 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability†
Emilio J.
Juarez-Perez
 ,
Luis K.
Ono
,
Luis K.
Ono
 ,
Maki
Maeda
,
Yan
Jiang
,
Maki
Maeda
,
Yan
Jiang
 ,
Zafer
Hawash
,
Zafer
Hawash
 and
Yabing
Qi
and
Yabing
Qi
 *
*
Energy Materials and Surface Sciences Unit (EMSSU), Okinawa Institute of Science and Technology Graduate University (OIST), 1919-1 Tancha, Onna-son, Okinawa 904-0495, Japan. E-mail: Yabing.Qi@OIST.jp
First published on 23rd April 2018
Abstract
Hybrid lead halide perovskites have emerged as promising active materials for photovoltaic cells. Although superb efficiencies have been achieved, it is widely recognized that long-term stability is a key challenge intimately determining the future development of perovskite-based photovoltaic technology. Herein, we present reversible and irreversible photodecomposition reactions of methylammonium lead iodide (MAPbI3). Simulated sunlight irradiation and temperature (40–80 °C) corresponding to solar cell working conditions lead to three degradation pathways: (1) CH3NH2 + HI (identified as the reversible path), (2) NH3 + CH3I (the irreversible or detrimental path), and (3) a reversible Pb(0) + I2(g) photodecomposition reaction. If only the reversible reactions (1) and (3) take place and reaction (2) can be avoided, encapsulated MAPbI3 can be regenerated during the off-illumination timeframe. Therefore, to further improve operational stability in hybrid perovskite solar cells, detailed understanding of how to mitigate photodegradation and thermal degradation processes is necessary. First, encapsulation of the device is necessary not only to avoid contact of the perovskite with ambient air, but also to prevent leakage of volatile products released from the perovskite. Second, careful selection of the organic cations in the compositional formula of the perovskite is necessary to avoid irreversible reactions. Third, selective contacts must be as chemically inert as possible toward the volatile released products. Finally, hybrid halide perovskite materials are speculated to undergo a dynamic formation and decomposition process; this can gradually decrease the crystalline grain size of the perovskite with time; therefore, efforts to deposit highly crystalline perovskites with large crystal sizes may fail to increase the long-term stability of photovoltaic devices.
Introduction
Hybrid lead halide perovskites have been intensively evaluated as light harvesting materials for photovoltaic cells since 2009.1 Currently, the long-term stability of perovskite solar cells is a major challenge that will require substantial effort to advance hybrid perovskite solar cells towards commercialization.2 Moreover, a significant number of research groups are achieving power conversion efficiencies of over 20% using hybrid perovskite devices;3 however, very few devices have succeeded in operational stability tests or shown lifetimes longer than 1000 hours at the maximum power point.3 However, promising attempts have been realized recently to improve the durability of these cells using different approaches such as chemically inert scaffolds and electrodes4 or mixed/multication hybrid perovskites.5 How hybrid perovskite suffers from degradation and what products are generated during the degradation are important questions to be answered in order to design stable perovskite-based solar cells. Furthermore, understanding reversible degradation routes or so-called self-healing in perovskite solar cells, in which the cell performance recovers its original value after resting in the dark, has recently received attention as a strategy to prolong device lifetimes.6 In the decomposition pathways of methylammonium-based perovskites, the degradation processes that must be avoided are irreversible degradation reactions that permanently limit the lifetimes of solar cells. Therefore, understanding the fundamental processes taking place in both reversible and irreversible degradation pathways is a step forward to enhance the stability of solar cells. Our group recently found that MAPbI3 perovskite degrades significantly faster upon exposure to I2 vapor than to H2O, O2, or light only.7 As highlighted by Wilks and Bär,8 we proposed that the internally generated I2 and its migration within MAPbI3 induced by solar cell operation and/or external stimuli (such as H2O, O2, light irradiation, applied bias, and heat) leads to self-sustaining and irreversible degradation reactions in perovskites independent of the device architecture.7 Additionally, we recently showed that CH3I and NH3 are generated as irreversible gas by-products during high temperature thermal degradation of MAPbI3.9 However, the conditions under which detrimental I2 is generated remain unclear.Interestingly, this puzzle is associated with a study published by Dawood et al. half a century ago regarding lead iodide (PbI2).10 PbI2 is a relevant material to the topic of perovskite solar cells. For example, PbI2 is a standard reagent choice to synthesize hybrid perovskite; also, it is the unique remaining solid degradation product after degradation of MAPbI3. Dawood et al. found that PbI2 suffers photodecomposition to iodine gas (I2) and lead (Pb0) upon interaction with visible light by observing the decrease in electrical conductivity under illumination wavelengths above the PbI2 band gap.10 Thus, they suggested the following stepwise PbI2 photodecomposition route: (I) photodecomposition of PbI2 takes place, assisted by a mechanism in which two excitons are generated (two electron–hole pairs); (II) these two excitons react, leading to the formation of a Pb0 atom; and (III) I2 molecule is formed and released, leaving two positively charged anion vacancy sites 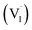 11 (see Fig. 1a).
