upon solvent removal at 120 °C overnight – a quinine-based cross-linked film denoted as F1 (F1a, X = Br; F1b, X = Cl) (Fig. 3A). The partial consumption of vinyl groups of 3a by reaction with thiol groups of 4 was confirmed by attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy through the decrease in the intensity of the bands associated with the stretching and bending vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (ca. 1600 cm−1 and ca. 830 cm−1, respectively) (see the ESI†). The incorporation of 4 to the cross-linked, insoluble films was confirmed by the appearance of a new band at ca. 1735 cm−1 associated with the stretching vibration of the C
C bond (ca. 1600 cm−1 and ca. 830 cm−1, respectively) (see the ESI†). The incorporation of 4 to the cross-linked, insoluble films was confirmed by the appearance of a new band at ca. 1735 cm−1 associated with the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, when compared to the FTIR spectrum of neat 3a. The nanoscale characterization of the films was carried out by PeakForce quantitative nanomechanical mapping (PF-QNM) measurements (Fig. 3B). The roughness of the bromide quaternized film F1a, as determined from topography maps, was found to be slightly higher than the roughness of the chloride quaternized film F1b but both were below 2–3 nm on average, showing a relatively flat surface at the nanoscale level. PF-QNM pictures of the mechanical modulus revealed the nanostructured nature of the films as illustrated in Fig. 3B. In both cases, a narrow distribution of the Young's modulus centered between 4 and 6 GPa was observed by PF-QNM (Fig. 3C), which is comparable to the Young's modulus of biocompatible polymers such as poly(lactic acid) and poly(glycolide).18 Nevertheless, the films F1a and F1b were found to be very brittle and hence of limited utility for practical applications.
O groups, when compared to the FTIR spectrum of neat 3a. The nanoscale characterization of the films was carried out by PeakForce quantitative nanomechanical mapping (PF-QNM) measurements (Fig. 3B). The roughness of the bromide quaternized film F1a, as determined from topography maps, was found to be slightly higher than the roughness of the chloride quaternized film F1b but both were below 2–3 nm on average, showing a relatively flat surface at the nanoscale level. PF-QNM pictures of the mechanical modulus revealed the nanostructured nature of the films as illustrated in Fig. 3B. In both cases, a narrow distribution of the Young's modulus centered between 4 and 6 GPa was observed by PF-QNM (Fig. 3C), which is comparable to the Young's modulus of biocompatible polymers such as poly(lactic acid) and poly(glycolide).18 Nevertheless, the films F1a and F1b were found to be very brittle and hence of limited utility for practical applications.
