Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage
Biao
Gao†
 a,
Xingxing
Li†
a,
Kang
Ding
a,
Chao
Huang
a,
Qingwei
Li
b,
Paul K.
Chu
c and
Kaifu
Huo
a,
Xingxing
Li†
a,
Kang
Ding
a,
Chao
Huang
a,
Qingwei
Li
b,
Paul K.
Chu
c and
Kaifu
Huo
 *ab
*ab
aThe State Key Laboratory of Refractories and Metallurgy, Institute of Advanced Materials and Nanotechnology, Wuhan University of Science and Technology, Wuhan 430081, China
bWuhan National Laboratory for Optoelectronics (WNLO), School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: kfhuo@hust.edu.cn
cDepartment of Physics, Department of Materials Science and Engineering, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, China
First published on 7th September 2018
Abstract
On the heels of the rapid development of portable electronics, electric vehicles, and renewable energy, electrochemical energy storage (EES) devices have become more prevalent. Electrode materials are key components for EES devices and largely determine their energy storage performance. Transition metal nitrides (TMNs) are promising electrode materials suitable for a wide range of EES devices including supercapacitors and rechargeable batteries due to their unique electronic structure, high conductivity, and large volumetric energy density, as well as good electrocatalytic activity. However, the practical implementation of TMNs in EES systems is hampered by their limited electrochemically active sites, unsatisfactory capacitance (capacity), and poor durability and flexibility. In comparison, TMN-based nanocomposites have been demonstrated to possess improved EES properties because they boast potential synergistic effects that ameliorate the electron and ion conductivity, prevent agglomeration, enhance the active sites, and improve the electrochemical stability. In this paper, recent advances pertaining to TMN-based hybrid materials are reviewed from the perspective of advanced EES systems with the focus on advanced supercapacitors and lithium–sulfur batteries. The challenge and future opportunities confronting TMN-based electrode materials in high-performance EES systems are also discussed.
1. Introduction
Owing to environmental pollution and depletion of fossil fuels, clean and renewable energy such as solar and wind energy is playing a more important role.1 However, the deployment of these sustainable energy technologies requires more efficient and reliable electrochemical energy storage (EES) systems that could offset this intermittency by storing the energy generated and making it available upon demand. Moreover, the rapid development of portable electronic devices and electric vehicles has spurred a remarkably increased interest in EES devices. Therefore, high-efficiency EES devices have been explored extensively in recent years and attracted increasing interest spanning from fundamental research to industrial applications.2,3Current EES systems include mainly supercapacitors (SCs), Li-ion batteries (LIBs), and Li–sulfur (Li–S) batteries, as well as Li-ion hybrid capacitors (LiHCs).4 In spite of different definitions, they have common features in the configurations. Generally, EES devices are composed of three indispensables: a negative electrode (anode), a positive electrode (cathode), and an aqueous/organic electrolyte.4,5 Since charges are stored on/in the electrodes in EES systems, the energy storage performance has been determined primarily by the electrode materials,6,7 and therefore, the development of high-efficiency electrode materials is vital to obtaining high energy and power densities as well as long cycle life for EES devices.
Various electrode materials have been developed for EES devices, for instance, carbon materials,5,8–11 metal oxides,12–15 carbides,16,17 nitrides,17–20 sulfides,21–23 phosphides24 and alloy elements25 and impressive advances have been achieved. Among them, group IVB–VIB early-transition-metal nitrides (early-TMNs) are remarkable electrode materials suitable for a wide range of EES devices including SCs, LIBs, Li–S batteries, and LiHCs because of their unique electronic structure, high conductivity, large volumetric energy density, and excellent catalytic activity.17,18 Different TMNs with various morphologies including nanoparticles (NPs), nanowires (NWs), nanorods (NRs), nanotubes (NTs), nanosheets (NSs) and nanohybrids of titanium nitride (TiN), vanadium nitride (VN), molybdenum nitride (MoN or Mo2N) and niobium nitride (Nb4N5 or NbN) have been fabricated and their EES characteristics have been studied.17,18,26–30 Nevertheless, the practical implementation of TMNs in EES systems has been stifled by the limited active sites, small capacitance (capacity), unsatisfactory durability, and brittleness. TMN-based nanocomposites with potential synergistic effects can prevent agglomeration, enhance the active sites, improve the electronic conductivity, and facilitate ion transport to boost the EES performance. Recently, several review papers have summarized advances pertaining to the synthesis and potential applications of TMNs in LIBs, SCs, fuel cells and electrocatalytic water splitting.17,18,20,31,32 However, the progress of TMN-based hybrid nanostructures in EES systems has not been discussed extensively. Moreover, the past few years have witnessed the rapid development of TMNs for EES devices including Li–S batteries and LiHCs and the progress has not been reviewed.
In this review, the structure and physical properties of TMNs are described and recent advances in TMN-based electrode materials in SCs, Li–S batteries, LIBs, and LiHCs are systematically summarized with the focus on high volumetric capacitance SCs and high-energy density Li–S batteries (Fig. 1). Finally, the challenges and future opportunities of TMN-based materials in high-performance EES systems are discussed.
 | ||
| Fig. 1 Nanostructured TMNs for typical energy storage devices such as SCs, LIBs, LiHCs and Li–S batteries. Copyright permission has been received for all the images in figures. | ||
2. Structure and physical properties of TMNs
Group IVB–VIB TMNs such as TiN, VN, NbN, MoN, Mo2N, and WN are interstitial compounds in which the nitrogen atoms are integrated into the interstitial sites of the parent metals.33Table 1 shows the structure and physical properties of common Group IVB–VIB TMNs including TiN, VN, NbN, Nb4N5, MoN, Mo2N and WN. TMNs typically have cubic, hexagonal closed packed and simple hexagonal structures (Fig. 2).17 Generally, TMNs possess an unusual combination of metallic, covalent, and ionic properties.19,33,34 The metallic and covalent characteristics endow early-TMNs with high conductivity (for example, the conductivity of VN is 1.44 × 105 S m−1),28 large hardness, high melting point, and Young's modulus (above 360 GPa).17 Therefore, TMNs are widely used as protective coatings on industrial components and diffusion-barrier layers in microelectronic devices.35,36 Moreover, the M–N bonding in TMNs causes expansion of the parent metal lattice and constriction of the metal d-band.17,37 The smaller deficiency in the d-band occupation and higher density of states of the metal near the Fermi level endow TMNs with noble metal-like catalytic activity. The appealing properties of TMNs with high electronic conductivity, catalytic activity and stable chemical properties play irreplaceable roles in advanced EES devices. Furthermore, TMNs have larger densities (for example, VN: 6.13 g cm−3) than carbon-based materials (generally <1.0 g cm−3) thus giving rise to a larger volumetric energy density in EES. TMNs with these unique properties are potential electrode materials in EES devices. In view of the thriving of TMNs, we will discuss the latest progress in the synthesis and applications of TMN-based electrode materials in high-performance EES devices such as SCs, Li–S batteries, LIBs, and LiHCs.| Parent | Nitride | Crystal structure | Density (g cm−3) | Melting point (°C) | Young's modulus (GPa) | Electrical conductivity (S m−1) |
|---|---|---|---|---|---|---|
| a d-decompose. | ||||||
| Ti | TiN | Cubic | 5.40 | 3050 | 420 | 3.70 × 106 |
| V | VN | Cubic | 6.13 | 2390 | 380 | 1.49 × 106 |
| Nb | NbN | Cubic | 8.47 | 2630da | 360 | 1.60 × 106 |
| Nb4N5 | Tetragonal | 7.51 | — | — | 2.31 × 103 | |
| Mo | Mo2N | Cubic | 9.46 | 790da(0.7 MPa) | ∼365 | 2.97 × 104 |
| MoN | Hexagonal | 7.06 | — | — | 2.31 × 103 | |
| MoN2 | Rhombohedral | 5.49 | — | — | — | |
| W | WN | Hexagonal | 17.7 | — | ∼430 | 6.97 × 103 |
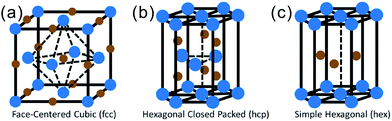 | ||
| Fig. 2 Common crystal structures in TMN compounds: (a) cubic; (b) hexagonal closed packed; and (c) simple hexagonal. The blue points represent transition metal atoms and the brown points represent nitrogen atoms.17 Reproduced with permission from ref. 17. Copyright 2016, Wiley-VCH. | ||
3. Synthesis of nanostructured TMNs and TMN based composites
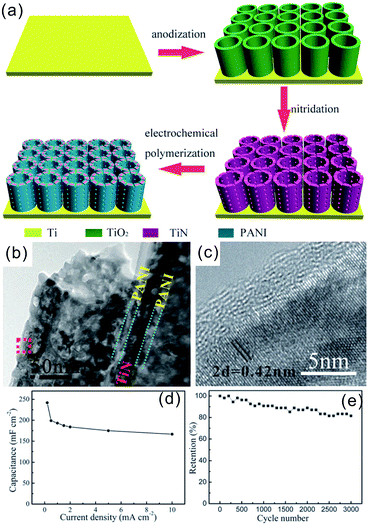
Nanostructured TMNs are generally produced from oxide precursors via post NH3 reduction annealing. This conversion process maintains the morphology of the oxides but produces abundant mesopores due to volume shrinkage from oxide to nitride as a result of the density difference between the oxide precursors and nitride products. For example, Gao et al. prepared mesoporous VN NWs by thermally nitriding V2O5 NWs38 and Lee et al. prepared mesoporous Mo3N2 NWs by nitridation of MoO3 NWs.39 Very recently, Bi et al. prepared (001) exposed VN mesocrystal NSs via nitriding a layered Na2V6O16 self-sacrificing template28 and Xiao et al. fabricated two-dimensional (2D) MoN NSs with subnanometer thickness and sheet size exceeding 20 μm via nitriding 2D h-MoO3.40 The topochemical conversion from transition metal oxides (TMOs) to TMNs could produce various TMN nanostructures such as VN, TiN, and Nb4N5 with different morphologies from zero dimension (0D) to three dimension (3D) because of the enriched morphologies of the TMOs.17,18,26,29,41TMNs/C hybrids were fabricated by mixing the existing TMNs with CNTs or coating carbon (C) on TMNs. For example, Xiao et al. prepared a VN NWs/CNT hybrid electrode by simple physical mixing of VN NWs and CNTs.42 Lu et al. obtained carbon coated TiN NWs by hydrothermal treatment of TiN NWs in glucose solution, followed by annealing at 800 °C in nitrogen.43 TMNs could be deposited on carbon to form TMNs/C hybrids. For example, Zhang et al. deposited a VN layer on a CNT array anchored on glassy carbon or Inconel substrates via magnetron sputtering of the vanadium target in mixed argon/nitrogen plasma.44 Yang et al. fabricated corn-like TiN nanoarrays on carbon cloth (CC) by atomic layer deposition (ALD) of TiO2 on the cobaltous dihydroxycarbonate (Co2(OH)2CO3) NW precursor followed by thermal nitridation and removal of the Co2(OH)2CO3 precursor in a diluted acid.45 Haldorai et al. prepared TiN nanopillars on graphene by refluxing the Ti(OBu)4 precursor and GO (graphene oxide) followed by thermal nitridation.46
Other TMN-based hybrid materials such as TMN supported TMOs (TMOs/TMNs), TMN supported conducting polymers (CPs/TMNs), and TMN supported nitrides (TMNs/TMNs) were fabricated by using TMNs as supporting materials to deposit or load other materials.47–49 For example, Peng et al. electrochemically polymerized polyaniline (PANI) on the outer/inner surface as well as wall nanoholes of TiN NT arrays (NTAs) to form a coaxial PANI/TiN/PANI hybrid nanostructure.48 In recent years, although tremendous progress has been achieved, there is still a great challenge to produce TMNs and their hybrid nanostructures with desired dimensions, morphologies and pore structures via low-cost and scalable methods.
4. Nanostructured TMN-based electrode materials for high-performance SCs
SCs, also referred to as electrochemical capacitors, have attracted increasing scientific and technological attention that bridges the gap between conventional electrostatic capacitors and batteries.50,51 SCs possess a high charge/discharge rate, long cycle life (more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), and high power density, and are widely used in portable electronics, regenerative braking of trams, hybrid electric vehicles and memory back-up devices.52–55 According to their charge–discharge mechanisms, SCs are classified as electrical double-layer capacitors (EDLCs) and pseudocapacitors.50,53,55 The former store charges via fast ion adsorption/desorption on large-surface-area carbon electrode materials such as carbon nanotubes (CNTs), activated carbon, or graphene,52,54 while the latter store charge mainly by fast and reversible redox reactions at or near the surface of the electrode materials, which have higher specific capacitances than carbon materials.56,57
000 cycles), and high power density, and are widely used in portable electronics, regenerative braking of trams, hybrid electric vehicles and memory back-up devices.52–55 According to their charge–discharge mechanisms, SCs are classified as electrical double-layer capacitors (EDLCs) and pseudocapacitors.50,53,55 The former store charges via fast ion adsorption/desorption on large-surface-area carbon electrode materials such as carbon nanotubes (CNTs), activated carbon, or graphene,52,54 while the latter store charge mainly by fast and reversible redox reactions at or near the surface of the electrode materials, which have higher specific capacitances than carbon materials.56,57
Since the capacitance of SCs is highly associated with the ion-accessible surface area of the electrode materials, nanostructured materials with a large effective area should possess improved capacitive storage performance. Nanostructured carbon materials such as CNTs, activated carbon, graphene, and three-dimensional carbon foams are employed as electrode materials in SCs. However, the low volumetric capacitance as a result of the small density (<1.0 g cm−3) and moderate gravimetric capacitance (generally <300 F g−1) has limited their application in portable and miniaturized capacitive storage.52 Pseudocapacitive materials including TMOs such as MnO2, WO3, and MoO3 as well as CPs like PANI and polypyrrole (PPy) exhibit higher specific capacitances (300–2000 F g−1).56,58 However, TMO or CP electrodes generally suffer from low electrical conductivity (e.g. conductivity of MnO2 is 10−5 to 10−6 S cm−1) or poor electrochemical stability.59–61
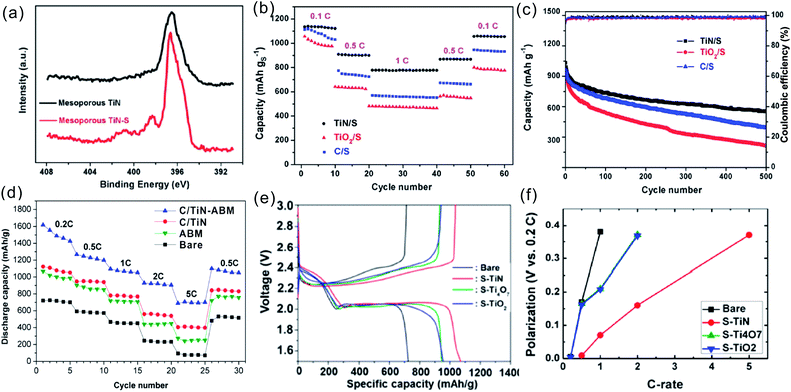
Nanostructured TMNs have emerged as promising electrode materials for SCs due to their intrinsic metallic conductivity, high pseudocapacitance and large density. Studies have demonstrated the use of TMNs in SCs and the remarkable capacitive properties of various nanostructured TMNs such as NPs, NWs or NTs of TiN,41,48 VN,62–64 Mo2N,65,66 MoN,40 Mo3N2,39 Nb4N5,27,29,67 WN,68 GaN,69 TiVN,70 and LaN71 have been reported. For example, Choi et al. reported that VN NPs exhibited a large specific capacitance of 1340 F g−1 in an aqueous KOH electrolyte.62 Lee et al. prepared mesoporous Mo3N2 NWs by nitridation of MoO3 NWs whose capacity was over 200 F g−1 even at a large charging/discharging rate of 200 mV s−1, better than that of MoO3 NWs.39 Recently, 2D structures such as MoN, VN, and TiN have been reported for SCs.28 For example, Bi et al. fabricated mesoporous VN NSs with the highest electrical conductivity of 1.44 × 105 S m−1 and flexible SCs based on VN NSs and polyvinyl alcohol (PVA)/LiCl gel with a superior volumetric capacitance of 1937 mF cm−3 at a current density of 5 mA cm−2.28 Xiao et al. fabricated 2D MoN NSs via nitriding MoO3 grown by the assistance of a NaCl crystal template.40,72 The MoN film electrodes obtained by vacuum filtration of the 2D MoN exhibited a high volumetric capacitance of 928 F cm−3 at 2 mV s−1 in H2SO4 electrolyte and excellent rate performance with a high capacitance of 200 F cm−3 even at an ultra-high scan rate of 20 V s−1. Moreover, this method can also be extended to fabricate other 2D nitrides such as W2N and V2N NSs. Because of their superior electrical conductivity and mechanical stability, nanostructured TMNs are also excellent conducting frameworks to load other electroactive materials for high-performance SCs. For example, highly conductive TiN NTs were used as backbones and supportive materials to load MnO2 (ref. 77) or PANI48 to achieve enhanced capacitive storage properties.
