Stabilizing NiCo2O4 hybrid architectures by reduced graphene oxide interlayers for improved cycling stability of hybrid supercapacitors†
Abstract
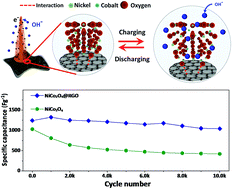
We demonstrate nickel cobaltite@reduced graphene oxide (NiCo2O4@rGO) hybrid architectures directly deposited on nickel-foam (NF). The rGO interlayers restrict the growth of NiCo2O4 nanoneedles into smaller and thinner dimensions compared to NiCo2O4 without rGO layers, providing kinetic and structural stability for hybrid architectures. Accordingly, the NiCo2O4@rGO hybrid on NF achieves a specific capacitance of 1427 F g−1 at 8 A g−1, a coulombic efficiency of 96.2%, and a capacitance retention of 83.8% over 10 000 cycles, which are greater than 1036 F g−1, 89.1%, and 40.8% of NiCo2O4 on NF in an aqueous 2 M KOH electrolyte. In order to enlarge the potential window of the aqueous system, hybrid supercapacitors (HSCs) are configured using the NiCo2O4@rGO hybrid on NF as a positive electrode and rGO on NF as a negative electrode. The HSCs exhibit a good cycling stability of 81.1% over 10 000 cycles, delivering maximum energy and power densities of 25.24 W h kg−1 and 21.42 kW kg−1.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...