Phosphorized polyoxometalate-etched iron-hydroxide porous nanotubes for efficient electrocatalytic oxygen evolution†
Abstract
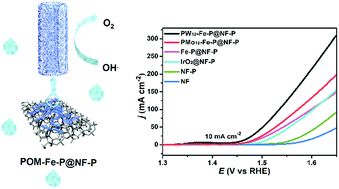
Splitting water into H2 and O2 attracts significant attention to meet the increasing need for energy. As the oxygen evolution reaction (OER) constitutes the bottleneck in water splitting, the development of efficient OER catalysts is highly desirable. As a potential electrocatalyst for this reaction, we demonstrate herein a facile two-step method to synthesize FeOOH/FePOx porous nanotubes, which involve the phosphorization of β-FeOOH nanotubes that are hydrothermally prepared with the assistance of polyoxometalates (POMs). The acidity of POMs readily modulates the pore size and surface area of the nanotubes. Benefitting from the porous and hollow feature to expose multiple active sites, the FeOOH/FePOx porous nanotubes can be used as a high-performance electrocatalyst, which offers an overpotential of 230 mV at a current density of 10 mA cm−2 with extraordinary stability in 300 h operation. Its superior performance exceeds that of the commercial catalyst (IrO2, 275 mV) under the same conditions and those of most reported Fe catalysts.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...