Although nanostructured TMNs have been demonstrated to be promising electrodes for SCs with high energy and power density, there are still some obstacles needed to be overcome to achieve high-performance capacitive storage, especially for flexible SCs. Firstly, nanostructured TMNs generally have small specific surface areas (generally <40 m2 g−1), resulting in limited electroactive sites for capacitive storage and compromised capacitive performance. Secondly, TMNs commonly suffer from severe capacitance decay during the long-term process in an aqueous acidic or alkaline electrolyte due to irreversible electrochemical oxidation.38,43 Thirdly, TMNs are inorganic nonmetallic ceramic materials which are brittle and have poor flexibility, and therefore they could not be directly applied in bendable and flexible SCs.43,78 To improve the specific capacitance, and cycle life, as well as mechanical flexibility, much effort has been devoted to the design and fabrication of TMN-based nanocomposites. In the following subsections, the latest progress associated with the development of TMN-based nanohybrids (mainly Ti, V, Nb, and Mo based nitrides) is described.
According to recent developments, TMN-based nanohybrids for SCs can be divided into three categories: (i) active TMNs deposited on metal or carbon substrates, (ii) freestanding or self-standing films of TMNs for flexible SCs, and (iii) TMNs as supporting frameworks for loading other electroactive materials.
4.1 Depositing active TMNs on metal or carbon substrates for SCs
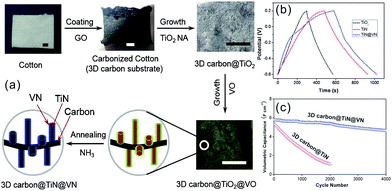
Direct growth or deposition of TMNs on conducting substrates can prevent agglomeration and provide channels for electron and ion transport to enhance the capacitive properties. These include coating and growing active materials of TMNs on metal substrates or carbon-based electrodes CC, carbon nanofibers (CNFs)/CNTs, and graphene.Xie et al. fabricated TiN nanopore arrays with a diameter of 80–100 nm and a thickness of 10.7 μm on a Ti substrate via electrochemical anodization of a Ti foil and further thermal nitridation. The areal specific capacitance of TiN nanopore arrays was 99.7 mF cm−2 at a current density of 0.20 mA cm−2 in 1 M H2SO4 solution, which is much higher than that of TiO2 nanoarrays (11.8 mF cm−2).79 Recently, we reported hierarchical TiN NP assembled (H-TiN) nanopillars grown on a Ti foil via nitriding hierarchical TiO2 nanopillar nanoarrays produced via hydrothermal treatment of anodic TiO2 NTs in pure water (Fig. 3a–d).41 The H-TiN nanopillar electrode exhibited a large volumetric capacitance of 120 F cm−3 at a current density of 0.83 A cm−3, which is almost 1.5-fold higher than that of TiN NT arrays. The improved volumetric capacitance of H-TiN nanopillars should be attributed to the higher number of active sites and larger surface area of H-TiN nanopillars as well as favourable electron/ion transport nanochannels. Such a structural design provides a promising method to improve specific capacitance via changing the morphology without increasing the volume of the electrode film. A symmetrical all-solid-state SC was assembled with two H-TiN nanopillars grown on Ti foil as electrodes and H3PO4/PVA as a gel-like electrolyte. The H-TiN nanopillar based device showed a high volumetric capacitance of 5.9 F cm−3 (based on the volume of the electrodes and electrolyte) at 0.02 A cm−3 and outstanding rate performance with a capacitance retention of 81.4% when the current density increased 10 times from 0.02 to 0.2 A cm−3. Recently, Cui et al. fabricated highly ordered Nb4N5 nanostructured arrays by anodizing the Nb foil and performing thermal nitridation (Fig. 3e).29 The Nb4N5 nanochannel electrode exhibited prominent electrical conductivity and electrochemical activity and a high areal capacitance of 225.8 mF cm−2 at a current density of 0.5 mA cm−2 in 1 M H2SO4 and also high rate capability with 60.8% retention from 0.5 to 10 mA cm−2 (Fig. 3f). Gao et al. synthesized mesoporous Nb4N5 quasi-aligned nanobelt (NB) arrays using the Nb foil as both the substrate and Nb precursor via hydrothermal growth of KNb3O8 arrays followed by protonation and thermal nitridation.67 The as-obtained Nb4N5 NBs showed an areal capacitance of 37.4 mF cm−2 at a current density of 0.2 mA cm−2 and robust rate performance with only 38% capacitance decay when the current density increased from 0.1 to 5 mA cm−2.
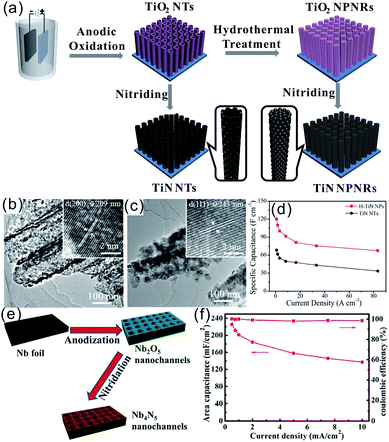 | ||
| Fig. 3 (a) Schematic diagram of the fabrication process of TiN NTs and H-TiN nanopillars; TEM images of the TiN NTs (b) and H-TiN nanopillars (c); (d) rate performance;41 (e and f) schematic illustration of the synthesis, rate performance and coulombic efficiency of Nb4N5.29 (a–d) Reproduced with permission from ref. 41. Copyright 2018, The Royal Society of Chemistry. (e and f) Reproduced with permission from ref. 29. Copyright 2016, Wiley-VCH. | ||
Despite these recent advances (Table 2), the conducting substrates (Nb and Ti) are usually much thicker and heavier than the coated TMN active materials. Even if the gravimetric specific capacitance based only on the active materials of TMNs is increased by the nanostructure design, the heavy metallic current collector gives rise to low volumetric and gravimetric capacitance of the whole SC device. Furthermore, the lower corrosive resistance of the metal substrate limits its implementation in acidic or alkaline electrolytes.
| Electrodes | Specific capacitance | Rate performance | Capacitance retention (%) after cycles | Ref. |
|---|---|---|---|---|
| TiN/Ti foil | 120 F cm−3 at 0.83 A cm−3 | 67 F cm−3 at 83 A cm−3 | 94.3% after 3000 cycles | 41 |
| TiN/Ti foil | 99.7 mF cm−2 at 0.20 mA cm−2 | 68.8 mF cm−2 at 4.00 mA cm−2 | 98.5% after 100 cycles | 79 |
| TiN/Ti fiber | 2.4 mF cm−1 at 10 mV s−1 | 0.83 mF cm−1 at 5 V s−1 | 80% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
80 |
| Nb4N5/Nb foil | 225.8 mF cm−2 at 0.5 mA cm−2 | 137.2 mF cm−2 at 10 mA cm−2 | 70.9% after 2000 cycles | 29 |
| Nb4N5/Nb foil | 37 mF cm−2 at 0.2 mA cm−2 | 23 mF cm−2 at 5 mA cm−2 | 80% after 1000 cycles | 67 |
| Ni–Mo–N/Ni foam | 2446 mC cm−2 at 2 mA cm−2 | 708 mC cm−2 at 100 mA cm−2 | 80.1% after 6000 cycles | 81 |
(1) Carbon cloth or carbon paper supported TMN electrodes for SCs. Carbon cloth (CC) is a flexible fabric consisting of carbon microfibers with good flexibility, high electronic conductivity, and excellent corrosion resistance, and is much lighter than metal foil thus acting as a good substrate to deposit or load TMNs for flexible SCs.82,83 Yang et al. fabricated corn-like TiN nanoarrays on CC via thermal nitridation of TiO2 nano-arrays on CC.45 A coin-like symmetrical capacitor comprising an organic electrolyte (1 mol L−1 LiClO4 in acetonitrile solution) and corn-like TiN electrodes was constructed. The TiN-based SCs showed a high power density of 150 W cm−3 at an energy density of 0.30 mW h cm−3 and had great potential in the instantaneous delivery of high power and energy.
Considering the flexibility and bendability of the CC and carbon paper substrates, growth of NFs or NRs of TMNs on the flexible CC or carbon paper is promising for flexible SCs. Lu et al. fabricated VN NWs on CC by nitriding hydrothermally formed TiO2 NWs.84 The CC supported VN NW electrode exhibited remarkable flexibility and a high specific capacitance of 298.5 F g−1 at a scan rate of 10 mV s−1 (based on the mass loading of VN NWs). Moreover, the VN NWs delivered outstanding rate performance with a capacitance retention of 71.5% when the scan rate was increased from 10 to 100 mV s−1 (Fig. 4a). The flexible asymmetric SC device based on CC supported VOx NWs (cathode) and VN NWs (anode) delivered a high volumetric capacitance of 1.6 F cm−3 (Fig. 4b) and retained excellent flexibility under repeated bending states (Fig. 4c). In addition to VN NWs, Lu et al. prepared TiN NWs on CC which exhibited a highest capacitance of 123 F g−1 at a scan rate of 10 mV s−1, which was almost 10 times higher than that of the corresponding TiO2 counterpart. The flexible SCs based on TiN NW electrodes grown on CC and LiCl/PVA gel-like electrolyte showed a volumetric capacitance of 0.33 F cm−3 at 2.5 mA cm−3 and maintained a good energy storage performance under different bending conditions (Fig. 4d–f).78
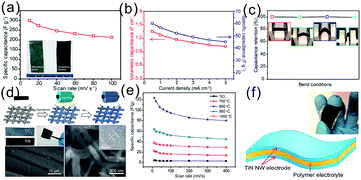 | ||
| Fig. 4 (a) Rate performance of the CC supported VN NW electrode. The insets in (a) are digital pictures of VOx and VN NWs grown on CC; (b) rate performance of the VOx//VN device; (c) cycling performance of the solid state device and the insets in (c) show the capacitance retention of the devices at different bending angles;84 (d) schematic diagram showing the process for preparing TiN NWs on CC and scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the TiN NW; (e) rate performance of TiO2 and TiN NW electrodes; (f) schematic diagram of an all solid-state TiN based SC. The inset is the digital picture of the device.78 (a–c) Reproduced with permission from ref. 84. Copyright 2013, American Chemical Society. (d–f) Reproduced with permission from ref. 78. Copyright 2012, American Chemical Society. | ||
Recently, He et al. prepared CC supported carbon coated W2N core–shell NWs (W2N@C/CC) by aerosol-assisted chemical vapor deposition followed by NH3 annealing.85 The W2N@C/CC electrodes exhibited a large areal specific capacitance of 693.0 mF cm−2 at 5 mV s−1 and outstanding rate capability with 59% capacitance retention when the current was increased from 5 to 50 mV s−1. The asymmetrical coin-cell type SC based on W2N@C CSA/CC (negative electrode) and nitrogen-doped graphene fabricated on commercial carbon paper (positive electrode) with an ionic liquid as the electrolyte exhibited a high capacitance of 170 mF cm−2 at 1 mA cm−2 with 85% capacitance retention when the current density increased to 10 mA cm−2.
The capacitive properties of CC supported Ni3N NSs and gallium nitride NW (GaN NW)/graphite paper (GP) nanocomposites were reported.69,87 The 3D Ni3N/carbon composite electrode showed large capacitances of 842 F g−1 at a scan rate of 10 mV s−1 and 252.6 F g−1 at 100 mV s−1. The composite electrode of GaN NW/GP had a large areal capacitance of 23.7 mF cm−2 at 10 mV s−1 and extraordinary cycling stability with 99% capacitance retention after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.69 The flexible symmetrical SC based on GaN NW/GP electrodes and H2SO4/PVA gel-like electrolyte showed high energy/power densities (0.65 μW h cm−2/45 mW cm−2).
000 cycles.69 The flexible symmetrical SC based on GaN NW/GP electrodes and H2SO4/PVA gel-like electrolyte showed high energy/power densities (0.65 μW h cm−2/45 mW cm−2).
Although the CC supported nanostructured TMN electrodes deliver promising flexible energy storage performance (Table 3), noncapacitive CC increases the device volume and weight, resulting in a lower specific capacitance and energy/power density considering the entire electrode and devices.
| Electrodes | Specific capacitance | Rate performance | Capacitance retention (%) after cycles | Ref. |
|---|---|---|---|---|
| TiN/CC | 159 F g−1 at 0.25 A g−1 | 124.5 F g−1 at 5 A g−1 | 91.7% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
43 |
| TiN/CC | 123 F g−1 at 10 mVs−1 | 79 F g−1 at 400 mVs−1 | 83.0% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
78 |
| TiON/CC | 38.3 F g−1 at 0.1 A g−1 | 16.0 F g−1 at 1 A g−1 | 83.0% after 8000 cycles | 88 |
| VN/CC | 298.5 F g−1 at 10 mV s−1 | 213.4 F g−1 at 100 mV s−1 | 87.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
84 |
| W2N/CC | 693.0 mF cm−2 at 5 mV s−1 | 540.5 mF cm−2 at 50 mV s−1 | 91% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
85 |
| Ni3N/CC | 990 F g−1 at 10 mA cm−2 | 800 F g−1 at 40 mA cm−2 | 81% after 200 cycles | 87 |
| Fe2N/CC | 169 F g−1 at 10 mV s−1 | 130 F g−1 at 10 mV s−1 | — | 89 |
| GaN/GP | 237 mF cm−2 at 0.1 mA cm−2 | 180.5 mF cm−2 at 5 mA cm−2 | 98% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
69 |
(2) Nano-carbon/TMN hybrid materials for SCs. Nano-carbon materials including 1D CNTs, 2D graphene, and 3D mesoporous carbon have large surface areas, excellent electrical properties, and high electrochemical stability and also provide abundant sites for loading active materials.90–92 Therefore, they are good substrates or supporters to introduce pseudocapacitive TMN materials for high performance SCs (Table 4).
| Electrodes | Specific capacitance | Rate performance | Capacitance retention (%) after cycles | Ref. |
|---|---|---|---|---|
| TiN/CNTs | 6.4 mF cm−2 at 100 mV s−1 | 4.3 mF cm−2 at 1000 mV s−1 | 86.5% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
86 |
| VN/CNT | 289 F g−1 at 20 mV s−1 | 230 F g−1 at 1000 mV s−1 | 64% after 600 cycles | 44 |
| VN/carbon fiber | 406.5 F g−1 at 0.5 A g−1 | 305.3 F g−1 at 5 A g−1 | 65.3% after 5000 cycles | 93 |
| VN/graphite foam | 177 C g−1 at 5 mV s−1 | 124 C g−1 at 5 mV s−1 | — | 98 |
| VN/carbon NS | 280 F g−1 at 1 A g−1 | 166 F g−1 at 10 A g−1 | 60% after 5000 cycles | 103 |
| VN@porous carbon nanospheres | 229.7 F g−1 at 1 A g−1 | 128.9 F g−1 at 16 A g−1 | 65.5% after 5000 cycles | 104 |
| VN@carbon fiber | 240.5 F g−1 at 0.5 A g−1 | 173.4 F g−1 at 5 A g−1 | 78.2% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
105 |
| VN/porous carbon | 255 F g−1 at 1 A g−1 | 175 F g−1 at 5 A g−1 | 66% after 1000 cycles | 107 |
| VTiN/carbon | 430.7 F g−1 at 0.5 A g−1 | 141.7 F g−1 at 10 A g−1 | 63% after 600 cycles | 106 |
| MoxN/carbon | 251 F g−1 at 0.5 A g−1 | 187 F g−1 at 5 A g−1 | 78.6% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
94 |
| Mo2N@C-rGO | 514.54 F g−1 at 0.25 A g−1 | 304.32 F g−1 at 10 A g−1 | 96% after 3000 cycles | 97 |
| FeN/carbon | 507 F g−1 at 0.5 A g−1 | 366 F g−1 at 20 A g−1 | — | 108 |
Zhang et al. deposited nanocrystalline VN on CNTs anchored on glassy carbon or Inconel substrates via magnetron sputtering of V in N2/Ar plasma (Fig. 5a and b).44 The VN functionalized CNT showed a respectable specific capacitance of 289 F g−1 in 1 M KOH electrolyte at a scan rate of 20 mV s−1. The well connected and highly electrically conductive VN/CNT arrays had an excellent rate capability with 80% capacitance retention at a high scan rate of 1000 mV s−1. Achour et al. designed and fabricated hierarchical composite electrodes consisting of porous nanostructured TiN grown on vertically aligned CNTs for micro-SC devices by reactive direct current sputtering.86 The electrodes deposited on silicon substrates had an areal capacitance of 18.3 mF cm−2 at 1 V s−1. Moreover, the areal capacitance of the TiN/CNT composite increased linearly with the thickness of the TiN layer. The porous TiN layer in the TiN/CNT composite with 1200 nm thickness exhibited a 360-fold increase in the areal capacitance compared to the pristine CNT electrode (Fig. 5c and d).