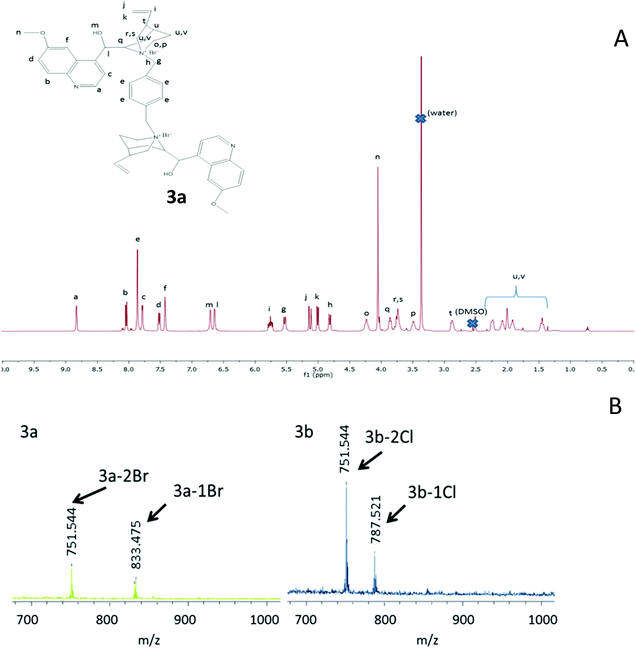 | ||
| Fig. 2 (A) 1H NMR spectrum of 3a (for assignment of peaks, see the ESI†). (B) MALDI-TOF spectra of 3a and 3b (see the text). | ||
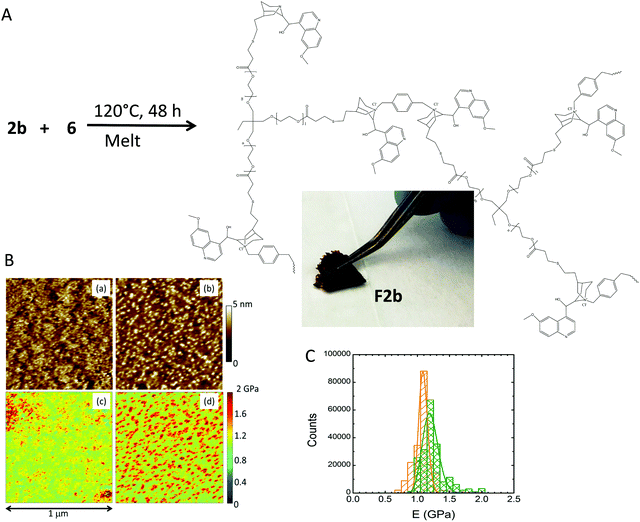
To increase film flexibility without involving high boiling point solvents, we turned our attention to a commercially available trifunctional thiolated cross-linker having short PEG segments in its chemical structure, 5 (Fig. 1). Compound 5 is amorphous at room temperature, without any sign of crystallinity and with a glass transition temperature (Tg) of −60 °C (ESI†). We realised that the incorporation of 5 into the quinine-based films could improve the resulting film flexibility upon cross-linking. Moreover, above room temperature, the viscosity of 5 is such that it can be manipulated as a liquid without involving external solvents. Consequently, we synthesized compound 6 (Fig. 4A) from 1 and 5via the UV-activated thiol–ene coupling reaction to be used as a quinine-containing cross-linker during the subsequent step of film formation via quaternization with 2b. The synthesis of 6 was carried out in DMSO at room temperature under UV irradiation for 3 h. 6 was isolated in 20% yield by simple precipitation in toluene and further drying under dynamic vacuum. The 1H NMR spectrum of 6 is shown in Fig. 4B. Assignment of the peaks was assisted by 1H–13C HSQC and 1H COSY NMR measurements performed on neat 5 and 6 (Fig. 4C). Confirmation of the successful formation of 6 was also gained by ATR-FTIR spectroscopy (see the ESI†). When compared to neat 5, compound 6 showed a value of Tg of −18 °C with no signs of crystallization. Consequently, the incorporation of quinine moieties into 5, even if increasing the value of Tg, still produces an amorphous compound that could improve the flexibility of the resulting cross-linked films. Thermogravimetric analysis measurements showed that 6 had no weight loss until 180 °C (see the ESI†). 6 was, to our delight, an excellent precursor of the valuable and active quinine-based film F2b (X = Cl) when quaternized in the melt with 2b, which has a melting point of ca. 100 °C. Film formation involved the mixing of 2b and 6 at 120 °C, followed by spreading the resulting blend on a thin glass that is maintained at that temperature for 48 h (see Fig. 5A and the ESI†). The cross-linking degree of F2b was easily tuned by changing the [2b]/[quinine] ratio (see the ESI†). In all cases investigated, [2b]/[quinine] ratio = 1/8 (denoted as film F2b-A), 1/4 (film F2b-B), 1/3 (film F2b-C) and 1/2 (film F2b-D), flexible cross-linked films were produced that were not soluble in DMSO, water or any other organic solvent. The insolubility of the films precluded their characterization by NMR techniques in solution. ATR-FTIR spectroscopy was used to monitor the changes in