11 (see Fig. 1a).
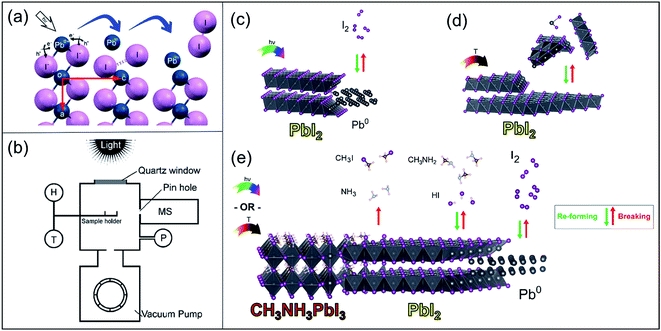 | ||
Fig. 1 Schematics of the photodecomposition, thermal evaporation and thermal degradation processes in PbI2 and MAPbI3. (a) (0k0) plane view of a layered PbI2 supercell. The figure depicts the two steps for the release of I2 and generation of Pb0 by the two-exciton mechanism. (b) Schematic of the experimental setup for controlled PbI2 and perovskite degradation experiments. H: electrical heater, T: thermocouple, P: crystal/cold cathode pressure gauge, MS: quadrupole mass spectrometer, top quartz window and Xe lamp or LED light sources with controlled on/off intervals. (c) PbI2 decomposition process driven by visible light (<530 nm) above the PbI2 band gap at 40 °C to 60 °C. (d) Temperature-assisted PbI2 evaporation at ∼70 °C in the dark, and (e) MAPbI3 photodecomposition and thermal degradation processes leading to irreversible decomposition to organic volatile gas species (CH3I + NH3), reversible decomposition (CH3NH2 + HI), and reversible generation of I2 and non-volatile Pb0 under illumination or mild heat conditions. The irreversibility of the process of releasing CH3I + NH3 is indicated by a one-directed arrow for the reaction. The database crystal phases used to depict the structures were ICSD-68819 for PbI2, ICSD-96501 for the cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m Pb0 phase and ICSD-238610 for tetragonal MAPbI3. Correlated partial occupation for the methylammonium cation in the MAPbI3 phase was solved using Supercell.12 m Pb0 phase and ICSD-238610 for tetragonal MAPbI3. Correlated partial occupation for the methylammonium cation in the MAPbI3 phase was solved using Supercell.12 | ||
By reexamining Dawood et al.'s work, here, we present the following two points: (1) systematical investigation of the light-only decomposition pathways of PbI2 by verifying that our experimental setup (Fig. 1b) can detect I2 release and then pursuing detection of gas release from MAPbI3; and (2) photodecomposition experiments on MAPbBr3, aiming to answer the question of why MAPbBr3 is more stable than MAPbI3 perovskite.
Our experiments were performed in a home-built small vacuum chamber in the absence of H2O and O2, eliminating all other possible external degradation factors. Inside this vacuum chamber, applied light and/or heat and measured temperatures were accomplished in situ directly on the sample holder, and the gaseous species released during photodecomposition were probed using a quadrupole mass spectrometer (MS) equipped with an electron multiplier detector. Based on this setup, the light-induced activation energies for I2 release from PbI2 and MAPbI3 were extracted and compared. Thin films of PbI2, MAPbI3 and MAPbBr3 materials before and after the photodecomposition procedure were studied by X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) to identify the remaining non-volatile degraded solid products. Finally, we also investigated whether photodegraded PbI2 films containing Pb0 could be recovered to PbI2 by exposure to I2 pellets at room temperature, which showed the reversibility of this photodecomposition. We propose that the decomposition of hybrid lead halide perovskite materials under mild visible illumination and temperature conditions is reversible; i.e., a continuously decomposing and re-forming dynamical process takes place which establishes a chemical equilibrium between the gas phase components and solid perovskite (Fig. 1e).