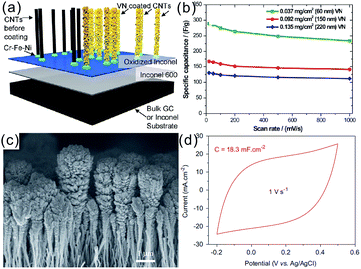 | ||
| Fig. 5 (a) Schematic illustration of the fabrication process and morphology of the 3D VN/CNT arrays; (b) rate performance of the VN/CNT arrays;44 (c) side-view SEM image of the TiN coated CNTs; (d) representative cyclic voltammogram (CV) curve of the TiN coated CNTs.86 (a and b) reproduced with permission from ref. 44. Copyright 2011, American Chemical Society. (c and d) Reproduced with permission from ref. 86. Copyright 2014, Elsevier. | ||
In addition to depositing TMNs on the surface of CNTs and CNFs, TMN NPs also were encapsulated into CNFs to improve the electrochemical properties. Wu et al. fabricated VN quantum dot/nitrogen-doped microporous carbon CNFs (VNQD/CNFs) by electrostatic spinning followed by thermal nitridation. 8–10 nm VNQDs with a content of 48 wt% were dispersed in the CNFs.93 The composite structure prevented the aggregation of VN NPs, improved the conductivity, and enhanced ion migration. Thus, the VNQD/CNF composite had a large specific capacitance of 406.5 F g−1 at 0.5 A g−1 in 6.0 M KOH solution and good rate capability with 75.1% capacitance retention when the current density was increased from 0.5 to 5 A g−1. Tan et al. fabricated MoxN dispersed CNFs (C/MoxN) with a diameter of 200 nm via electrostatic spinning and annealing in N2/NH3.94 The C/MoxN electrode showed a capacitance of 251 F g−1 at 0.5 A g−1 and a capacity retention of 70.9% when the current density was increased 10 times from 0.5 to 5 A g−1. The symmetrical SCs of C/MoxN//C/MoxN devices had an energy density of 4.51 W h kg−1 and a power density of 250 W kg−1.
Compared to 1D CNTs or CNFs, 2D graphene has a larger theoretical surface area of 2630 m2 g−1, higher conductivity, better mechanical strength, and flexibility,95 and so graphene is a promising supporting framework for TMNs to enhance the capacitive properties. Moreover, the mass loading of TMNs in TMNs/graphene is higher than that of the TMNs/CNT because 2D graphene provides a larger surface area to incorporate TMNs.96 The GO obtained by “Hummers'” method is rich in functional groups on its surface, which is beneficial for in situ coupling with kinds of TMO nanostructures to form various TMO/rGO composites. The TiN nanopillars/reduced graphene oxide (TiN/rGO) obtained from refluxing the Ti(OBu)4 precursor and GO followed by thermal nitridation showed a capacitance of 415 F g−1 at 0.5 A g−1 and a good rate performance of 275 F g−1 at 5 A g−1.46 Vadahanambi et al. fabricated a 3D architecture of a carbon sheathed Mo2N-reduced graphene oxide (Mo2N@C-rGO) hybrid as shown in the TEM image (Fig. 6a).97 The Mo2N@C-rGO electrodes showed high capacitances of 514.54 F g−1 (0.25 A g−1) and 304.32 F g−1 (10 A g−1) (Fig. 6b) as well as remarkable cyclability with a capacitance retention of 96% after 3000 cycles.97 Wang et al. prepared porous VN hierarchical nanostructures by NH3 annealing of 3D graphite foam supported V2O5 nanostructures. The graphene supported VN had capacitances of 177, 90, and 154 C g−1 in 1 M KOH, 1 M LiCl, and 1 M LiPF6, respectively.98 Recently, Zhu et al. reported all TMN-based solid-sate asymmetrical SCs with the TiN cathode, Fe2N anode and PVA/LiCl solid electrolyte (Fig. 6c and d).89 The porous TiN and Fe2N NPs were both grown on vertically aligned graphene nanosheet (GNS) substrates. The asymmetrical SCs had a capacitance of 58 F g−1 after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 4 A g−1 and a high volumetric energy density of 0.51 mW h cm−3 and a power density of 211.4 mW cm−3 at a large current density of 8 A g−1.
000 cycles at 4 A g−1 and a high volumetric energy density of 0.51 mW h cm−3 and a power density of 211.4 mW cm−3 at a large current density of 8 A g−1.
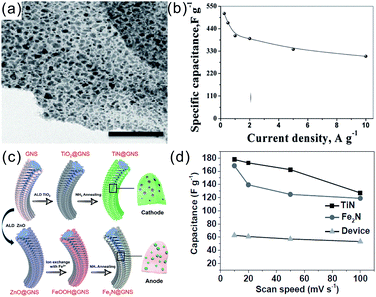 | ||
| Fig. 6 (a) Low-resolution TEM picture of Mo2N/rGO; (b) rate performance of the Mo2N/rGO electrode;97 (c) schematic diagram of the preparation process of the TMN cathode and anode materials; (d) rate performance of the electrode and all-nitride-based solid-sate asymmetrical device.89 (a and d) Reproduced with permission from ref. 97. Copyright 2018, The Royal Society of Chemistry. (c and d) Reproduced with permission from ref. 89. Copyright 2015, Wiley-VCH. | ||
With regard to nanostructured TMN electrodes in SCs, cycling stability is one of the main concerns due to the irreversible electrochemical oxidation of TMNs during cycling resulting in severe capacitance decay in the long-term process. A carbon coating can improve the cycling performance of TMN electrodes.38,43 Our group reported nitrogen-doped carbon encapsulated mesoporous VN (MVN@NC) NWs for long-life SC electrodes, which are shown in Fig. 7a and b. The mesoporous VN NWs had abundant active sites for charge storage and the NC shell suppressed the electrochemical dissolution of the inner MVN NWs in an alkaline electrolyte, leading to excellent capacitive properties. The MVN NWs had a high areal capacitance of 282 mF cm−2 and excellent long-term stability with 91.8% capacitance retention after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in a KOH electrolyte (Fig. 7c), which was superior to that of pure MVN NWs (6.7%).38 Lu et al. demonstrated that carbon shell coated TiN NWs boosted the stability with a high capacitance retention of 91.7% after 15
000 cycles in a KOH electrolyte (Fig. 7c), which was superior to that of pure MVN NWs (6.7%).38 Lu et al. demonstrated that carbon shell coated TiN NWs boosted the stability with a high capacitance retention of 91.7% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which was much better than that of uncoated TiN NWs (9.1%) (Fig. 7d and e).43 Grote et al. also showed that a carbon coating on TiN NT arrays led to better cycle stability compared to the bare TiN NT arrays.99 Yuan et al. fabricated 2D-layered carbon wrapped TiN (C@TiN) via one-step nitridation of transition metal carbides (MXenes).100 The carbon atoms generated during nitridation were deposited on the surface of TiN NSs producing a thin carbon coating (<5 nm). The in situ grown carbon layer not only prevented the electrochemical oxidation of TiN by the electrolyte but also worked as the cushion to accommodate the structural deformation. The C@TiN showed a high capacitance retention of 93.1% after 10
000 cycles, which was much better than that of uncoated TiN NWs (9.1%) (Fig. 7d and e).43 Grote et al. also showed that a carbon coating on TiN NT arrays led to better cycle stability compared to the bare TiN NT arrays.99 Yuan et al. fabricated 2D-layered carbon wrapped TiN (C@TiN) via one-step nitridation of transition metal carbides (MXenes).100 The carbon atoms generated during nitridation were deposited on the surface of TiN NSs producing a thin carbon coating (<5 nm). The in situ grown carbon layer not only prevented the electrochemical oxidation of TiN by the electrolyte but also worked as the cushion to accommodate the structural deformation. The C@TiN showed a high capacitance retention of 93.1% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1, which was better than that of pure TiN (29.2%).100 Zhang et al. directly grew carbon coated well-aligned VN NWs on CNT fibers which showed a high areal capacitance of 715 mF cm−2 at 1 mA cm−2 in a three electrode system. Moreover, the carbon-coated VN NWs exhibited improved cycling stability with a capacitance retention of about 90% after 10
000 cycles at 2 A g−1, which was better than that of pure TiN (29.2%).100 Zhang et al. directly grew carbon coated well-aligned VN NWs on CNT fibers which showed a high areal capacitance of 715 mF cm−2 at 1 mA cm−2 in a three electrode system. Moreover, the carbon-coated VN NWs exhibited improved cycling stability with a capacitance retention of about 90% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles compared to pure VN NW arrays (the capacitance retention was below 20%) since the carbon layer remitted electrochemical oxidation and delamination from the inner VN cores.101 Very recently, we reported carbon-encapsulated VN nanodot intercalated graphene nanosheets (VN@C/G), which were prepared by one-step thermal nitridation of polyaniline intercalated V2O5 NSs in NH3. The sandwiched V2O5 layer was converted into 3–5 nm VN nanodots which were encapsulated and intercalated into the in situ carbonized graphene layer forming pillared lamellar NSs. The VN@C/G NSs showed an ultrahigh volumetric capacitance of 1048.8 F cm−3 at 1.1 A cm−3 and a rate capability of 546 F cm−3 at 210 A cm−3 surpassing those of carbon, TMOs and other metal nitrides. Moreover, the VN@C/G NSs exhibited outstanding cycling stability with 90% capacitance retention after 10
000 cycles compared to pure VN NW arrays (the capacitance retention was below 20%) since the carbon layer remitted electrochemical oxidation and delamination from the inner VN cores.101 Very recently, we reported carbon-encapsulated VN nanodot intercalated graphene nanosheets (VN@C/G), which were prepared by one-step thermal nitridation of polyaniline intercalated V2O5 NSs in NH3. The sandwiched V2O5 layer was converted into 3–5 nm VN nanodots which were encapsulated and intercalated into the in situ carbonized graphene layer forming pillared lamellar NSs. The VN@C/G NSs showed an ultrahigh volumetric capacitance of 1048.8 F cm−3 at 1.1 A cm−3 and a rate capability of 546 F cm−3 at 210 A cm−3 surpassing those of carbon, TMOs and other metal nitrides. Moreover, the VN@C/G NSs exhibited outstanding cycling stability with 90% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.102
000 cycles.102
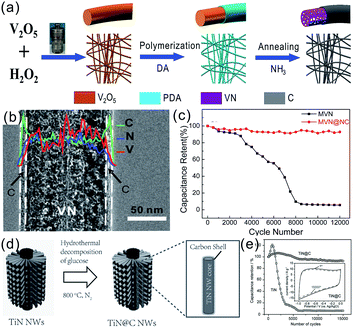 | ||
Fig. 7 (a) Schematic of the fabrication process of MVN@NC NWs;38 (b) TEM images of MVN@NC NWs; (c) cycling performance of the MVN NW and MVN@NC NW film electrodes in a 6 M KOH electrolyte at a scan rate of 200 mV s−1; (d) schematic of the process of carbon coating on the TiN NWs; (e) cycling performance of the TiN (squares) and TiN@C (circles) NW electrodes in the CV test for 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (100 mV s−1).43 (a–c) Reproduced with permission from ref. 38. Copyright 2015, Wiley-VCH. (d and e) Reproduced with permission from ref. 43. Copyright 2015, Wiley-VCH. 000 cycles (100 mV s−1).43 (a–c) Reproduced with permission from ref. 38. Copyright 2015, Wiley-VCH. (d and e) Reproduced with permission from ref. 43. Copyright 2015, Wiley-VCH. | ||
Although graphene and TMN hybrids show obvious synergetic effects in increasing the capacitance and improving the cycling stability of TMNs, graphene is easily restacked during the hybridization process and TMNs are easily aggregated at a high mass loading giving rise to decreased electrochemical charge storage properties.
4.2 Self-supported TMN-based composite film electrodes
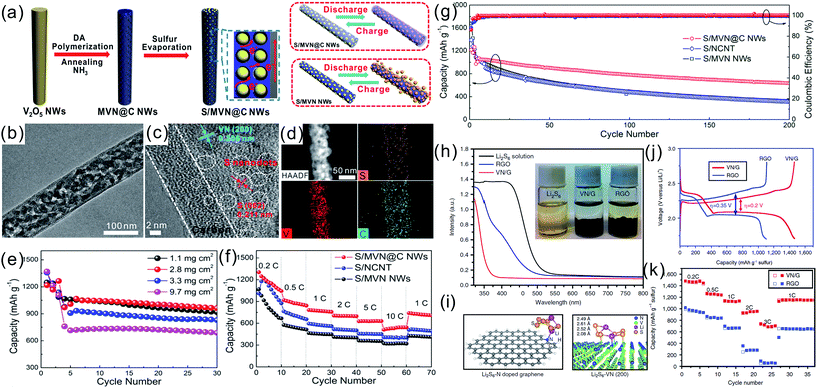
TMNs are inorganic nonmetallic ceramic materials which are brittle and have poor flexibility and thus they could not be directly applied in bendable and flexible SCs. Recently, binder-free films composed of 1D TMNs active materials and highly flexible 1D CNTs or 2D graphene have been reported to demonstrate potential in high volumetric electrochemical performance SCs. Binder-free film electrodes which avoid current collectors, conductive additives, and binders reduce the device weight, resulting in high volumetric energy and power density.55Our group fabricated a flexible freestanding mesoporous VN NWs (MVN NWs)/CNT hybrid electrode by simply vacuum-filtering the VN NW and CNT mixture (Fig. 8a–d).42 The binder-free MVN/CNT film exploits the synergistic effects of the electrochemical performance of MVN NWs and the high conductivity and mechanical consolidation of CNTs. The CV curves indicated that the capacitance of the MVN/CNT film electrode was larger than those of the CNT and MVN NW electrodes. The areal capacitance of the freestanding MVN/CNT hybrid film was 178 mF cm−2 at a current density of 1.1 mA cm−2 (Fig. 8e). The interpenetrating MVN/CNT and the mesoporous structure of VN enable effective electrolyte transport and active-site accessibility, giving rise to better utilization and larger specific capacitance. The all solid-state symmetrical SC devices composed of the H3PO4/PVA gel as the electrolyte showed a volumetric capacitance of 7.9 F cm−3 at a current density of 0.025 A cm−3 and a considerable rate performance with a capacitance of 4 F cm−3 when the current was increased 20 times from 0.025 to 0.5 A cm−3 (Fig. 8f and g).
