One-pot synthesis of self-supported hierarchical urchin-like Ni3S2 with ultrahigh areal pseudocapacitance
Abstract
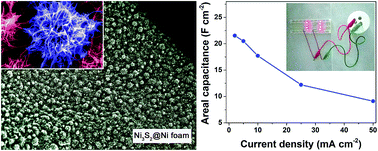
Recently, nickel sulfides have been widely investigated as an electrode material for pseudocapacitors. Among them, self-supported nickel sulfides have been intensively studied; yet, their structures are rather limited to nanosheet or nanorod arrays. Herein, a facile one-step hydrothermal method was developed to synthesize self-supported Ni3S2 on a Ni foam substrate with an urchin-like hierarchical structure consisting of nanowire subunits with a diameter of about 20–30 nm, thus combining the advantages of both hierarchical and self-supported structures. Because of this unique structure, when the sample was applied as an electrode for supercapacitors, it demonstrated an ultrahigh areal capacitance of 21.54 F cm−2 at a current rate of 2 mA cm−2, and even at a very high current rate of 50 mA cm−2, a reversible capacitance of 5.40 F cm−2 can still be retained after 1000 charge–discharge cycles. When the sample was employed as the cathode to build an asymmetric supercapacitor (ASC) with activated carbon as the anode, the device exhibited an areal capacitance of 1.26 F cm−2 at a current rate of 2 mA cm−2 and a coulombic efficiency of 97.7%. Furthermore, this device showed great potential for practical application by lighting up 8 red light-emitting diodes.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...