Results and discussion
PbI2 degradation under illumination and dark conditions
First, we performed photodecomposition experiments on PbI2 (Fig. 1c) in our home-designed experimental setup (Fig. 1b) to verify the adequacy of this setup for I2 detection prior to comparison with the photodecomposition of MAPbI3. Two important findings by Dawood et al.10 were: (1) I2 gas and metallic Pb0 generation as a consequence of PbI2 photodegradation with an activation energy (Ea) of 4.7 kcal mol−1, and (2) a threshold wavelength of 520 nm, where only higher photon energies initiate the photodecomposition of PbI2. These findings were also observed in our moisture-free and anaerobic setup; however, I2 gas release was detected at temperatures as low as 40 °C to 60 °C. We probed four different pulsed light sources: three LED light sources, red (617 nm), blue (470 nm), and white (450 + 550 nm), and Xe lamp-based simulated solar irradiation (0.55 Sun). Also, an experiment under dark conditions while heating the sample stage to a similar temperature as during the illuminated experiments was carried out to decouple the photodecomposition and thermal degradation (or evaporation) processes (Fig. 1d). Details of the experimental procedures can be found in the Methods section and the ESI.† Next, the experimental Ea for I2 release during the PbI2 photodecomposition process was extracted in order to determine the feasibility of this photodecomposition reaction. In our analysis, the corresponding I2 releases using different light sources were estimated from the MS data trace pulses; see Table 1.| Light source | Wavelength (nm) | Temperature (°C) | Light intensity (mW cm−2) | E a (kcal mol−1) |
|---|---|---|---|---|
| a Details of the Ea determination and wavelength spectra distribution for each light source are shown in ESI Sections S4 and S5, respectively. | ||||
| White LED | 450 + 550 | 50 to 70 | 80 to 110 | 57 |
| Blue LED | 470 | 45 to 62 | 71 to 120 | 45 |
| Xe lamp | ∼Sun | 35 to 78 | 55 | 9 |
E a showed strong dependence on the magnitude and wavelength distribution of the employed light source. The simulated sunlight (Xe lamp, Table 1), which has a broader spectral wavelength, led to the smallest Ea of ∼9 kcal mol−1 compared to the narrow wavelength intervals generated by LEDs (Ea ∼ 45 kcal and 57 kcal mol−1 for blue and white LEDs, respectively). Interestingly, despite the different photodegradation conditions employed in the current study (i.e., high vacuum and absence of O2), the extracted Ea corresponding to PbI2 degradation under Xe lamp illumination (55.2 mW cm−2) is in good agreement with the Ea value reported by Dawood et al. (4.7 kcal mol−1).10
Further investigations using XRD and XPS techniques revealed that Pb0 was the remaining non-volatile product of the photodecomposition of PbI2. More interestingly, this decomposed Pb0 product also showed a reversible recovery process back to PbI2 after brief exposure to I2 gas (ESI Sections S7 and S8†).
In summary, the chemical and physical processes extracted from all the sets of degradation and recovery experiments on PbI2 powders and thin films are summarized by eqn (1) and illustrated in Fig. 1c. Under illumination with photon energies higher than 2.34 eV (<530 nm), photodecomposition takes place; however, the process is reversible if Pb0 is exposed again to I2(g):
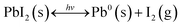 | (1) |
Under dark conditions at moderate temperatures (∼70 °C) in high vacuum (∼10−6 Torr), PbI2 sublimates in the form of molecules or clusters, as depicted in Fig. 1d and described in eqn (2) below:
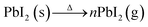 | (2) |
Notably, no traces of released I2 gas were observed under dark and mild temperature conditions.