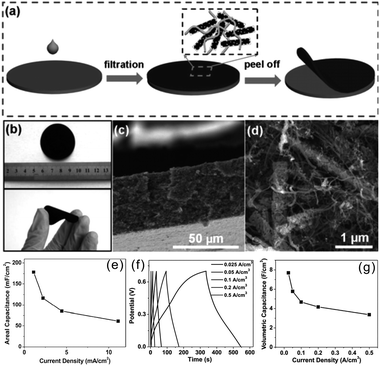 | ||
| Fig. 8 (a) Schematic showing the synthesis of mesoporous VN NWs; (b) digital pictures of the flexible electrode film; (c) low and (d) high-resolution cross-view SEM images of the film; (e) rate performance of the MVN/CNT based electrode; (f) galvanostatic charging/discharging (GCD) curves and (g) rate performance of the MVN/CNT based devices.42 (a–g) Reproduced with permission from ref. 42. Copyright 2013, Wiley-VCH. | ||
Compared with 1D CNTs, 2D graphene has a larger specific surface area, is more flexible, and can more easily form bendable electrodes.5 Recently, we developed an electrostatic self-assembly method combined with NH3 annealing to fabricate alternately stacked mesoporous Mo2N NB and rGO NS (MMNNBs/rGO) composite film electrodes.109 The detailed assembly process to assemble the flexible SCs is described in Fig. 9a. The as-prepared α-MoO3 NBs were positively charged by decoration with poly diallyldimethylammonium chloride under vigorous stirring and the positively charged MoO3 NB dispersion was electrostatically assembled with negatively charged-GO to form the MoO3/GO composite. The composite film was fabricated by vacuum filtration and peeling from the filter membrane. The MMNNBs/rGO films were prepared by annealing the MoO3/GO composite film in NH3 (Fig. 9b). Owing to the highly uniform composite and layer-by-layer structure, the MMNNBs/rGO film had outstanding flexibility. The as-prepared MMNNBs/rGO hybrid electrode had a high Mo2N mass loading of 95.6% and areal capacitances of 142 and 98 mF cm−2 at 1 and 150 mA cm−2 in 1 M H2SO4, respectively (Fig. 9c). The outstanding electrochemical performance was attributed to the synergetic effects of the mesoporous Mo2N NBs and alternately stacked and interconnected rGO networks. The flexible all-solid-state SCs produced by sandwiching two film electrodes of the MMNNBs/rGO hybrid in conjunction with the H3PO4/PVA gel electrolyte showed a high volumetric capacitance of 15.4 F cm−3 at 0.1 mA cm−3 and a capacitance retention of 64% when the current density was increased to 1 mA cm−3 (Fig. 9d). The high rate performance of the Mo2N based all-solid-state SCs was attributed to the small resistance (7.6 Ω cm−2) as shown in Fig. 9e. The freestanding and flexible Nb4N5/rGO composite film with a high Nb4N5 content of 91% was also fabricated by our group,27,110 The Nb4N5/rGO hybrid film showed a high areal capacitance of 141 mF cm−2 at 1 mA cm−2 and good rate performance with a capacitance of 60 mF cm−2 when the current density was increased 100 times from 1 to 100 mA cm−2. The symmetrical all-solid-state SCs based on a paper-like Nb4N5/rGO film electrode and the PVA/H2SO4 electrolyte showed a high volumetric capacitance (based on the whole device) of 19 F cm−3 at 0.1 mA cm−3 and favorable rate performance with a high volumetric capacitance of 5 F cm−3 at 5 mA cm−3. The devices exhibit high mechanical flexibility with the capacity unchanged after repeated bending.
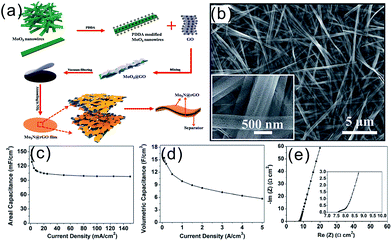 | ||
| Fig. 9 (a) Schematic illustration of the fabrication procedures for the MMNNBs/rGO flexible electrodes and SCs; (b) SEM images of the MMNNBs/rGO composites; rate performance of the electrode (c) and device (d); (e) Nyquist plot.109 (a–e) Reproduced with permission from ref. 109. Copyright 2015, The Royal Society of Chemistry. | ||
Although TMNs/CNT or TMNs/rGO composite films have promising applications in flexible SCs, they generally suffer from limitations such as inherent aggregation of CNTs/rGO, nonuniform mixture, and unstable junctions between the VN NWs and CNTs thus hampering further development to attain higher capacity, durability, and flexibility in practice. In this respect, our group fabricated paper-like self-supported MVN@NC NW electrodes consisting of 3D intertwined ultralong MVN@NC NWs by vacuum filtration of polydopamine coated V2O5 NWs followed by NH3 annealing.38 The VN content in the freestanding MVN@NC NWs film was as high as 84.5 wt% and the NC shell suppressed the electrochemical oxidation and dissolution of the inner MVN alkaline electrolyte thereby giving long-term cycling stability. The freestanding MVN@NC NW film electrode showed a high areal capacitance of 282 mF cm−2 and excellent long-term stability with 91.8% capacitance retention after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in a KOH electrolyte. The all-solid-state flexible SCs assembled by sandwiching two flexible MVN@NC NW film electrodes with the PVA/KOH gel-like electrolyte boasted a large volumetric capacitance of 10.9 F cm−3, energy density of 0.97 mW h cm−3, and power density of 2.72 W cm−3 at a current density of 0.051 A cm−3 based on the entire cell. By virtue of the excellent mechanical flexibility, high capacitance and large energy/power density, the self-supported MVN@NC NW paper-like electrodes have large potential in portable and wearable flexible electronics.
000 cycles in a KOH electrolyte. The all-solid-state flexible SCs assembled by sandwiching two flexible MVN@NC NW film electrodes with the PVA/KOH gel-like electrolyte boasted a large volumetric capacitance of 10.9 F cm−3, energy density of 0.97 mW h cm−3, and power density of 2.72 W cm−3 at a current density of 0.051 A cm−3 based on the entire cell. By virtue of the excellent mechanical flexibility, high capacitance and large energy/power density, the self-supported MVN@NC NW paper-like electrodes have large potential in portable and wearable flexible electronics.
The abovementioned TMN-based freestanding and binder-free film electrodes have promising applications in flexible SCs (Table 5). However, it is difficult to fabricate a paper-like electrode by a low-cost and scalable method. Developing some advanced technologies to produce freestanding electrode films with high volumetric and areal capacitance is highly desirable.
| Electrodes | Specific capacitance | Rate performance | Capacitance retention (%) after cycles | Ref. |
|---|---|---|---|---|
| VN@N doped carbon | 196 F g−1 at 1.44 mA cm−2 | 123.48 F g−1 at 144 mA cm−2 | 91.8% after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
38 |
| VN/CNT | 178 mF cm−2 at 1.1 mA cm−2 | 53 mF cm−2 at 11 mA cm−2 | 82% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
42 |
| Mo2N/graphene | 142 mF cm−2 at 1 mA cm−2 | 98 mF cm−2 at 150 mA cm−2 | 85.7% after 4000 cycles | 109 |
| Nb4N5/rGO | 175 F g−1 at 1 A g−1 | 131 F g−1 at 10 A g−1 | 90% after 6000 cycles | 27 |
| TiN@carbon | 167 F g−1 at 1 A g−1 | 95 F g−1 at 50 A g−1 | 70% after 6000 cycles | 99 |
4.3 TMNs as supportive materials for high-performance electrode materials
Owing to the superior electrical conductivity and mechanical stability, nanostructured TMNs constitute a promising electronic conducting framework to load TMOs, CPs, and even other TMNs to achieve enhanced capacitive properties for high-performance SCs. In the hybrid nanostructures, TMNs offer mechanical support and fast electron transfer according to the sorts of pseudo-capacitive materials. TMN hybrids can be classified as TMOs/TMNs, CPs/TMNs, and TMNs/TMNs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 10e). Recently, Wang et al. deposited ultrathin TiN shells on NiCo2O4 nanofiber arrays grown on carbon fiber cloth substrates by ALD.111 The conformal ultrathin TiN coating facilitated transportation of electrons at the electrode/electrolyte interface and acted as a mechanical buffer to prevent the structural deformation of the underlying NiCo2O4 nanofiber arrays during cycling. Additionally, the TiN shell contributed pseudocapacitively through the faradaic reactions. The core–shell NiCo2O4@TiN composite arrays displayed a high specific capacitance of 998 mF cm−2 at 2 mA cm−2 and a capacitance retention of 59% when the current density was increased from 2 to 20 mA cm−2. The symmetrical all-solid-state SCs based on the hybrid NiCo2O4@TiN electrodes had a power density of 58.2 mW cm−3 at an energy density of 0.061 mW h cm−3, which was the highest values of any NiCo2O4-based all-solid-state SC reported. In addition to TMOs, other hydroxide/TMNs including Ni(OH)2/TiN and NixCo2x(OH)6x/TiN as well as TMN/MoS2 hybrid electrodes were reported as high-performance electrodes materials for SCs.112–114 Some of the recently reported TMN supported TMO electrodes and their capacitive properties are summarized in Table 6.
000 cycles (Fig. 10e). Recently, Wang et al. deposited ultrathin TiN shells on NiCo2O4 nanofiber arrays grown on carbon fiber cloth substrates by ALD.111 The conformal ultrathin TiN coating facilitated transportation of electrons at the electrode/electrolyte interface and acted as a mechanical buffer to prevent the structural deformation of the underlying NiCo2O4 nanofiber arrays during cycling. Additionally, the TiN shell contributed pseudocapacitively through the faradaic reactions. The core–shell NiCo2O4@TiN composite arrays displayed a high specific capacitance of 998 mF cm−2 at 2 mA cm−2 and a capacitance retention of 59% when the current density was increased from 2 to 20 mA cm−2. The symmetrical all-solid-state SCs based on the hybrid NiCo2O4@TiN electrodes had a power density of 58.2 mW cm−3 at an energy density of 0.061 mW h cm−3, which was the highest values of any NiCo2O4-based all-solid-state SC reported. In addition to TMOs, other hydroxide/TMNs including Ni(OH)2/TiN and NixCo2x(OH)6x/TiN as well as TMN/MoS2 hybrid electrodes were reported as high-performance electrodes materials for SCs.112–114 Some of the recently reported TMN supported TMO electrodes and their capacitive properties are summarized in Table 6.
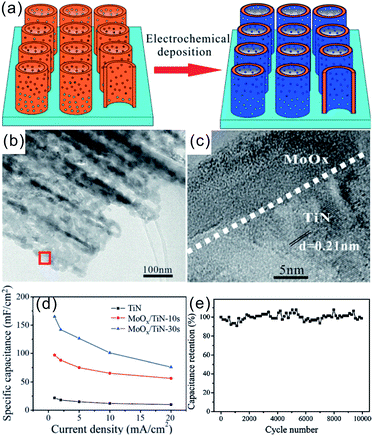 | ||
| Fig. 10 (a) Schematics of the fabrication procedures of MoOx/TiN/MoOx NTAs; (b) TEM and (c) HR-TEM images of MoOx/TiN-10s; (d) rate capability (based on areal capacitance) of the TiN, MoOx/TiN-10s, and MoOx/TiN-30s electrodes (deposition time of 10 s and 30 s); (d) cycling properties of the MoOx/TiN-10s electrode at a current density of 2 mA cm−2 in 1 M Na2SO4 solution.47 (a–e) Reproduced with permission from ref. 47. Copyright 2014, Wiley-VCH. | ||
| Electrodes | Specific capacitance | Rate performance | Capacitance retention (%) after cycles | Ref. |
|---|---|---|---|---|
| TMO/TMN | ||||
| MoOx/TiN | 323 F g−1 at 1 mA cm−2 | 193.8 F g−1 at 20 mA cm−2 | 100% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
47 |
| MnO2/TiN | 681.0 F g−1 at 2 A g−1 | 390.2 F g−1 at 400 A g−1 | 97% after 1000 cycles | 77 |
| MnO2–TiN | 853.3 F g−1 at 1.0 A g−1 | 225 F g−1 at 15 A g−1 | 91.7% after 1000 cycles | 122 |
| TiNxOy/MnO2 | 95.5 mF cm−2 at 0.5 mA cm−2 | 77 mF cm−2 at 20 mA cm−2 | 93.88% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
123 |
| Co(OH)2/VN | 62.4 F g−1 at 0.2 A g−1 | 34.4 F g−1 at 10 A g−1 | 80% after 4000 cycles | 124 |
| NiCo2O4@TiN | 998 mF cm−2 at 2 mA cm−2 | 582 mF cm−2 at 20 mA cm−2 | 70% after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
111 |
| NixCo2x(OH)(6x)/TiN | 2543 F g−1 at 5 mV s−1 | 660 F g−1 at 500 mV s−1 | 93.75% after 5000 cycles | 113 |
| TiN@NiCo2O4 | 1200 F g−1 at 2.0 A g−1 | 763.2 F g−1 at 100 A g−1 | 92.2% after 5000 cycles | 125 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| CP/TMN | ||||
| PANI/TiN/PANI | 242 mF cm−2 at 0.2 mA cm−2 | 167 mF cm−2 at 10 mA cm−2 | 83% after 3000 cycles | 48 |
| PPy–TiN | 1265 F g−1 at 0.6 A g−1 | 459 F g−1 at 15 A g−1 | 72.6% after 2000 cycles | 117 |
| PANI/C/TiN | 1093 F g−1 at 1.0 A g−1 | 708 F g−1 at 5.0 A g−1 | 98% after 2000 cycles | 118 |
| PPy/TiN/PANI | 1471.9 F g−1 at 0.5 A g−1 | 1077.4 F g−1 at 10 A g−1 | 38.8% after 1000 cycles | 119 |
| PANI/TiN | 1064 F g−1 at 1 A g−1 | 787.5 F g−1 at 5 A g−1 | 95% after 200 cycles | 126 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| TMN/TMN | ||||
| TiN@VN | 198.3 F g−1 at 5 A g−1 | 0.52 F cm−2 at 10 mA cm−2 | 95% after 2000 cycles | 49 |
| TiN/VN | 247.5 F g−1 at 2 mV s−1 | 160.8 F g−1 at 50 mV s−1 | 88% after 500 cycles | 121 |
| Ti@TiN/MON | 736.6 mF cm−2 at 10 mV s−1 | 552.45 mF cm−2 at 100 mV s−1 | — | 127 |
| MoNx/TiN | 121.5 mF cm−2 at 0.3 mA cm−2 | 112.6 F g−1 at 15 A g−1 | 93.8% after 1000 cycles | 128 |
| γ-Mo2N/Co3Mo3N | 109.9 F g−1 at 10 mVs−1 | — | 67% after 4000 cycles | 129 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Others | ||||
| TiN@MoS2 | 662.2 mF cm−2 at 1 mA cm−2 | 525 mF cm−2 at 10 mA cm−2 | 100% after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
114 |
| PMo12/PANI/TiN | 469 F g−1 at 1.0 A g−1 | 317 F g−1 at 10 A g−1 | 64% after 1000 cycles | 120 |
 | ||
| Fig. 11 (a) Schematic of the fabrication process of coaxial PANI/TiN/PANI-NTAs NTAs; (b) TEM and (c) high-resolution TEM images of the coaxial PANI/TiN/PANI NT; (d) rate performance of the PANI/TiN/PANI-NTA electrode; (e) cycling performance of the PANI/TiN/PANI-NTA electrode at a current density of 0.2 mA cm−2.48 (a–e) Reproduced with permission from ref. 48. Copyright 2013, The Royal Society of Chemistry. | ||
5. TMNs for Li–S batteries
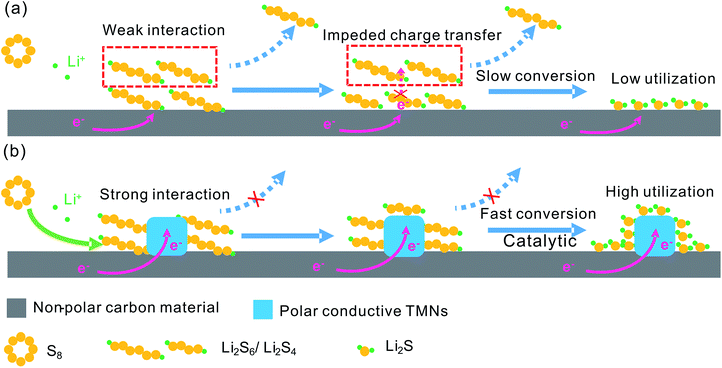
Li–S batteries have been considered as one of the most promising next-generation energy storage systems due to their high theoretical energy density of 2600 W h kg−1.130–134 Moreover, S is naturally abundant, inexpensive, and environmentally friendly. However, the practical implementation of Li–S batteries is plagued by several challenges. Firstly, S and the discharge product Li2S are electronically and ionically insulating resulting in slow reaction kinetics. Secondly, S suffers from an 80% volume change during charging and discharging involving phase transitions of solid S–liquid polysulfide (LiPS) intermediates–solid sulfides. Thirdly, the long-chain soluble LiPSs (Li2Sn, 3 ≤ n ≤ 8) dissolve into the electrolyte and shuttle from the cathode to the anode during cycling causing loss of the S cathode and passivation of the lithium–metal surface with the insoluble solid Li2S2/Li2S.14,135–137To overcome these hurdles, one of the promising approaches is encapsulation of S within conductive supporters to form S-based composite cathodes. Incorporation of S into porous carbon materials forming S/C composites has been widely investigated to improve the electrical conductivity of cathodes and alleviate LiPS shuttling through physical confinement.131,132 However, the weak interaction between nonpolar carbon and polar LiPS species inevitably causes effusion and irreversible loss of LiPSs from the cathodes (Fig. 13a) resulting in rapid capacity fading during cycling. Polar metal oxides such as SiO2, TiO2, MnO2, and some metal sulfides have recently been explored as host materials for S cathodes in Li–S batteries and the S/metal oxide (or sulfide) composites have demonstrated enhanced cycle stability via chemical anchoring of LiPSs on the cathodes.14,131,138–141 However, if the chemically anchored LiPSs are not quickly converted into insoluble Li2S during the reduction process, the adsorbed LiPSs will accumulate around the host surface decreasing the LiPS adsorption/trapping efficiency and irreversible loss of LiPSs, especially for the S cathode with a high S mass loading. Moreover, the low conductivity of most metal oxides/sulfides causes a low S utilization ratio and poor rate performance.14,138 To improve the electrochemical properties of S cathodes and restrain the shuttling of LiPSs, a promising way is to design highly conductive host materials with abundant polar active sites to adsorb LiPSs and high catalytic activity to catalytically promote LiPS conversion to solid Li2S2/Li2S, and vice versa.
