CoO-modified Co4N as a heterostructured electrocatalyst for highly efficient overall water splitting in neutral media†
Abstract
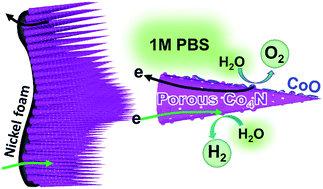
Obtaining a high-performance nonprecious electrocatalyst for oxygen and hydrogen evolution reactions (OER and HER) under mild conditions is highly desirable yet challenging. Transition metal nitrides are emerging as cost-effective substitutes for state-of-the-art noble metal based catalysts, but large OER overpotentials plague these nitrides. Herein, we developed the heterostructure of CoO-domain-anchored Co4N nanowhiskers as an effective bifunctional electrocatalyst for both OER and HER in neutral media. The exterior CoO targets OER, and the CO4N matrix is active for HER. The periodically-aligned {111} crystalline planes between Co4N and CoO provide good electron transport through their interface. The heterostructure performs dual functions of elevating activity and diminishing interior resistance of the catalyst. Moreover, the porous metallic Co4N nanowhiskers were integrated on a nickel foam, and this configuration contributes an arterial channel for collecting current as well as a spacious surface area for increasing the reaction cross section for heterocatalysis. These advantages actuate our bifunctional 3D catalytic electrode to achieve low OER and HER overpotentials in neutral systems. It finally provides an unprecedentedly low cell voltage (1.79 V for 10 mA cm−2) for catalyzing overall water splitting in neutral media.


 Please wait while we load your content...
Please wait while we load your content...