Degradation of MAPbI3 and MAPbBr3 under illumination and dark conditions
In the previous section, the photodecomposition of PbI2 and its volatile (I2) and non-volatile (Pb0) decomposition products as well as its recovery routes were established based on our home-built setup. Identical experimental conditions were applied to MAPbI3 perovskite. In addition, we studied the photodecomposition of MAPbBr3 for comparison purposes, as this material has been reported to show higher stability than MAPbI3.7,13,14 Currently, the origin of this difference remains largely elusive, and studies of this relative stability have also yielded contradictory results.15MAPbI3 was introduced in the vacuum chamber; after pumping down the system (∼10−6 Torr), a set of experiments with Xe lamp illumination light pulses and heating-only pulses under dark conditions were carried out. Sample temperatures and recorded MS traces are displayed in Fig. 2a and b for each experiment. Firstly, it is interesting to note that the hybrid perovskites required much longer times (∼72 h) to reach a similar level of high vacuum conditions compared to PbI2 (∼6 h). The MS data helped clarify the reason for this phenomenon. One of the most important findings from this experiment is that in contrast with PbI2, MAPbI3 powder releases I2 independently of light or dark conditions. The rate of I2 generation was relatively constant during the light/dark pulse conditions (Fig. 2a), whereas I2 was only generated at high temperature pulses (>∼60 °C) applied during heating-in-the-dark conditions (Fig. 2b). Under mild temperature conditions (<∼60 °C), I2 release is minimized. This indicates that MAPbI3 continues to degrade for some time after it is exposed to the light source. Unlike PbI2, MAPbI3 does not have a wavelength threshold where I2 is released by photodecomposition. If this threshold exists due to the band gap of MAPbI3, an infrared light source should be used; therefore, we preferred to directly heat the sample in dark conditions. Furthermore, in addition to I2 release, MAPbI3 perovskite continuously released organic gas components (CH3NH2, HI, CH3I and NH3) under vacuum conditions. Upon insertion of MAPbI3 into the vacuum chamber, the background signals associated with MAPbI3 degradation detected by MS were observed to increase. This increase was further enhanced with increase in the number of the light or temperature pulses. Furthermore, mass peaks corresponding to dimethylformamide (DMF) solvent could be observed.16 These occluded solvent molecules in the perovskite were expected to be detected because the perovskite powder samples were prepared using a protocol similar to the typical spin coating deposition method; see the Methods section and ESI Section 1.†Ea values for I2 release in MAPbI3 were extracted for comparison with the PbI2 values; see Table 2.
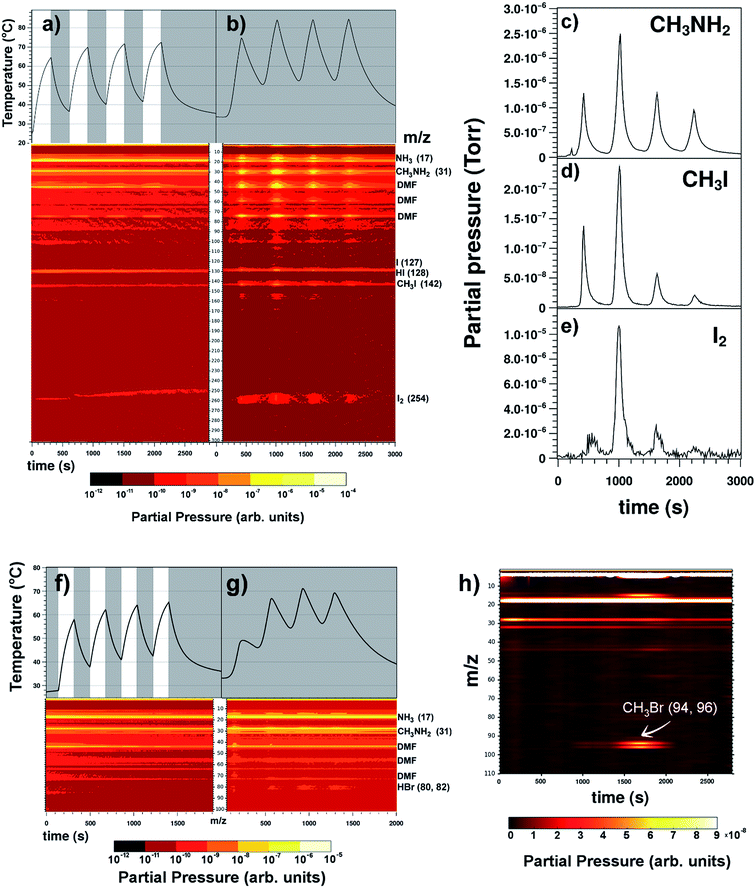 | ||
| Fig. 2 Mass spectrometry profiles of MAPbI3 and MAPbBr3 decomposition products during illumination and heating-in-the-dark pulse experiments. (a) Light/dark intervals (5 min each) on MAPbI3 perovskite using a Xe lamp delivering 55 mW cm−2 of light power. White and grey areas represent the light and dark pulse durations, respectively. The black lines correspond to the sample temperature. (b) Heating on/off intervals (5 min each) on the MAPbI3 sample under dark conditions. Species of interest detected in MS are labeled on the right side. The right panel shows calibrated mass traces for (c) CH3NH2, (d) CH3I and (e) I2 during the