the vibration bands upon film formation, when compared to neat 6. Unfortunately, due to the presence of multiple overlapping IR bands, it was not possible to assign specific quaternization vibration bands. Film F2b-D showed a value of Tg of −14 °C with no signs of crystallinity. The nanoscale characterization of the quinine-based films was carried out by PF-QNM measurements and was compared with that of compound 6 (Fig. 5B). The roughness of the melt-quaternized film D was found to be slightly higher than that shown by a soluble film of neat compound 6, although both films showed a relatively flat surface at the nanoscale level (roughness < 2–3 nm). The nanostructured nature of film D is clearly visible in Fig. 5B. The melt-quaternized film D showed an average value of Young's modulus of 1.3 GPa, slightly higher than that of the film of compound 6 (1.1 GPa) due to its network, cross-linked structure. A significant reduction in the Young's modulus of film D was observed when compared to films F1a and F1b obtained by cross-linking in DMSO solution via UV-activated thiol–ene coupling. In fact, the value of the Young's modulus of film D was similar to that found in polymers such as poly(quinoline) and poly(p-xylylene), as well as biological materials like bacteriophage capsids.19
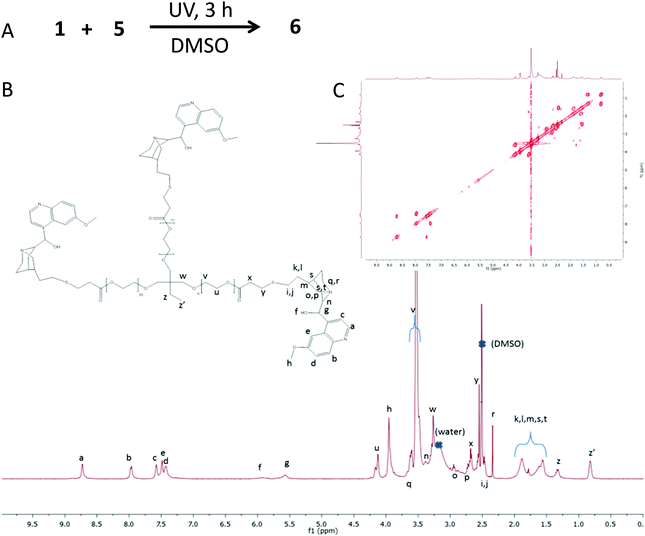 | ||
| Fig. 4 (A) Illustration of reaction conditions employed to synthetize 6 from 1 and 5via UV-activated thiol–ene coupling reaction. (B) 1H NMR spectrum of 6. (C) 1H COSY spectrum of 6. | ||
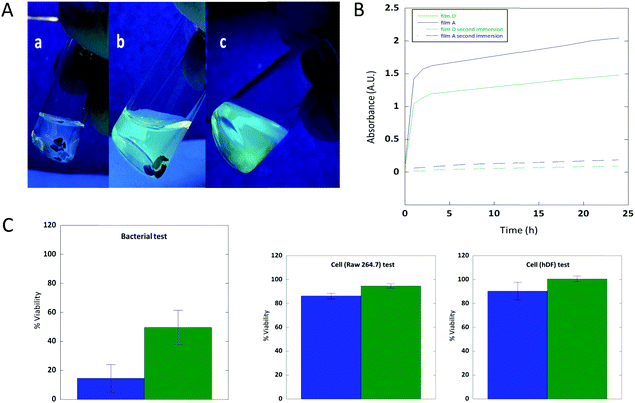
One of the main advantages of the second strategy towards quinine-based cross-linked coatings is that active films are directly obtained, as illustrated in Fig. 6A. We observed fluorescence upon immersion of film D in water, which we attribute to the delivery of residual unreacted 6 having fluorescent quinine moieties, because unbonded 2b that should also be released is a non-fluorescent compound.20 Similarly, if a drop of water is deposited on neat film D, it was found to turn fluorescent very quickly (see the ESI†). UV-vis spectroscopy measurements revealed an absorption band located at 330 nm, arising from quinine moieties of 6.21Fig. 6A shows the fluorescence intensity after immersion of film D in water, after 2 h with the film still remaining at the bottom of the vial, and after 24 h of immersion with final removal of film D from the water. Fig. 6B shows experimental delivery curves corresponding to two consecutive immersion cycles of 24 h for films A and D. The delivery process was almost complete after 24 h and a fast delivery was observed in the first two hours for both films. As expected, at a given time, the fluorescence intensity increases upon decreasing the [2b]/[quinine] ratio (i.e., upon decreasing the cross-linking degree).