TMNs are desirable host materials for S cathodes in Li–S batteries because of their high conductivity, good mechanical stability, strong chemical polarity, and noble metal-like catalytic activity.144 The high conductivity of TMNs offers fast electron transfer and the strong chemical interaction between TMNs and LiPSs to trap the LiPSs. The high electrocatalytic activity of TMNs catalytically accelerates the LiPSs–Li2S2/Li2S conversion to enhance the redox kinetics and mitigate LiPS loss (Fig. 13b). In addition to acting as the host materials in S cathodes, TMN-based films are also utilized as interlayers in Li–S batteries to restrain the shuttling of LiPSs and improve the battery performance.145,146 The recent progress in TMNs in Li–S batteries is summarized in Table 7.
| Electrode | Morphology | S content (%) (areal loading, mg cm−2) | Reversible capacity (mA h g−1) | Capacity retention | Large rate capability (mA h g−1) | Ref. |
|---|---|---|---|---|---|---|
| Host | ||||||
| TiN | Mesoporous particles | 58.8(1.0) | 644 after 500 cycles at 0.5C | 65.2% | 776 at 1C | 142 |
| TiN | Particles | 75(1.2) | 780 after 50 cycles at 1C | 70.0% | 700 at 5C | 143 |
| TiN | Nanopowder | 58.8(1.0) | 660 after 200 cycles at 0.5C | 59.0% | 555 at 5C | 147 |
| TiN | Hollow porous tubes | 73.8(1) | 840 after 450 cycles at 0.5C | 93.3% | 694 at 2C | 148 |
| VN | NBs | 78.2(1.2) | 837 after 1000 cycles at 1C | 76.0% | 812 at 5C | 149 |
| VN/C | N-carbon coated mesoporous NWs | 57.2(2.8) | 636 after 200 cycles at 1C | 61.2% | 543 at 10C | 152 |
| VN | Nanoribbon on graphene | −(3.0) | 1252 after 100 cycles at 0.2C | 85.0% | 701 at 3C | 153 |
| VN | Nanoarray on porous carbon fiber | −(9.5) | 1052 after 250 cycles at 0.1C | 80.3% | 591 at 5C | 154 |
| Co4N | Mesoporous spheres | 72.3(1.5–2) | 930 after 500 cycles at 1.0C | 84.5% | 882 at 2C | 145 |
| WN | Nano-plates | −(8) | 980 after 100 cycles at 0.1C | — | 665 at 1C | 151 |
| Mo2N | Mesoporous particles | 48.2(1.1) | 771 after 200 cycles at 0.5C | 77.5% | — | 150 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Interlayers | ||||||
| TiN | TiO2–TiN nanopillars | 88.0(1.2) | 927 after 300 cycles at 0.3C | 91.9% | 682 at 2C | 146 |
| TiN | NPs on graphene | 80.0(2.0) | 550 after 1000 cycles at 2.0C | 85.0% | 578 at 5C | 155 |
5.1 Nanostructured TMNs for high-performance S cathodes in Li–S batteries
 | ||
| Fig. 14 (a) N 1s XPS spectra of TiN and TiN–S; (b) rate performance and (c) cycling stability of the TiN/S, TiO2/S and C/S;142 (d) rate capability of the bare cathode and C/TiN cathode compared to the carbon interlayer (ABM) and C/TiN cathode with ABM (C/TiN-ABM); (e) GCD profiles at 0.2C; (f) polarization potential of the second plateau of the bare and C/TiN cathodes at 0.2C.143 Reproduced with permission from ref. 142. Copyright 2016, Wiley-VCH. (d–f) Reproduced with permission from ref. 143. Copyright 2017, American Chemical Society. | ||
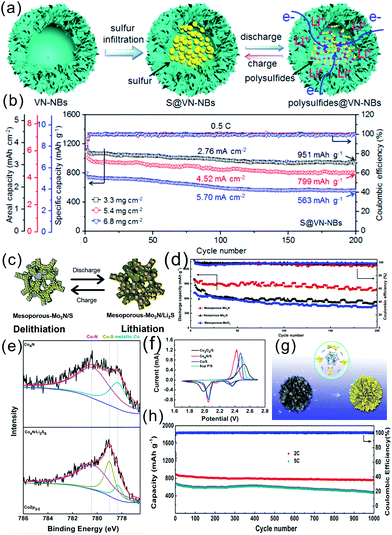
Very recently, Ma et al. showed that porous-shell VN nanobubbles (VN-NBs) could serve as an efficient S host in Li–S batteries (Fig. 15a).149 The highly conductive polar VN not only promoted fast transfer of electrons and chemical adsorption of soluble LiPSs, but also catalytically accelerated fast conversion of LiPSs. S was encapsulated in VN-NBs (S@VN-NBs) with a high mass loading of 5.4 mg cm−2 and the materials showed a high areal/specific capacity of 5.81 mA h cm−2/1076 mA h g−1 (Fig. 15b), superior rate capability of 632 mA h g−1 at 5.0C, and long cycling stability with a capacity decay of 0.024% per cycle after 1000 cycles.
 | ||
| Fig. 15 (a) Schematics of the fabrication of S@VN-NB composites; (b) cycling capability of the S@VN-NB cathodes with a S areal loading of 3.3, 5.4, and 6.8 mg cm−2, respectively;149 (c) possible mechanism of using mesoporous-Mo2N as the S host during charging–discharging; (d) cycling performance and coulombic efficiency of the mesoporous-Mo2N/S, nonporous-Mo2N/S and mesoporous-MoO3/S cathodes at 0.5C for 200 cycles;150 (e) fine XPS spectra of Co 2p before and after adsorbing Li2S6; (f) CV curves of Co3O4/S, Co4N/S, Co/S, and Sup P/S; (g) schematic illustration of a Co4N sphere and its interaction with LiPSs during the GCD process; (h) cycling performance of the Co4N/S at 2C and 4C.145 (a and b) Reproduced with permission from ref. 149. Copyright 2017, American Chemical Society. (c and d) Reproduced with permission from ref. 150. Copyright 2018, Elsevier. (e–h) Reproduced with permission from ref. 145. Copyright 2017, American Chemical Society. | ||
Other TMNs such as Co4N spheres, WN nanoplates, mesoporous Mo2N, and Mo2N nanorods have been investigated as host materials for S cathodes.145,150,151 For example, Jiang et al. reported a mesoporous, conductive Mo2N cathode with a polar surface as the host material for Li–S batteries.150 The mesoporous-Mo2N/S cathode showed a high capacity of 995 mA h g−1, and cycling stability with 91.9% capacity retention after 100 cycles, which are superior to those of nonporous-Mo2N/S and mesoporous-MoO3/S (Fig. 15c and d). Deng et al. fabricated mesoporous Co4N spheres as effective cathode hosts for S cathodes in Li–S batteries and X-ray photoelectron spectroscopy (XPS) confirmed the presence of Co–S bonding after adsorbing Li2S6 (ref. 145) (Fig. 15e). Co4N served as a conductive Lewis base “catalyst” matrix to facilitate oxidization and phase transition reactions from short-chain to long-chain LiPSs and to S8 (Fig. 15f and g). With a S content of 72.3 wt% in the composite, the Co4N@S showed a high specific discharge capacity of 1659 mA h g−1 at 0.1C which was close to the theoretical capacity. Moreover, at high rates of 2 and 5C, the discharge capacity stabilized at 805 and 585 mA h g−1 after 300 cycles (Fig. 15h).
Despite the great promise, the small surface area and low pore volume of TMN host materials cannot efficiently suppress the outward diffusion of Li2Sn, especially with a large S concentration, thus leading to unsatisfactory capacity retention and cycle life.
 | ||
| Fig. 16 (a) Schematic diagram illustrating the preparation procedure and cycling process of S/MVN@C NW cathodes; (b) TEM and (c) HR-TEM images of S/MVN@C NWs (S NPs marked by the white dotted line); (d) STEM images of S/MVN@C NWs and corresponding elemental maps of S, V and C; (e) cycling performance of S/MVN@C NWs with different S areal mass loadings; rate performances (f) and (g) cycling performance of S/CNT, S/VN and S/MVN@C NWs;152 (h) ultraviolet/visible absorption spectra and adsorption performance of RGO and VN/G; (i) side view of a Li2S6 molecule on a nitrogen-doped graphene surface and VN (200) surface; (j) GCD curves of the VN/G and RGO cathodes at 0.2C; (k) rate performance of the RGO and VN/G cathodes.153 (a–g) Reproduced with permission from ref. 152. Copyright 2017, Elsevier. (h–k) Reproduced with permission from ref. 153. Copyright 2017, Nature Publishing Group. | ||
Sun et al. synthesized a conductive porous VN nanoribbon/graphene (VN/G) hybrid as the S host.153 VN provided a strong chemical affinity for LiPSs (Fig. 16h and i) and the conductive network facilitated the transportation of electrons and Li ions. The hybrid cathode showed a high specific capacity of 1461 mA h g−1 after 100 cycles at 0.2C as well as a high rate performance of 956 mA h g−1 at 2C (Fig. 16j and k). Very recently, Zhong et al. reported a porous carbon fibers/VN array (PCF/VN) composite as a S scaffold,154 in which the PCF with a highly porous structure provided a large space to accommodate active S and possessed cross-linked maze channels to physically immobilize the LiPS species. The VN NB arrays demonstrated the strong ability to chemically anchor LiPSs to retard the shuttle effect. The PCF/VN/S electrode displayed a high reversible capacity of 1310.8 mA h g−1 at 0.1C, and a good cyclability of 1052.5 mA h g−1 after 250 cycles with a capacity retention of 80.3%.
5.2 TMNs as functional interlayers for Li–S batteries
TMNs could act as functional interlayers coated on the separator or inserted between the separator and the sulfur cathode to improve the electrochemical properties of Li–S batteries. Deng et al. designed rGO-supported TiN-nanoparticle (TiN/rGO) cover layers on the separator by an in situ synthesis method.155 The multifunctional TiN-based layer created an airtight chamber for the S conversion reactions at a macroscopic level to effectively block the shuttling effect during discharging/charging (Fig. 17a). When pure S powders were directly used as the active materials with an areal loading of 2.8 mg cm−2. The discharge specific capacity of the first cycle was 1580 mA h g−1 at 0.05C. The S cathode could be operated at 2C for over 1000 cycles with a low capacity decay rate of 0.015% per cycle. Recently, Zhou et al. reported twinborn TiO2–TiN heterostructure-modified separators enabling long life Li–S batteries. The TiO2–TiN heterostructure combined the merits of highly adsorptive TiO2 and conducting TiN and achieved smooth trapping–diffusion–conversion of LiPSs across the interface.146 With the catalytic conversion effect of TiN, the LiPSs were rapidly converted to insoluble Li2S2 and Li2S avoiding the accumulation of LiPSs in the electrolyte and further inhibiting the shuttle effect (Fig. 17b). Fast diffusion of LiPSs from TiO2 to TiN helped to achieve both high trapping efficiency and fast conversion. The cell with this interlayer exhibited a capacity of 927 mA h g−1 after 300 cycles at 0.3C (Fig. 17c), and a capacity retention of 73% and 67% over 2000 cycles at 1C for S areal mass loadings of 3.1 and 4.3 mg cm−2, respectively.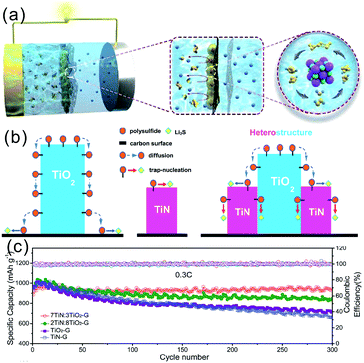 | ||
| Fig. 17 (a) The schematic of the multifunctional TiN/rGO cover layer to create a macro-chamber for S redox reactions for lithium–sulfur batteries;155 (b) schematic illustration of the LiPS conversion procedure on TiN, TiO2 and the TiO2–TiN heterostructure surface; (c) cycling performance samples with different TiN contents at 0.3C over 300 cycles.146 (a) Reproduced with permission from ref. 155. Copyright 2017, The Royal Society of Chemistry. (b–c) Reproduced with permission from ref. 146. Copyright 2017, The Royal Society of Chemistry. | ||
TMN-based host materials show enhanced electrochemical characteristics for S cathodes and a brief summary of TMN-based materials for Li–S batteries is shown in Table 7. However, the pore volume and active surface area of TMN host materials need to be optimized to improve the S mass loading, especially areal mass loading, and enhance the catalytic efficiency and redox kinetics. Moreover, the process in which TMNs catalytically accelerate the LiPSs–Li2S2/Li2S conversion is not well understood and a better understanding is vital to optimize the S cathodes and battery performance.
6. TMNs for LIBs and LiHCs
Different from SCs which store charge via surface ion adsorption/desorption or surface redox reactions, LIBs store energy in the bulk of active materials through the Li+ intercalation/de-intercalation reactions.92 Currently, LIBs dominate EES systems and are the mainstream power supply devices for mobile electronics and electric vehicles due to their high energy density, long-term lifespan (>500 cycles), and no/little memory effect.156–158 The electrochemical performance of LIBs depends strongly on the electrode materials.158,159 Generally, anode materials react with Li by three mechanisms, namely (i) insertion, (ii) alloying, and (iii) conversion.160,161 Graphite is widely used as a commercial anode in LIBs via the insertion/desertion mechanism. Nevertheless, the small theoretical capacity of 372 mA g−1 cannot satisfy the requirement for a high energy density. TMNs are promising electrode materials for Li storage due to their high conductivities, large theoretical capacities, and good Li ion diffusion capabilities.18,19,162,163 Moreover, the conversion reaction of TMNs results in the formation of metal NPs embedded in the Li3N matrix, which is a lithium superionic conductor (6 × 103 S cm−1 at ambient temperature).18 In this subsection, we will mainly discuss the latest progress pertaining to TMN-based composites in LIBs and LiHCs.Fu et al. confirmed the electrochemical reaction mechanism of VN with Li forming V and Li3N by ex situ X-ray diffraction, high-resolution transmission electron microscopy and selected area electron diffraction as well as in situ spectroelectrochemical measurements.164 However, similar to the conversion reaction of TMOs with Li, TMNs suffer from large volume changes (for example, 240% for VN calculated based on the density of VN, Li3N, and V) during cycling, resulting in particle pulverization, severe particle–particle electronic contact loss, and poor cycle performance.20,165 Combining TMNs with a carbon matrix is a promising strategy to improve the Li storage performance. Wang et al. prepared hybrid 2D–0D graphene-VN quantum dots (G-VNQDs) by a hydrothermal reaction of GO with NH4VO3 and subsequent treatment in NH3 (Fig. 18a and b).158 The VN quantum dots with sizes of 2–5 nm were homogeneously dispersed on graphene and the 2D–0D G-VNQD hybrid prevented the aggregation of VN during cycling, accommodated the volume changes of VN effectively, and enhanced electron transfer and conversion reaction kinetics with Li. Electrochemical results showed that G-VNQDs had a higher specific capacity of 715 mA h g−1 at 0.2C (1C = 372 mA h g−1) than the single component of either VN (365 mA h g −1) or NG (433 mA h g−1). Moreover, the G-VNQDs delivered a good cycle performance with a large capacity of 280 mA h g−1 at 5C after 800 cycles (Fig. 18c and d).