heating intervals under dark conditions. ESI Fig. S14† shows the calculated CH3I/CH3NH2 molar ratios. (f) Light/dark intervals (3 min each) on the MAPbBr3 perovskite sample. (g) Heating on/off intervals (3 min each) on the MAPbBr3 sample. (h) m/z traces registered simultaneously during the thermal degradation of MAPbBr3 using a heating rate of 20 °C min−1 under He atmosphere in a TG/DTA equipment using the same setting as recently published for MAPbI3 perovskite.9 Release of CH3Br (m/z = 94 and 96 amu traces) was observed during the thermal degradation. ESI Fig. S13f† shows the fragmentation pattern for CH3Br molecule. | ||
| Light/dark conditions | Temperature (°C) | Light intensity (mW cm−2) | E a (kcal mol−1) |
|---|---|---|---|
| a Details of the Ea determination and wavelength spectra for each light source are shown in ESI Sections S4 and S5, respectively. | |||
| Xe lamp | 35 to 72 | 55 | 6 |
| Heat in dark | 60 to 84 | 0 | 18 |
Interestingly, the Ea value corresponding to I2 release in MAPbI3 (∼6 kcal mol−1) was slightly lower than that in PbI2 (∼9 kcal mol−1), which indicates that I2 release in MAPbI3 is even slightly more favorable than in PbI2. Because I2 release was observed in dark conditions, Ea was also calculated for MAPbI3 from the signals of I2 release during the heating-in-the-dark experiments. We noted that the Ea in the heating-in-the-dark conditions was around three times higher than that under light exposure, indicating that a light-driven process is dominant during MAPbI3 degradation.
The similarities and differences found for MAPbI3 and PbI2 during these light/heat stress experiments have two possible causes (considering that the MAPbI3 material system is to some extent represented by PbI2 with intercalated MA+ cations): (i) in contrast to PbI2, MAPbI3 shows a smaller band gap, suggesting that additional photons with lower energies are effective for exciton and/or free charge generation at the same illumination power, and (ii) I2 release in MAPbI3 does not require a two-exciton mechanism as in PbI2 because [PbI6] octahedral distortion produces shorter I–I bond distances that are consistent with the formation of neutral I2 defects,17 which potentially facilitates the release of I2.18
For the sake of completeness, the same photodecomposition and thermal decomposition experiments performed on PbI2 and MAPbI3 were applied to MAPbBr3 to elucidate its decomposition products (e.g., is Br2 generated? What organic molecules are released from degraded MAPbBr3?) and associate it with the reported disparity in the stability of MAPbBr3 compared to MAPbI3. Comparing the experimental observations of the photodecomposition of MAPbI3 and MAPbBr3 perovskites, the main difference was that under vacuum and near room temperature conditions, MAPbI3 showed numerous degradation gas products (i.e., CH3I and NH3; CH3NH2 and HI; and I2); however, MAPbBr3 only released CH3NH2 and HBr (and solvent); see Fig. 2f and g. We emphasize that the CH3Br, NH3, and Br2 gaseous species were detected below the signal-sensitivity threshold of MS under vacuum conditions in a low temperature range (30 °C to 70 °C). On the other hand, under almost atmospherically inert He pressure conditions and at high temperatures (∼300 °C), MAPbI3 and MAPbBr3 underwent similar degradation processes of releasing CH3I/NH3 gas products: (MAPbI3)9 and CH3Br/NH3 (MAPbBr3) (Fig. 2h), respectively. Table 3 summarizes both the experimental conditions and detected products from the degradation tests carried out on perovskites.