Cell and bacterial tests were carried out to determine the bioactivity of the elution media from the active melt-quaternized quinine-based films (see Fig. 6C and the ESI†). Remarkably, when the elution media from films A and D were incubated at 37 °C for 72 h with mouse macrophage cell line Raw 264.7 and human dermal fibroblast (hDF) cells, cell viability above 80% was observed in both cases indicating the non-cytotoxic activity of both films. On the other hand, the elution media from films A and D were found to exhibit antibacterial properties against E. coli when incubated at 37 °C for 4 h as illustrated in Fig. 6C. Films A and D reduced the bacterial viability below 20 and 50%, respectively, which can be explained by the fact that the lowest cross-linked film delivers a higher amount of active compound when compared to the highest cross-linked one. These results are of great interest for the potential development of new active bio-based products based on cinchona alkaloids. According to the results shown in Fig. 6B, one cannot expect significant antibacterial activity after two immersion cycles of 24 h due to complete elution of the active compounds.
3. Conclusions
We have introduced an innovative synthetic strategy toward active nanostructured cinchona alkaloid-based coatings by means of simple melt quaternization/spreading procedures at a relatively low temperature (120 °C) without the involvement of any solvent. Through this ground-breaking concept, we have produced active, flexible and nanostructured quinine-based films (Tg = −14 °C, and Young's modulus = 1.3 GPa). These films are able to release antimicrobial compounds against E. coli that are noncytotoxic against mouse macrophage and human dermal fibroblast cells. Remarkably, these kinds of active cinchona alkaloid-based coatings are facile to prepare, making this strategy one of the most facile reported procedures to date to produce active bio-based films with potential use in the fields of food packaging, health care and medicine.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by the Spanish Ministry “Ministerio de Economia y Competitividad”, MAT2015-63704-P (MINECO/FEDER, UE) and the Basque Government, IT-654-13 is acknowledged. A. L.-S. is grateful to the University of the Basque Country for his UPV/EHU pre-doctoral grant, and to both MPC and Gipuzkoako Foru Aldundia for financial support during his stay at KTH. The authors thank José I. Miranda, Antonio Veloso and Daniel E. Martínez-Tong for excellent technical assistance.Notes and references
- R. M. Donlan, Emerging Infect. Dis., 2001, 7, 277–281 CrossRef CAS PubMed.
- P. L. Tran, N. Lowry, T. Campbell, T. W. Reid, D. R. Webster, E. Tobin, A. Aslani, T. Mosley, J. Dertien, J. A. Colmer-Hamood and A. N. Hamood, Antimicrob. Agents Chemother., 2012, 56, 972–978 CrossRef CAS PubMed.
- V. K. Vendra, L. Wu and S. Krishnan, in Nanomaterials for the Life Sciences Vol. 5: Nanostructured Thin Films and Surfaces. ed. C. S. S. R. Kumar, Wiley-VCH, Weinheim, 2010, ch. 1, pp. 1–39 Search PubMed.
- S. Jepsen, T. Berglundh, R. Genco, A. M. Aass, K. Demirel, J. Derks, E. Figuero, J. L. Giovannoli, M. Goldstein, F. Lambert, A. Ortiz-Vigon, I. Polyzois, G. E. Salvi, F. Schwarz, G. Serino, C. Tomasi and N. U. Zitzmann, J. Clin. Periodontol., 2015, 42, S152–S157 CrossRef PubMed.
- K. Chaloupka, Y. Malam and A. M. Seifalian, Trends Biotechnol., 2010, 28, 580–588 CrossRef CAS PubMed.
- Y. Chang, S.-C. Liao, A. Higuchi, R.-C. Ruaan, C.-W. Chu and W.-Y. Chen, Langmuir, 2008, 24, 5453–5458 CrossRef CAS PubMed.