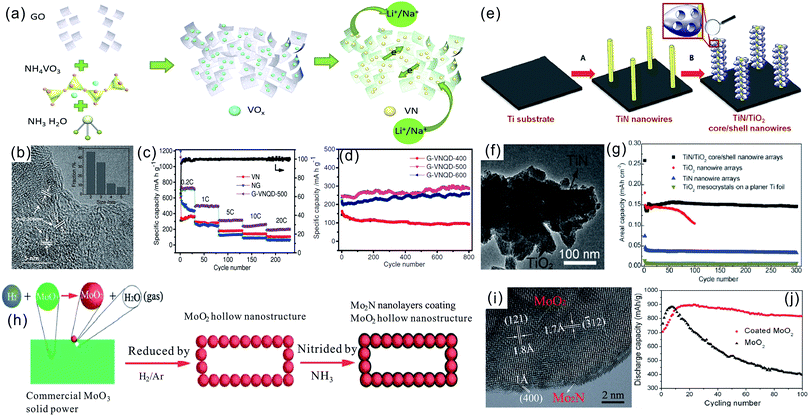 | ||
| Fig. 18 (a) Schematic illustration of the fabrication of hybrid 2D–0D graphene-VN quantum dots; (b) HR-TEM images of G-VNQD-500 (nitriding temperature is 500 °C); (c) rate performance of VN, NG, and G-VNQD-500; (d) cycling stability of G-VNQDs at a rate of 5C for 800 cycles;158 (e) schematic illustration of the experimental procedures of the synthesis for the TiN/TiO2 NWs on Ti; (f) HR-TEM image and (g) cycling performance of the TiN/TiO2 NWs at a current density of 0.11 mA cm−2;169 (h) schematic illustration of the formation of Mo2N nanolayer coated MoO2 hollow nanostructures by a controlled stepwise method; (i) HR-TEM characterization of the Mo2N nanolayer coated MoO2 hollow nanostructures; (j) cycling stability of the MoO2 hollow nanostructures before and after Mo2N nanolayer coating at 100 mA g−1.171 (a–d) Reproduced with permission from ref. 158. Copyright 2016, Wiley-VCH. (e–g) Reproduced with permission from ref. 169. Copyright 2017, Elsevier. (h–j) Reproduced with permission from ref. 171. Copyright 2013, The Royal Society of Chemistry. | ||
In addition to being the active materials of electrodes in LIBs, TMNs have been widely applied in LIBs as supporting materials to ameliorate the structural stability and improve the electrical conductivity of other electrode materials because they possess good electrical conductivity, high hardness, and high chemical and thermal stability.166–168 For example, Kim et al. reported that TiN acted as an inactive material to deposit Si to maintain the electrochemical stability of the nanocomposites.166 Tong et al. improved the first reversible capacity and Li storage properties of SnS2 by growing SnS2 NSs on porous VN substrates grown on CC. VN provided mechanical stability and shortened the Li-ion and electron pathways. The 3D porous VN/SnS2 NSs yielded a high specific capacity of 819 mA h g−1 at a current density of 0.65 A g−1 and an excellent capacity retention of 97% after 100 cycles.167 Wen et al. reported that core/shell TiN/TiO2 NW arrays exhibited pseudocapacitance-dominated charge storage of Li ions (Fig. 18e and f).169 The single-crystal TiN NW cores served as the highly conductive nanostructured current collectors and the branched shell consisting of nanoporous anatase TiO2 mesocrystals was the active material. The gravimetric specific capacity of TiO2 in the TiN/TiO2 NW arrays was 188 mA h g−1 (0.146 mA h cm−2) at 0.11 mA cm−2 (corresponding to 1C on the basis of the mass of TiO2). At a large current density of 1.0 mA cm−2 and after 1000 cycles, the discharge capacity was maintained at 0.081 mA h cm−2 (Fig. 18g).
Apart from utilizing TMNs such as TiN as the conducting supporters and current collectors to improve the electrochemical properties of active materials in LIBs, TMNs are also used as the inactive and conductive protective coatings for electrode materials to improve the Li storage properties.168 For example, Zhang et al. prepared a TiN/Li4Ti5O12 composite via high-energy ball-milling of a Li4Ti5O12 and TiN powder mixture.170 In the TiN/Li4Ti5O12 composite, an amorphous TiN layer with a thickness of several manometers was coated on the Li4Ti5O12 particles. Although the capacity of the TiN/Li4Ti5O12 was similar to that of the pristine Li4Ti5O12, the TiN/Li4Ti5O12 showed a higher capacity of 130 mA h g−1 at a high current density of 20C (1C = 175 mA g−1) compared to pure Li4Ti5O12 (<13 mA h g−1). The excellent rate performance of TiN/Li4Ti5O12 can be attributed to the high electron conductivity of the TiN and good Li ion conducting ability because of the formation of the Li3N phase during the conversion reaction of the TiN coating. Recently, Liu et al. reported the fabrication of a hollow MoO2 nanostructure coated with a Mo2N nanolayer, showing a capacity of 815 mA h g−1 after 100 cycles (Fig. 18h–j). The Mo2N layer not only provided the electrode with better electrical conductivity, but also mitigated structural deterioration, thus ensuring the superior rate capability and cycle life of the batteries, respectively.171Table 8 summarizes the specific capacity and rate performance of different TMN electrode materials in LIBs.
| Types of materials | Morphology | Specific capacity (mA h g−1) | Rate performance (mA h g−1) | Capacitance retention (mA h g−1) after cycles | Ref. |
|---|---|---|---|---|---|
| a mA h cm−2. b mA h cm−3. | |||||
| TiN | |||||
| Si/TiN | Particles | 300 at 0.25 mA cm−2 | — | — | 166 |
| TiN/TiO2 | Core–shell NWs | 0.146a at 0.11 mA cm−2 | 0.055a at 11.0 mA cm−2 | 0.08a after 1000 cycles at 1.0 mA cm−2 | 169 |
| TiN | NWs on CC | 567 at 0.335 A g−1 | 288 at 1.675 A g−1 | 455 after 100 cycles at 0.335 A g−1 | 177 |
| TiO2@TiN | NWs on CC | 537 at 335 mA g−1 | 136 at 10 A g−1 | 443 after 200 cycles at 335 mA g−1 | 178 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| VN | |||||
| VN | Hollow spheres | 720 at 0.05 A g−1 | 460 at 0.05 A g−1 | 460 after 70 cycles at 4 A g−1 | 26 |
| VN | NPs on graphene | 715 at 240 mA g−1 | 201 at 24 A g−1 | 280 after 800 cycles at 6 A g−1 | 158 |
| VN | Thin films | 1156 at 28 μA h cm−2 | — | 800 after 50 cycles at 28 μA h cm−2 | 164 |
| SnS2/VN | NSs on porous VN | 819 at 0.65 A g−1 | 349 at 4 A g−1 | 231 after 650 cycles at 13 A g−1 | 167 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Others | |||||
| MoNx | Thin films | 0.118a at 100 μA cm−2 | — | 0.069a after 100 cycles at 0.1 mA cm−2 | 179 |
| Mo2N coated MoO2 | Hollow structures | 815 at 0.1 A g−1 | 415 at 5 A g−1 | 815 after 100 cycles at 0.1 A g−1 | 171 |
| Fe2N/C | Carbon coated nanocubes | 1295b/0.1 A g−1 | 356/10.0 A g−1 | 365 after 2500 cycles at 10.0 A g−1 | 180 |
| Fe3N/Fe3O4 | NPs | 1250/0.05 A g−1 | 404/1.6 A g−1 | 739 after 60 cycles at 0.05 A g−1 | 168 |
| GaN@Cu | Nanorods | 980 mA h g−1/0.25 A g−1 | — | 509 after 3000 cycles at 10 A g−1 | 181 |
Considering the large Li storage capacity, high conductivity, and excellent Li ion conductivity, TMNs are desirable anode materials for LiHCs to provide both higher energy and power densities.172,173 LiHCs consisting of battery-type anodes and SC-like cathodes have emerged as a new type of hybrid energy storage device that bridges the gap between LIBs and SCs.160,161 In LiHCs, the large capacity offered by the battery-type anodes and capacitor-like cathodes yields a fast charge–discharge rate and high power density. Therefore, LiHCs can deliver a larger energy density than SCs and higher power density than LIBs and have potential applications in the EES of hybrid electric vehicles.
Wang et al. reported LiHCs employing a 3D VN–RGO composite anode and a porous carbon NR cathode. The 3D VN–RGO composites were prepared by combining VN NWs with rGO (Fig. 19a). The VN NWs provided large Li ion storage pseudocapacitance while the connected rGO network with high conductivity accelerated electron transfer. The 3D VN–RGO composite exhibited a large Li-ion storage capacity of 640 mA h g −1 at 0.1 A g−1 and a fast charge/discharge rate of 270 mA h g−1 at 5 A g−1 (Fig. 19b). The LiHCs displayed a high capacity of 73 and 28.9 F g−1 (based on the total mass of cathodic and anodic active materials) at 0.1 and 5 A g−1 (Fig. 19c) and an ultrahigh energy density of 162 and 64 Wh kg−1 at a power density of 0.2 and 10 kW kg−1, respectively.174 Cui et al. reported NbN/nitrogen-doped graphene nanocomposites as anode materials for LiHCs. The NbN/NG hybrid electrode delivered a good rate performance of 116 mA h g−1 at a current density of 2000 mA g−1.175 The LiHCs comprising the NbN/NG hybrid anode and activated carbon cathode had high energy densities of 122.7–98.4 W h kg−1 at power densities of 100–2000 W kg−1, respectively. Yan et al. also utilized porous NbN as the anode and activated carbon as the cathode to assemble the device of LiHCs, which exhibited a high energy density of 149 W h kg−1 and a high power density of 45 kW kg−1 as well as an excellent stability with 95% capacitance retention after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.0 A g−1.176Table 9 summarizes the latest progress in LiHCs based on TMNs. In order to meet the practical applications, much efforts should be devoted to design and optimize TMNs anodes and carbon cathodes to improve the energy/power density of LiHCs.
000 cycles at 1.0 A g−1.176Table 9 summarizes the latest progress in LiHCs based on TMNs. In order to meet the practical applications, much efforts should be devoted to design and optimize TMNs anodes and carbon cathodes to improve the energy/power density of LiHCs.
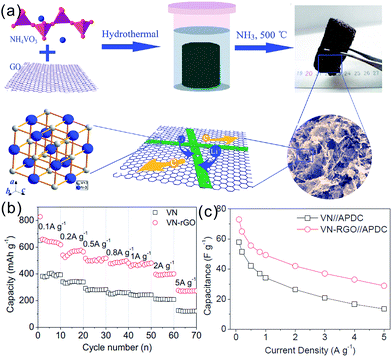 | ||
| Fig. 19 (a) Schematic showing the fabrication of the 3D porous VN–RGO hybrid by the hydrothermal treatment and annealing process in NH3; (b) rate capability of VN NWs and 3D VN–RGO composite electrodes at various current densities ranging from 0.1 to 5 A g−1; (c) specific capacitance values of 3D VN–RGO//APDC (activated polyaniline-derived carbon) LiHCs at different current densities.174 (a–c) Reproduced with permission from ref. 174. Copyright 2015, Wiley-VCH. | ||
| Anode | Specific capacitance | Rate performance | Capacitance retention after cycles | Energy density (W h kg −1) vs. power density (W kg −1) | Ref. |
|---|---|---|---|---|---|
| VN NW/rGO | 73 F g−1 at 0.1 A g−1 | 28.9 F g−1 at 5 A g−1 | 83% after 1000 cycles at 2 A g−1 | 162 vs. 200 | 174 |
| NbN NPs/N-graphene | 260 mA h g−1 at 0.1 A g−1 | 116 mA h g−1 at 2 A g−1 | 81.7% after 1000 cycles at 0.5 A g−1 | 122.7–98.4 vs. 100–2000 | 175 |
| Porous NbN | 67.2 F g−1 at 0.1 A g−1 | 20.7 F g−1 at 5 A g−1 | 95% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.0 A g−1 000 cycles at 1.0 A g−1 |
149 vs. 200 | 176 |
7. Conclusion and outlook
Electrical energy storage is a key technology for a clean energy economy. It enables society-changing advances, especially in portable electronic devices such as smart phones and tablet computers, electric vehicles, and renewable energy grid storage. Electrodes are the key components in EES devices and determine their energy storage performance. TMNs are emerging candidates for high-performance EES devices including SCs, Li–S batteries, LIBs, and LiHCs due to their interesting morphology, high conductivity, mechanical stability, high volumetric energy density, and good catalytic activity. Compared to their bulk counterparts, nanostructured TMNs deliver better electrochemical performance such as higher capacity and better electrocatalytic activity due to their smaller particle size and larger surface area thus enabling shorter diffusion paths for electrons and ions and providing sufficient contact between the active materials and electrolytes or reactants. In this review, we discuss the recent progress in TMN-based nanohybrids of Ti, V, Nb, Mo and W in SCs, Li–S batteries, LIBs and LiHCs.For SCs, we discuss TMN-based hybrids as the active materials to synergistically improve the capacitive storage performance. Combining TMNs with nano-carbons including CNTs, CNFs and graphene enhances the electrochemical utilization, improves the specific capacitance, and ameliorates the cycle stability and mechanical flexibility. Thus, high areal or volumetric SCs with high rate capability and good mechanical flexibility can be constructed with TMN-based hybrid electrode materials. Highly conductive and mechanically stable TMNs are appealing supporters or backbones for other active SC materials to enhance the capacitive storage performance. For Li–S batteries, the slow conversion between soluble LiPSs and insoluble Li2S2/Li2S is the main cause for the shuttling of LiPSs in the electrolyte. Nanostructured TMNs are the ideal S host materials because they have high conductivity, a strong affinity for LiPSs, and good mechanical stability. More importantly, they can catalytically propel the LiPSs–Li2S2/Li2S conversion due to their high catalytic activity. With regard to LIBs and LiHCs, nanostructured TMNs show large capacity and high rate capability. Moreover, TMNs act as supportive materials to load electrochemically active materials to enhance the battery performance. The promising Li storage properties and rapid Li ion storage kinetics of TMN-based electrode materials render them suitable for application in LiHCs with both high energy and power densities and have great potential in EES devices.
Although considerable advances have been made in TMN-based electrode materials, there is still substantial room for the improvement and development of advanced TMNs with better electrochemical performance. Several fundamental issues need to be resolved in order that TMNs can be widely implemented in next-generation high-performance EES devices.
(1) The electrochemical performances of TMNs are associated with their structure and surface areas. However, current TMNs and TMN-based composites generally have small surface areas and insufficient active sites. The development of novel TMN nanostructures such as 2D TMNs can improve the electrochemical properties and promote the applications of TMNs in EES devices, especially high volumetric capacitance SCs. Moreover, ternary or mixed phase TMNs show improved capacitance (capacity) and excellent rate capability compared to single nitrides, but few have been reported for LIBs and SCs. Compared to TMOs and carbon materials, the detailed electrochemical reactions of TMNs in EES devices are poorly understood from the perspective of thermodynamics and kinetics. The development of data mining and high-throughput methodologies to predict and design high-performance multi-component TMNs for EES should be actively pursued.
