Constructing stable ordered ion channels for a solid electrolyte membrane with high ionic conductivity by combining the advantages of liquid crystal and ionic liquid†
Abstract
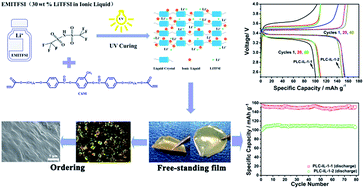
The advantages of solid-state polymer electrolytes (SPEs) such as ease of fabrication, high safety, good compatibility with Li metals make SPEs one of the most promising electrolyte materials for next generation high-performance and high-safety lithium-ion batteries (LIBs). However, the traditionally used PEO-based electrolytes usually exhibit very low ionic conductivity due to the high crystallinity of PEO, which impedes the commercialization of solid-state LIBs using PEO-based polymer electrolytes. In this study, an ‘alternative’ solid electrolyte material was prepared using a nematic liquid crystal (LC) and an ionic liquid (IL). Specifically, the LC with ordered layered nanostructures was polymerized and immobilized via UV-irradiation, while IL was sufficiently inserted into the ordered ion channels for fast transport ions. It should be noted that such a free-standing electrolyte film with stable ordered channels has been confirmed through the characterizations of DSC, POM, SEM and XRD. As a result, the solid electrolyte film shows superior comprehensive electrochemical performance in terms of a very high room temperature ionic conductivity (2.14 × 10−2 S cm−1), wide electrochemical window (4.8 V), and very good compatibility with lithium metal. Furthermore, the LiFePO4/Li cell using the ordered electrolyte film shows an average discharge capacity of 150 mA h g−1. Undoubtedly, our study provides a new solid electrolyte material that has the potential to be used in the next generation of high safety and high energy density LIBs.


 Please wait while we load your content...
Please wait while we load your content...