- (a) A. d. l. S. Pereira, S. Sheikh, C. Blaszykowski, O. Pop-Georgievski, K. Fedorov, M. Thompson and C. Rodriguez-Emmenegger, Biomacromolecules, 2016, 17, 1179–1185 CrossRef PubMed; (b) G. Subbiahdoss, B. Pidhatika, G. Coullerez, M. Charnley, R. Kuijer, H. C. van der Mei, M. Textor and H. J. Busscher, Eur. Cells Mater., 2010, 19, 205–213 CrossRef CAS.
- Z. Li, D. Lee, X. Sheng, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 9820–9823 CrossRef CAS PubMed.
- T. S. Kaufman and E. A. Rúveda, Angew. Chem., Int. Ed., 2005, 44, 854–885 CrossRef CAS PubMed.
- D. A. Casteel in Burger's Medicinal Chemistry and Drug Discovery, ed. M. E. Wolff, Wiley, New York, 5th edn, 1997, ch. 59, vol. 5, p. 16 Search PubMed.
- R. Wolf, A. Baroni, R. Greco, F. Corrado, E. Ruocco, M. A. Tufano and V. Ruocco, Dermatol. Online J., 2003, 9, 3 Search PubMed.
- S. A. Kharal, Q. Hussain and A. S. Fakhuruddin, J. Pak. Med. Assoc., 2009, 59, 208–212 Search PubMed.
- R. Wolf, M. A. Tufano, V. Ruocco, E. Grimaldi, E. Ruocco, G. Donnarumma and A. Baroni, Int. J. Dermatol., 2006, 45, 661–663 CrossRef CAS PubMed.
- J. Lv, Y. Qian, T. Liu and Y. Wang, Bioorg. Med. Chem. Lett., 2007, 17, 4102–4106 CrossRef CAS PubMed.
- Frontiers in Biomaterials, Vol. 3, Chitosan Based Materials and its Applications. ed. G. L. Dotto, S. P. Campana-Filho and L. A. de Almeida Pinto, Bentham Science Publishers, Sharjah, 2017, pp. 249–271 Search PubMed.
- (a) M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons, Inc., New Jersey, 6th edn, 2007, ch. 10, p. 555 Search PubMed; (b) R. Lambert, A.-L. Wirotius and D. Taton, ACS Macro Lett., 2017, 6, 489–494 CrossRef CAS.
- (a) A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC; (b) M. Claudino, M. Jonsson and M. Johansson, RSC Adv., 2014, 4, 10317–10329 RSC; (c) I. Perez-Baena, I. Asenjo-Sanz, A. Arbe, A. J. Moreno, F. Lo Verso, J. Colmenero and J. A. Pomposo, Macromolecules, 2014, 47, 8270–8280 CrossRef CAS.
- (a) J. C. Middleton and A. J. Tipton, Biomaterials, 2000, 21, 2335–2346 CrossRef CAS PubMed; (b) A. Södergård and M. Stolt, Prog. Polym. Sci., 2002, 27, 1123–1163 CrossRef.
- (a) M. E. Dokukin and I. Sokolov, Langmuir, 2012, 28, 16060–16071 CrossRef CAS PubMed; (b) I. L. Ivanovska, P. J. de Pablo, B. Ibarra, G. Sgalari, F. C. MacKintosh, J. L. Carrascosa, C. F. Schmidt and G. J. L. Wuite, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 7600–7605 CrossRef CAS PubMed; (c) Polymer Data Handbook, ed. J. E. Mark, 2nd edn, University Press, New York, 2009, pp. 1014–1017 and pp. 1184–1187 Search PubMed.
- All our attempts to determine the molar ratio of 6 to 2b by NMR spectroscopy failed.
- (a) R. L. O’Brien, J. G. Olenick and F. E. Hahn, Proc. Natl. Acad. Sci. U. S. A., 1966, 55, 1511–1517 CrossRef; (b) R. I. Allen, K. J. Box, J. E. A. Comer, C. Peake and K. Y. Tam, J. Pharm. Biomed. Anal., 1998, 17, 699–712 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, instrumentation, synthetic details and additional results. See DOI: 10.1039/c7tb02739g |
| This journal is © The Royal Society of Chemistry 2018 |