(2) Although there have been many studies on TMNs as capacitive electrodes in SCs, the origin and intrinsic mechanisms responsible for the large pseudocapacitive properties of TMNs are not fully understood. Generally, TMNs are prepared by nitridation of metal oxide precursors via a topochemical reaction but this process unavoidably results in the presence of remaining oxygen in the bulk of TMNs, resulting in composition differences in the product. However, the influence of the oxygen concentrations and microstructures of metal oxynitrides in TMNs on the capacitive properties remains unclear. On the other hand, most TMNs have larger specific capacitance in an aqueous acidic or basic electrolyte but they have poor cycling stability in these electrolytes and so TMNs must be modified or coated with other protective layers to improve the cyclability. However, the capacitance decay mechanism of TMNs is not well understood. Advanced characterization techniques such as in situ Raman scattering, X-ray absorption near edge structure and infrared spectroscopy, as well as theoretical simulation can provide some insights. Additionally, the development of flexible devices based on TMN electrodes is highly desirable and needs to be the prospective research.
(3) TMNs are promising S host materials which not only offer a strong affinity for LiPSs, but also catalytically propel the LiPSs–Li2S2/Li2S conversion. Designing TMNs with a large surface area and high catalytic activity is crucial to enhancing the redox kinetics between the captured LiPSs and insoluble Li2S2/Li2S. This is expected to effectively suppress the shuttling of LiPSs and improve the battery performance. However, the detailed electrocatalytic mechanism is unclear in this stage. A better understanding of the catalytic mechanism is expected to provide a design principle for catalytic S host materials. In situ characterization and theoretical calculation are needed to fathom the catalytic process of TMNs from LiPSs and Li2S2/Li2S.
(4) TMNs deliver good Li storage performance, suggesting their potential in high-rate LIBs and LiHCs due to their high conductivity, high ion conductivity and good mechanical stability. However, data reported so far for TMNs in Li ion storage are mainly based on a half-cell using Li foil as the counter electrode. Important parameters such as the tap density of electrode materials and the mass loading of active materials as well as the electrode thickness are not given in detail, thereby making it difficult to compare the Li storage properties with the actual energy and power densities of these devices. Although the capacity fading of some TMN anodes are less remarkable in a half cell, achieving stable cycle life in a high-energy full cell is a tough challenge. Efforts should also be devoted to exploring their potential applications in new energy storage systems such as Na/K ion batteries taking advantage of the merits of TMNs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51572100, 21875080, 51504171 and 61434001), Major Project of Technology Innovation of Hubei Province (2018AAA011), HUST Key Interdisciplinary Team Project (2016JCTD101), Fundamental Research Funds for the Central Universities (HUST: 2015QN071), Wuhan Yellow Crane Talents Program, and City University of Hong Kong Applied Research Grant (ARG) No. 9667122 and Hong Kong Research Grants Council (RGC) General Research Funds (GRF) No. CityU 11205617.Notes and references
- Z. Abdmouleh, A. Gastli, L. Ben-Brahim, M. Haouari and N. A. Al-Emadi, Renewable Energy, 2017, 113, 266–280 CrossRef.
- Q. Wei, F. Xiong, S. Tan, L. Huang, E. H. Lan, B. Dunn and L. Mai, Adv. Mater., 2017, 29, 1602300 CrossRef PubMed.
- C. Chellaswamy and R. Ramesh, Renewable Sustainable Energy Rev., 2017, 76, 824–838 CrossRef.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. Shen, Adv. Sci., 2018, 5, 1700322 CrossRef PubMed.
- J. Mao, J. Iocozzia, J. Huang, K. Meng, Y. Lai and Z. Lin, Energy Environ. Sci., 2018, 11, 772–799 RSC.
- A. S. Aricò, P. Bruce, B. Scrosati, J. M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef PubMed.
- C. Liu, X. Yan, F. Hu, G. Gao, G. Wu and X. Yang, Adv. Mater., 2018, 30, 1705713 CrossRef PubMed.
- P. Simon and Y. Gogotsi, Acc. Chem. Res., 2013, 46, 1094–1103 CrossRef CAS PubMed.
- J. Azadmanjiri, V. K. Srivastava, P. Kumar, M. Nikzad, J. Wang and A. Yu, J. Phys. Chem. A, 2018, 6, 702–734 CAS.
- H. P. Feng, L. Tang, G. Zeng, J. Tang, Y. Deng, M. Yan, Y. Liu, Y. Zhou, X. Ren and S. Chen, J. Mater. Chem. A, 2018, 6, 7310–7337 RSC.
- T. Lv, M. Liu, D. Zhu, L. Gan and T. Chen, Adv. Mater., 2018, 30, 1705489 CrossRef PubMed.
- M. Zheng, H. Tang, L. Li, Q. Hu, L. Zhang, H. Xue and H. Pang, Adv. Sci., 2018, 5, 1700592 CrossRef PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- X. Liu, J. Q. Huang, Q. Zhang and L. Mai, Adv. Mater., 2017, 29, 1601759 CrossRef PubMed.
- J. Wei, X. Li, H. Xue, J. Shao, R. Zhu and H. Pang, Adv. Mater. Interfaces, 2018, 5, 1701509 CrossRef.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- Y. Zhong, X. Xia, F. Shi, J. Zhan, J. Tu and H. J. Fan, Adv. Sci., 2016, 3, 1500286 CrossRef PubMed.
- M. S. Balogun, Y. Huang, W. Qiu, H. Yang, H. Ji and Y. Tong, Mater. Today, 2017, 20, 425–451 CrossRef CAS.
- S. Dong, X. Chen, X. Zhang and G. Cui, Coord. Chem. Rev., 2013, 257, 1946–1956 CrossRef CAS.
- M. S. Balogun, W. Qiu, W. Wang, P. Fang, X. Lu and Y. Tong, J. Mater. Chem. A, 2015, 3, 1364–1387 RSC.
- N. Choudhary, M. A. Islam, J. H. Kim, T. J. Ko, A. Schropp, L. Hurtado, D. Weitzman, L. Zhai and Y. Jung, Nano Today, 2018, 19, 16–40 CrossRef CAS.
- Y. Y. Xin and W. L. Xiong, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- P. Kulkarni, S. K. Nataraj, R. G. Balakrishna, D. H. Nagaraju and M. V. Reddy, J. Mater. Chem. A, 2017, 5, 22040–22094 RSC.
- J. Wang, Z. Liu, Y. Zheng, L. Cui, W. Yang and J. Liu, J. Mater. Chem. A, 2017, 5, 22913–22932 RSC.
- W. Li, X. Sun and Y. Yu, Small Methods, 2017, 1, 1600037 CrossRef.
- D. Zhao, Z. Cui, S. Wang, J. Qin and M. Cao, J. Mater. Chem. A, 2016, 4, 7914–7923 RSC.
- C. Huang, Y. Yang, J. Fu, J. Wu, H. Song, X. Zhang, B. Gao, P. K. Chu and K. Huo, J. Nanosci. Nanotechnol., 2018, 18, 30–38 CrossRef CAS PubMed.
- W. Bi, Z. Hu, X. Li, C. Wu, J. Wu, Y. Wu and Y. Xie, Nano Res., 2015, 8, 193–200 CrossRef CAS.
- H. Cui, G. Zhu, X. Liu, F. Liu, Y. Xie, C. Yang, T. Lin, H. Gu and F. Huang, Adv. Sci., 2016, 2, 1500126 CrossRef PubMed.
- T. Xu, H. Zhang, L. Fang, J. Yang, K. Wu and Y. Wang, ACS Nano, 2015, 9, 6817–6825 CrossRef PubMed.
- J. Xie and Y. Xie, Chem. – Eur. J., 2016, 22, 3588–3598 CrossRef CAS PubMed.
- W. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC.
- K. Schwarz, Crit. Rev. Solid State Mater. Sci., 1987, 13, 211–257 CrossRef CAS.
- S. V. Didziulis, K. D. Butcher and S. S. Perry, Inorg. Chem., 2003, 42, 7766–7781 CrossRef CAS PubMed.
- P. Parkin, Chem. Soc. Rev., 1996, 25, 199–207 RSC.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed.
- W. Lengauer, Transition Metal Carbides, Nitrides,and Carbonitrides, Wiley-VCH Verlag GmbH, 2000 Search PubMed.
- B. Gao, X. Li, X. Guo, X. Zhang, X. Peng, L. Wang, J. Fu, P. K. Chu and K. Huo, Adv. Mater. Interfaces, 2015, 2, 1500211 CrossRef.
- K. H. Lee, Y. W. Lee, A. R. Ko, G. Cao and K. W. Park, J. Am. Ceram. Soc., 2013, 96, 37–39 CrossRef CAS.
- X. Xiao, H. Yu, H. Jin, M. Wu, Y. Fang, J. Sun, Z. Hu, T. Li, J. Wu and L. Huang, ACS Nano, 2017, 11, 2180–2186 CrossRef CAS PubMed.
- P. Qin, X. Li, B. Gao, J. Fu, L. Xia, X. Zhang, K. Huo, W. Shen and P. K. Chu, Nanoscale, 2018, 10, 8728–8734 RSC.
- X. Xiao, X. Peng, H. Jin, T. Li, C. Zhang, B. Gao, B. Hu, K. Huo and J. Zhou, Adv. Mater., 2013, 25, 5091–5097 CrossRef CAS PubMed.
- X. Lu, T. Liu, T. Zhai, G. Wang, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Adv. Energy Mater., 2014, 4, 1300994 CrossRef.
- L. Zhang, C. M. B. Holt, E. J. Luber, B. C. Olsen, H. Wang, M. Danaie, X. Cui, X. Tan, V. W. Lui, W. P. Kalisvaart and D. Mitlin, J. Phys. Chem. C, 2011, 115, 24381–24393 CrossRef CAS.
- P. Yang, D. Chao, C. Zhu, X. Xia, Y. Zhang, X. Wang, S. Peng, B. K. Tay, Z. X. Shen, W. Mai and H. J. Fan, Adv. Sci., 2016, 3, 1500299 CrossRef PubMed.
- Y. Haldorai, D. Arreaga-Salas, C. S. Rak, Y. S. Huh, Y. K. Han and W. Voit, Electrochim. Acta, 2016, 220, 465–474 CrossRef CAS.
- X. Peng, K. Huo, J. Fu, B. Gao, L. Wang, L. Hu, X. Zhang and P. K. Chu, ChemElectroChem, 2015, 2, 512–517 CrossRef CAS.
- X. Peng, K. Huo, J. Fu, X. Zhang, B. Gao and P. K. Chu, Chem. Commun., 2013, 49, 10172–10174 RSC.
- H. Pang, S. J. Ee, Y. Dong, X. Dong and P. Chen, ChemElectroChem, 2014, 1, 1027–1029 CrossRef CAS.
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- A. González, E. Goikolea, J. A. Barrena and R. Mysyk, Renewable Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef.
- Q. Wang, J. Yan and Z. Fan, Energy Environ. Sci., 2016, 9, 729–762 RSC.
- Q. Li, Y. Xu, S. Zheng, X. Guo, H. Xue and H. Pang, Small, 2018, 1800426 CrossRef PubMed.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- D. P. dubal, N. R. Chodankar, D. H. Kim and P. Gomez-Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- I. Shown, A. Ganguly, L. C. Chen and K. H. Chen, Energy Sci. Eng., 2015, 3, 2–26 CrossRef CAS.
- M. Huang, F. Li, F. Dong, Y. Zhang and L. Zhang, J. Mater. Chem. A, 2015, 3, 21380–21423 RSC.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed.
- J. Kang, S. Zhang and Z. Zhang, Adv. Mater., 2017, 29, 1700515 CrossRef PubMed.
- K. Zhang, X. Han, Z. Hu, X. Zhang, Z. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
- J. Wang, F. Kang and B. Wei, Prog. Mater. Sci., 2015, 74, 51–124 CrossRef CAS.
- D. Choi, G. E. Blomgren and P. N. Kumta, Adv. Mater., 2006, 18, 1178–1182 CrossRef CAS.
- D. Shu, C. Lv, F. Cheng, C. He, K. Yang, J. Nan and L. Long, Int. J. Electrochem. Sci., 2013, 8, 1209–1225 CAS.
- A. M. Glushenkov, D. Hulicova-Jurcakova, D. Llewellyn, G. Q. Lu and Y. Chen, Chem. Mater., 2010, 22, 914–921 CrossRef CAS.
- P. Priyanka, P. G. Rasmussen and L. T. Thompson, J. Power Sources, 2012, 207, 212–215 CrossRef.
- J. Liu, K. Huang, H. Tang and M. Lei, Int. J. Hydrogen Energy, 2016, 41, 996–1001 CrossRef CAS.
- B. Gao, X. Xiao, J. Su, X. Zhang, X. Peng, J. Fu and P. K. Chu, Appl. Surf. Sci., 2016, 383, 57–63 CrossRef CAS.
- X. Ma and W. Zhang, ChemistrySelect, 2017, 2, 8726–8730 CrossRef CAS.
- S. Wang, L. Zhang, C. Sun, Y. Shao, Y. Wu, J. Lv and X. Hao, Adv. Mater., 2016, 28, 3768–3776 CrossRef CAS PubMed.
- A. Achour, R. Lucio-Porto, M. Chaker, A. Arman, A. Ahmadpourian, M. A. Soussou, M. Boujtita, L. L. Brizoual, M. A. Djouadi and T. Brousse, Electrochem. Commun., 2017, 77, 40–43 CrossRef CAS.
- W. Zhang, X. Ma, A. Loh, X. Li, F. C. Walsh and L. Kong, ACS Energy Lett., 2017, 2, 336–341 CrossRef CAS.
- X. Xiao, H. Song, S. Lin, Y. Zhou, X. Zhan, Z. Hu, Q. Zhang, J. Sun, B. Yang, T. Li, L. Jiao, J. Zhou, J. Tang and Y. Gogotsi, Nat. Commun., 2016, 7, 11296 CrossRef CAS PubMed.
- D. Choi and P. N. Kumta, J. Am. Ceram. Soc., 2011, 94, 2371–2378 CrossRef CAS.
- L. E. Toth, Transition Metal Carbides and Nitrides, Academic Press, 1971 Search PubMed.
- R. Riedel, Handbook of Ceramic Hard Materials, Wiley-VCH Verlag GmbH, 2000 Search PubMed.
- X. Zhou, D. Gall and S. V. Khare, J. Alloys Compd., 2014, 595, 80–86 CrossRef CAS.
- S. Dong, X. Chen, L. Gu, X. Zhou, L. Li, Z. Liu, P. Han, H. Xu, J. Yao, H. Wang, X. Zhang, C. Shang, G. Cui and L. Chen, Energy Environ. Sci., 2011, 4, 3502–3508 RSC.
- X. Lu, G. Wang, T. Zhai, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Nano Lett., 2012, 12, 5376–5381 CrossRef CAS PubMed.
- Y. Xie, Y. Wang and H. Du, Mater. Sci. Eng., B, 2013, 178, 1443–1451 CrossRef CAS.
- P. Sun, R. Lin, Z. Wang, M. Qiu, Z. Chai, B. Zhang, H. Meng, S. Tan, C. Zhao and W. Mai, Nano Energy, 2017, 31, 432–440 CrossRef CAS.
- Y. Ruan, L. Lv, Z. Li, C. Wang and J. Jiang, Nanoscale, 2017, 9, 18032–18041 RSC.
- S. Xie, S. Liu, F. Cheng and X. Lu, ChemElectroChem, 2018, 5, 5571–5582 Search PubMed.
- X. Lu, M. Yu, G. Wang, Y. Tong and Y. Li, Energy Environ. Sci., 2014, 7, 2160–2181 RSC.
- X. Lu, M. Yu, T. Zhai, G. Wang, S. Xie, T. Liu, C. Liang, Y. Tong and Y. Li, Nano Lett., 2013, 13, 2628–2633 CrossRef CAS PubMed.
- G. He, M. Ling, X. Han, D. I. A. E. Amaiem, Y. Shao, Y. Li, W. Li, S. Ji, B. Li and Y. Lu, Energy Storage Mater., 2017, 9, 119–125 CrossRef.