| Perovskite type | Inert atmosphere: vacuum, T ∼ room | Inert atmosphere: ∼1 atm, helium T ∼ 300 °C |
|---|---|---|
| a This work. b Ref. 9. c Ref. 19. d Ref. 20, assuming that an iodine-transfer polymerization reaction was involved on the CH3I monomer to form radical species such as CH3CH2˙, which can propagate to form a longer polyethylene chain, as observed in the work by Ke et al. | ||
| MAPbI3 | (1) CH3NH2 + HIa,c | NH3 + CH3Ib |
| (2) NH3 + CH3Ia,c,d | ||
| (3) I2a | ||
| MAPbBr3 | CH3NH2 + HBra | NH3 + CH3Bra |
In summary, the chemical processes extracted from all the sets of degradation and recovery experiments on halide perovskite powders and thin films of MAPbI3 are summarized by eqn (3) and (4) and illustrated in Fig. 1e. Under illumination or dark, low-heating conditions compatible with photovoltaic operation, the photodecomposition and thermal decomposition reactions occurring are:
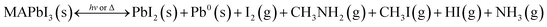 | (3) |
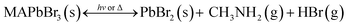 | (4) |
CH3X/NH3 (X = I or Br) molecules are reported to be the thermodynamically driven products of degradation of methylammonium cations;19 this degradation is irreversible to reform MA+.9 In contrast, the release of CH3NH2 + HX is considered to be reversible because of its high reactivity in neutralizing back to MA+ and X−. In fact, CH3NH2/HI gases have been demonstrated as excellent reagents that can be used to directly synthesize MAPbI3 perovskite.21,22 Therefore, under encapsulated conditions, it is not correct to consider CH3NH2 + HI released gases as degradation products of perovskites because they can resynthesize MAPbI3. A unique situation where CH3NH2 + HX can be considered as degradation products is when perovskites are placed in an open system (e.g. non-encapsulated solar cells) where back reaction is obviously inhibited because the released gases are permanently leaked. As highlighted in our work, we determined CH3NH2 + HI release to be a benign or reversible pathway of degradation because it does not lead to permanent degradation, but to a chemical equilibrium of formation and destruction of perovskite (Fig. 1e).
On the other hand, the back formation to MAX or MAPbX3 from the released CH3X + NH3 molecules is thermodynamically unfavorable and prone to form non-primary ammonium salts, as previously reported.9 Therefore, we assign the CH3X + NH3 release as an authentic detrimental pathway for perovskite degradation. If such a degradation path is taking place even in smaller proportions, as represented in Fig. 2d and elsewhere,19 it would result in short time stability of methylammonium-based hybrid perovskite solar cells even if careful encapsulation is employed. In view of the above points, it can be understood that MAPbBr3 is more stable than MAPbI3 because this detrimental path releasing CH3Br + NH3 was not observed at low temperatures, i.e., 40 °C to 80 °C (see Table 3 and eqn (4)). Consequently, an encapsulated sample of methylammonium-based bromide perovskite would be more stable than I-based perovskite under near ambient conditions.
Photostability and thermal stability of pristine perovskite: implications for its operational stability in solar cell devices
Perovskite thin films employed in photovoltaic devices under working conditions can follow a different degradation path compared to that of the pristine polycrystalline powder samples used in this study. In fact, in this study on photodecomposition and thermal decomposition of perovskites, the light harvester material was intentionally placed under optimistic and favorable conditions for perovskite stability, avoiding contact with any other compound (e.g., HTL, ETL, dopants used in HTL, moisture or oxygen) and without any applied bias. At this point, it is important to recall that the chemical synthesis procedure of pristine perovskite as well as the temperature and illumination conditions during the tests can be considered to resemble the working conditions to which perovskite is deposited in devices.With regard to selective contacts, the side reactions that will be suffered by a specific HTL used in photovoltaic devices upon exposure to the gas by-products (HI, CH3I, I2, CH3NH2 and NH3) released by MAPbI3, for example, during working conditions (eqn (3)) are out of the scope of this work. However, it is suggested that, rarely, the spiro functional group in the spiro-MeOTAD molecule, which is widely used as an HTL, can resist the attack of the above chemical gas agents over a photovoltaic device lifetime of ∼20 years. Therefore, avoiding contact with other compounds related only to the device helps reveal the intrinsic pathways of photodecomposition and thermal decomposition in perovskite. It can be reasonably considered that selective contacts cannot avoid these intrinsic decomposition paths in perovskites, which are driven by light and temperature. In contrast, decomposition can increase due to unknown side reactions that are specific for each selective layer case.
