Lithiophilic Co/Co4N nanoparticles embedded in hollow N-doped carbon nanocubes stabilizing lithium metal anodes for Li–air batteries†
Abstract
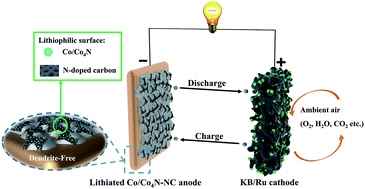
The metallic Li electrode is considered as the most promising anode candidate for next-generation rechargeable batteries due to its super-high energy density. However, the safety and passivation issue, low coulombic efficiency and short cycle-life derived from uneven Li dendrites and an unstable solid electrolyte interphase (SEI) have hindered the practical application of Li metal batteries, especially Li–air batteries. Herein, we have synthesized Co4N-doped Co nanoparticles embedded into hollow nitrogen (N)-doped carbon nanocubes (Co/Co4N-NC) through the pyrolysis of a metal–organic framework (MOF), and further applied it as the Li plating matrix. The high content of lithiophilic Co4N, pyridinic and pyrrolic N species in Co/Co4N-NC can effectively ensure the uniform distribution of Li. In addition, the intercalation reaction in graphitized carbon coupled with electrodeposition reaction in the porous framework can further enhance the Li storage capacity of Co/Co4N-NC. Moreover, we have also demonstrated the formation of a stable SEI layer on the Co/Co4N-NC electrode during the Li plating process. Hence, the Co/Co4N-NC electrode can significantly suppress dendritic Li and shows a high coulombic efficiency of 98.5% over 300 cycles. More importantly, a Li–air battery using the lithiated Co/Co4N-NC electrode displays better cycle performance than a Li–air cell with a Li-anode in ambient air.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...