- A. Achour, J. B. Ducros, R. L. Porto, M. Boujtita, E. Gautron, L. L. Brizoual, M. A. Djouadi and T. Brousse, Nano Energy, 2014, 7, 104–113 CrossRef CAS.
- M. S. Balogun, Y. Zeng, C. Li, W. Qiu, Y. Luo, A. Onasanya, T. K. Olaniyi and Y. Tong, J. Mater. Chem. A, 2016, 4, 9844–9849 RSC.
- J. Zhang, A. L. Hector, S. Soulé, Q. Zhang and X. Zhao, J. Mater. Chem. A, 2018, 6, 5208–5216 RSC.
- C. Zhu, P. Yang, D. Chao, X. Wang, X. Zhang, S. Chen, B. K. Tay, H. Huang, H. Zhang, W. Mai and H. Fan, Adv. Mater., 2015, 27, 4566–4571 CrossRef CAS PubMed.
- L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 43, 797–828 RSC.
- K. Chen, S. Song, F. Liu and D. Xue, Chem. Soc. Rev., 2015, 44, 6230–6257 RSC.
- Y. Wu and F. Ran, J. Power Sources, 2017, 344, 1–10 CrossRef CAS.
- Y. Tan, L. Meng, Y. Wang, W. Dong, L. Kong, L. Kang and F. Ran, Electrochim. Acta, 2018, 277, 41–49 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- Y. Shao, M. F. El-Kady, L. J. Wang, Q. Zhang, Y. Li, H. Wang, M. F. Mousaviae and R. B. Kane, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC.
- V. Sridhar and P. Hark, New J. Chem., 2018, 42, 5668–5673 RSC.
- B. Wang, Z. Chen, G. Lu, T. Wang and Y. Ge, Mater. Res. Bull., 2016, 76, 37–40 CrossRef CAS.
- F. Grote, H. Zhao and Y. Lei, J. Mater. Chem. A, 2015, 3, 3465–3470 RSC.
- W. Yuan, L. Cheng, H. Wu, Y. Zhang, S. Lv and X. Guo, Chem. Commun., 2018, 54, 2755–2758 RSC.
- Q. Zhang, W. Xu, Z. Pan, J. Sun, J. Zhao, J. Zhang, C. Zhang, L. Tang, J. Luo, B. Song, Z. Zhang, W. Lu, Q. Li, Y. Zhang and T. Yao, Nano Lett., 2017, 17, 2719–2726 CrossRef CAS PubMed.
- Q. Li, Y. Chen, J. Zhang, W. Tian, L. Wang, Z. Ren, X. Ren, X. Li, B. Gao, X. Peng, P. K. Chu and K. Huo, Nano Energy, 2018, 51, 128–136 CrossRef CAS.
- Y. Tan, L. Ying, Z. Tang, W. Zhe, L. Kong, K. Long, L. Zhen and F. Ran, Sci. Rep., 2018, 8, 2915 CrossRef PubMed.
- Y. Liu, L. Liu, L. Kong, L. Kang and F. Ran, Electrochim. Acta, 2016, 211, 469–477 CrossRef CAS.
- F. Ran, Y. Wu, M. Jiang, Y. Tan, Y. Liu, L. Kong, L. Kang and S. Chen, Dalton Trans., 2018, 47, 4128–4138 RSC.
- Y. Xu, J. Wang, B. Ding, L. Shen, H. Dou and X. Zhang, ChemElectroChem, 2016, 2, 2020–2026 CrossRef.
- Y. Yang, K. Shen, Y. Liu, Y. Tan, X. Zhao, J. Wu, X. Niu and F. Ran, Nano-Micro Lett., 2017, 9, 6 CrossRef PubMed.
- A. Sliwak, A. Moyseowicz and G. Gryglewicz, J. Mater. Chem. A, 2017, 5, 5680–5684 RSC.
- G. Ma, Z. Wang, B. Gao, T. Ding, Q. Zhong, X. Peng, J. Su, B. Hu, L. Yuan, P. K. Chu and K. Huo, J. Mater. Chem. A, 2015, 3, 14617–14624 RSC.
- H. Song, J. Fu, K. Ding, C. Huang, K. Wu, X. Zhang, B. Gao, K. Huo, X. Peng and P. K. Chu, J. Power Sources, 2016, 328, 599–606 CrossRef CAS.
- R. Wang, C. Xia, N. Wei and H. N. Alshareef, Electrochim. Acta, 2016, 196, 611–621 CrossRef CAS.
- H. Yi, X. Chen, H. Wang and X. Wang, Electrochim. Acta, 2014, 116, 372–378 CrossRef CAS.
- C. Shang, S. Dong, S. Wang, D. Xiao, P. Han, X. Wang, L. Gu and G. Cui, ACS Nano, 2013, 7, 5430–5436 CrossRef CAS PubMed.
- M. Yu, S. Zhao, H. Feng, L. Hu, X. Zhang, Y. Zeng, Y. Tong and X. Lu, ACS Energy Lett., 2017, 2, 1862–1868 CrossRef CAS.
- Y. Shi, L. Peng, Y. Ding, Y. Zhao and G. Yu, Chem. Soc. Rev., 2015, 44, 6684–6696 RSC.
- Z. Li, G. Ma, R. Ge, F. Qin, X. Dong, W. Meng, T. Liu, J. Tong, F. Jiang, Y. Zhou, K. Li, X. Min, K. Huo and Y. Zhou, Angew. Chem., Int. Ed., 2016, 55, 979–982 CrossRef CAS PubMed.
- H. Du, Y. Xie, C. Xia, W. Wang and F. Tian, New J. Chem., 2014, 38, 1284–1293 RSC.
- Y. Xie, C. Xia, H. Du and W. Wang, J. Power Sources, 2015, 286, 561–570 CrossRef CAS.
- Y. Xie and D. Wang, J. Alloys Compd., 2016, 665, 323–332 CrossRef CAS.
- L. Lu and Y. Xie, New J. Chem., 2016, 41, 335–346 RSC.
- X. Zhou, C. Shang, L. Gu, S. Dong, X. Chen, P. Han, L. Li, J. Yao, Z. Liu, H. Xu, Y. Zhu and G. Cui, ACS Appl. Mater. Interfaces, 2011, 3, 3058–3063 CrossRef CAS PubMed.
- Y. Xie and X. Fang, Electrochim. Acta, 2014, 120, 273–283 CrossRef CAS.
- J. Zhang, Y. Li, Y. Zhang, X. Qian, R. Niu, R. Hu, X. Zhu, X. Wang and J. Zhu, Nano Energy, 2018, 43, 91–102 CrossRef CAS.
- R. Wang, X. Yan, J. Lang, Z. Zheng and P. Zhang, J. Mater. Chem. A, 2014, 2, 12724–12732 RSC.
- M. Liu, T. Yang, J. Chen, L. Su, K. C. Chou and X. Hou, J. Alloys Compd., 2016, 692, 605–613 CrossRef.
- C. Xia, Y. Xie, W. Wang and H. Du, Synth. Met., 2014, 192, 93–100 CrossRef CAS.
- D. Ruan, R. Lin, K. Jiang, X. Yu, Y. Zhu, Y. Fu, Z. Wang, H. Yan and W. Mai, ACS Appl. Mater. Interfaces, 2017, 9, 29699–29706 CrossRef CAS PubMed.
- Y. Xie and F. Tian, Mater. Sci. Eng., B, 2017, 215, 64–70 CrossRef CAS.
- C. Chen, D. Zhao, D. Xu and X. Wang, Mater. Chem. Phys., 2006, 95, 84–88 CrossRef CAS.
- R. Younesi, G. M. Veith, P. Johansson, K. Edstrom and T. Vegge, Energy Environ. Sci., 2015, 8, 1905–1922 RSC.
- R. Fang, S. Zhao, Z. Sun, D. Wang, H. M. Cheng and F. Li, Adv. Mater., 2017, 29, 1606823 CrossRef PubMed.
- Z. W. Seh, Y. Sun, Q. Zhang and Y. Cui, Chem. Soc. Rev., 2016, 45, 5605–5634 RSC.
- H. Peng, J. Huang, X. Cheng and Q. Zhang, Adv. Mater., 2017, 38, 1700260 Search PubMed.
- O. Ogoke, G. Wu, X. Wang, A. Casimir, L. Ma, T. Wu and J. Lu, J. Mater. Chem. A, 2017, 5, 448–469 RSC.
- Z. Cheng, H. Pan, H. Zhong, Z. Xiao, X. Li and R. Wang, Adv. Funct. Mater., 2018, 1707597 CrossRef.
- A. Manthiram, S. H. Chung and C. Zu, Adv. Mater., 2015, 27, 1980–2006 CrossRef CAS PubMed.
- M. A. Pope and I. A. Aksay, Adv. Energy Mater., 2015, 5, 1500124 CrossRef.
- K. Cao, H. Liu, Y. Li, Y. Wang and L. Jiao, Energy Storage Mater., 2017, 9, 78–84 CrossRef.
- S. Rehman, S. Guo and Y. Hou, Adv. Mater., 2016, 28, 3167–3172 CrossRef CAS PubMed.
- H. Lin, L. Yang, X. Jiang, G. Li, T. Zhang, Q. Yao, G. W. Zheng and J. Y. Lee, Energy Environ. Sci., 2017, 10, 1476–1486 RSC.
- Y. Wei, Z. Kong, Y. Pan, Y. Cao, D. Long, J. Wang, W. Qiao and L. Ling, J. Mater. Chem. A, 2018, 6, 5899–5909 RSC.
- Z. Cui, C. Zu, W. Zhou, A. Manthiram and J. B. Goodenough, Adv. Mater., 2016, 28, 6926–6931 CrossRef CAS PubMed.
- T. G. Jeong, S. C. Dong, H. Song, J. Choi, S. A. Park, S. H. Oh, H. Kim, Y. Jung and Y. T. Kim, ACS Energy Lett., 2017, 2, 327–333 CrossRef CAS.
- Y. C. Jeong, J. H. Kim, S. Nam, C. R. Park and S. J. Yang, Adv. Funct. Mater., 2018, 1707411 CrossRef.
- D. Deng, F. Xue, Y. Jia, J. Ye, C. Bai, M. Zheng and Q. Dong, ACS Nano, 2017, 11, 6031–6039 CrossRef CAS PubMed.
- T. Zhou, W. Lv, J. Li, G. Zhou, Y. Zhao, S. Fan, B. Liu, B. Li, F. Kang and Q. Yang, Energy Environ. Sci., 2017, 10, 1694–1703 RSC.
- Z. Hao, L. Yuan, C. Chen, J. Xiang, Y. Li, Z. Huang, P. Hu and Y. Huang, J. Mater. Chem. A, 2016, 4, 17711–17717 RSC.
- D. Deng, T. An, Y. Li, Q. Wu, M. Zheng and Q. Dong, J. Mater. Chem. A, 2016, 4, 16184–16190 RSC.
- L. Ma, H. Yuan, W. Zhang, G. Zhu, Y. Wang, Y. Hu, P. Zhao, R. Chen, T. Chen, J. Liu, Z. Hu and Z. Jin, Nano Lett., 2017, 17, 7839–7846 CrossRef CAS PubMed.
- G. Jiang, F. Xu, S. Yang, J. Wu, B. Wei and H. Wang, J. Power Sources, 2018, 395, 77–84 CrossRef CAS.
- N. Mosavati, S. O. Salley and K. Y. S. Ng, J. Power Sources, 2017, 340, 210–216 CrossRef CAS.
- X. Li, K. Ding, B. Gao, Q. Li, Y. Li, J. Fu, X. Zhang, P. K. Chu and K. Huo, Nano Energy, 2017, 40, 655–662 CrossRef CAS.
- Z. Sun, J. Zhang, L. Yin, G. Hu, R. Fang, H. M. Cheng and F. Li, Nat. Commun., 2017, 8, 14627 CrossRef PubMed.
- Y. Zhong, D. Chao, S. Deng, J. Zhan, R. Y. Fang, Y. Xia, Y. Wang, X. Wang, X. Xia and J. Tu, Adv. Funct. Mater., 2018, 1706391 CrossRef.
- D. Deng, J. Lei, F. Xue, C. Bai, X. Lin, J. Ye, M. Zheng and Q. Dong, J. Mater. Chem. A, 2017, 5, 23497–23505 RSC.
- W. Xi, Q. Weng, Y. Yang, Y. Bando and D. Golberg, Chem. Soc. Rev., 2016, 47, 4042–4073 Search PubMed.
- M. S. Palacin, Chem. Soc. Rev., 2009, 38, 2565–2575 RSC.
- L. Wang, J. Sun, R. Song, S. Yang and H. Song, Adv. Energy Mater., 2016, 6, 1502067 CrossRef.
- L. Zhou, K. Zhang, Z. Hu, Z. Tao, L. Mai, Y. M. Kang, S. L. Chou and J. Chen, Adv. Energy Mater., 2018, 8, 1701415 CrossRef.
- M. S. Balogun, W. Qiu, Y. Luo, M. Hui, W. Mai, A. Onasanya, T. K. Olaniyi and Y. Tong, Nano Res., 2016, 9, 2823–2851 CrossRef CAS.
- K. Feng, M. Li, W. Liu, A. G. Kashkooli, X. Xiao, M. Cai and Z. Chen, Small, 2018, 14, 1702737 CrossRef PubMed.
- S. H. Elder, F. J. DiSalvo, L. Topor and A. Navrotsky, Chem. Mater., 1993, 5, 1545–1553 CrossRef CAS.
- F. Gillot, J. Oró-Solé and M. R. Palacín, J. Mater. Chem., 2011, 21, 9997–10002 RSC.
- Q. Sun and Z. Fu, Electrochim. Acta, 2008, 54, 403–409 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- I. S. Kim, P. N. Lumta and G. E. Blomgren, Electrochem. Solid-State Lett., 2000, 3, 493–496 CrossRef CAS.
- M. S. Balogun, W. Qiu, J. Jian, Y. Huang, Y. Luo, H. Yang, C. Liang, X. Lu and Y. Tong, ACS Appl. Mater. Interfaces, 2015, 7, 23205–23215 CrossRef CAS PubMed.
- Y. Li, Y. Yan, H. Ming and J. Zheng, Appl. Surf. Sci., 2014, 305, 683–688 CrossRef CAS.
- W. Wen, J. M. Wu, Y. Z. Jiang, L. L. Lai and J. Song, Chem, 2017, 2, 404–416 CAS.
- J. Zhang, J. Zhang, W. Cai, F. Zhang, L. Yu, Z. Wu and Z. Zhang, J. Power Sources, 2012, 211, 133–139 CrossRef CAS.
- J. Liu, S. Tang, Y. Lu, G. Cai, S. Liang, W. Wang and X. Chen, Energy Environ. Sci., 2013, 6, 2691–2697 RSC.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gómez-Romero, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- R. Wang, J. Lang, P. Zhang, Z. Lin and X. Yan, Adv. Funct. Mater., 2015, 25, 2270–2278 CrossRef CAS.
- M. Liu, L. Zhang, P. Han, X. Han, H. Du, X. Yue, Z. Zhang, H. Zhang and G. Cui, Part. Part. Syst. Charact., 2016, 32, 1006–1011 CrossRef.
- P. Wang, R. Wang, J. Lang, X. Zhang, Z. Chen and X. Yan, J. Mater. Chem. A, 2016, 4, 9760–9766 RSC.
- M. S. Balogun, M. Yu, C. Li, T. Zhai, Y. Liu, X. Lu and Y. Tong, J. Mater. Chem. A, 2014, 2, 10825–10829 RSC.
- M. S. Balogun, C. Li, Y. Zeng, M. Yu, Q. Wu, M. Wu, X. Lu and Y. Tong, J. Power Sources, 2014, 272, 946–953 CrossRef CAS.
- D. K. Nandi, U. K. Sen, D. Choudhury, S. Mitra and S. K. Sarkar, ACS Appl. Mater. Interfaces, 2014, 6, 6606–6615 CrossRef CAS PubMed.
- Y. Dong, B. Wang, K. Zhao, Y. Yu, X. Wang, L. Mai and S. Jin, Nano Lett., 2017, 17, 5740–5746 CrossRef CAS PubMed.
- S. Ni, P. Huang, D. Chao, G. Yuan, L. Zhang, F. Zhao and J. Li, Adv. Funct. Mater., 2017, 27, 1701808 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |