Facile construction of crosslinked all-carbon-backbone anion-exchange membranes with robust durability†
Abstract
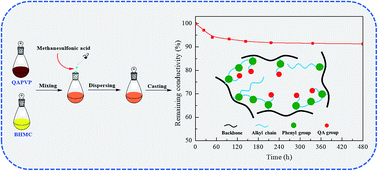
With the aim of preparing highly alkaline stable anion-exchange membranes (AEMs) with desirable ionic exchange capacity (IEC) and swelling, a series of crosslinked poly(4-vinylphenol) (PVP)-based membranes was prepared. A facile crosslinking method using bis(hydroxymethyl)-4-cresol (BHMC) as the crosslinker was introduced in the preparation of AEMs to avoid sacrificing specific functional groups such as –OH in the backbone. It is worth mentioning that the whole procedure for membrane preparation was efficient and easy to handle. The as-prepared membranes exhibited pretty low water uptake and swelling by the introduction of a crosslinked structure. Microphase separation to different extents could be observed in the membranes with different degrees of crosslinking via transmission electron microscopy (TEM) and small angle X-ray scattering (SAXS). The QAPVP-5% membrane with an ionic exchange capacity (IEC) of 2.12 meq g−1 displayed the highest ionic conductivity of 78 mS cm−1 at 80 °C. Moreover, the all-carbon backbone endowed an excellent alkaline stability of the membranes, and the QAPVP-5% membrane still retained 91.3% of its original conductivity after immersing in a 2 M aqueous KOH solution at 80 °C for 480 h. A peak power density of 122 mW cm−2 was also obtained by a single cell equipped with the QAPVP-5% membrane.


 Please wait while we load your content...
Please wait while we load your content...