Recent advances toward practical use of halide perovskite nanocrystals
Abstract
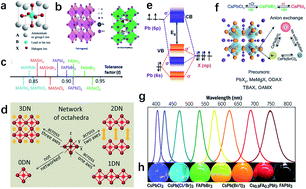
Halide perovskite nanocrystals (NCs) and quantum dots (QDs) have received considerable attention, due to their superior photoluminescence quantum yields close to unity, variable morphologies, and tunable optical bandgaps achieved by modifying their composition, size and dimensionality. Their potential applications in solar cells, LEDs and photodetectors have driven research efforts to validate the feasibility of practical use of these materials in future optoelectronics and electronics. From the perspective of commercial applications, there are three issues of serious concern, namely, the toxicity of Pb, chemical instability, and the limited yield of NCs/QDs. In this review, we mainly focus on the recent research progress in the areas of relevance. First, we summarize the development of Pb-free perovskite NCs in the context of exploiting new materials. Second, we review feasible strategies to improve the stability of NCs and QDs during their preparation and incorporation. Third, batch synthesis methods are reviewed, which are focused on the reproducibility and yields towards mass production. Finally, a brief outlook is provided to forecast potential development to address the challenges in the future.
- This article is part of the themed collections: Recent Review Articles and Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...