On the other hand, the voltage applied to thin films is a relevant parameter to consider when measuring photodegradation and thermal degradation in halide perovskites. With regard to degradation paths for perovskite under applied bias, additional experimental work was carried out in a specially designed MAPbI3 thin-film device consisting of two-gold electrode contacts (spaced by 70 microns) deposited on MAPbI3. Controlled bias voltage was applied on the gold electrodes, generating an electric field corresponding to that typically generated in perovskite solar cells (∼0.71 V μm−1). In this case, the perovskite layer was protected from moisture and oxygen by a top CYTOP layer. The core levels of MAPbI3 thin films were investigated using XPS mapping on lateral devices (Fig. 3). As can be seen in Fig. 3a, a uniform distribution of Pb2+ and I− was found for MAPbI3. However, after applying the electric field (Fig. 3b), a clear decrease as well as a non-uniform distribution of Pb2+ and I− was observed. XPS spectra (Fig. 3c) were also collected from the area between the electrodes (using a 27 μm detector slit). Depletion of Pb0 and Pb2+ was clearly observed on the negative electrode side, where a reduction process takes place in the perovskite under the externally applied electric field. Similarly, a broadening of the FWHM belonging to I 3d core level (from 1.0 to 1.4 eV) was observed after applying the electric field. The broadening of the I 3d FWHM indicates the formation of new iodine species, such as CH3I, HI and I2. Overall, the XPS results in this experiment are consistent with the observations described in the case of unbiased pristine perovskites. However, the most impressive observation in these biased thin-film perovskite experiments is the violent in situ release of gases observed in the form of “bubbles” formed under the CYTOP transparent layer. The CYTOP layer effectively impedes the release of these volatile gases from perovskite to the ambient environment (Fig. 3d and the video film deposited in the ESI† file).
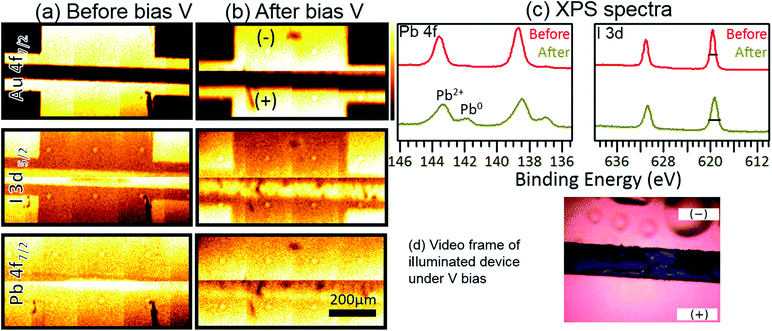 | ||
| Fig. 3 XPS mapping and spectra (Al-Kα = 1486.6 eV) of MAPbI3 thin films before and after applying a voltage bias. (a, b) XPS maps of Au 4f7/2, I 3d5/2, and Pb 4f7/2 core levels and (c) XPS spectra of Pb 4f and I 3d core levels before and after application of an electrical bias. The FWHM values of the I 3d peak are 1 eV and 1.4 eV before and after application of bias, respectively. The bias voltage applied was 50 V across 70 μm, ε = 50/70 = 0.71 V μm−1. Under conventional cell operation at the maximum power point, the bias voltage would be 0.8 V across 0.5 μm of perovskite, ε = 0.8/0.5 = 1.6 V μm−1. The Pb0/Pb2+ composition ratio is 0.20 in the biased degradation sample. This is similar to the 0.18 composition ratio obtained from an unbiased sample (see Table S2 and Fig. S15b†). The composition ratio for I 3d in the biased degraded sample is 1.8. This ratio is 1.6 for the unbiased degraded sample (see Table S2 and Fig. S17a†). (d) Video frame of the illuminated device under V bias showing the release of volatile gases in the negative electrode and trapped gas bubbles under the transparent CYTOP layer. See the ESI† file for a full video of the experiment. | ||
The challenges associated with these chemical instability issues found in MAPbI3 perovskite must be mitigated in perovskite solar cells. Below, four guidelines/recommendations based on the outputs of the above degradation study may aid the design of better devices with increased operational stability:
(1) Careful selection of cations for the A site in the perovskite structure, replacing MA+ with a suitable mixture of Cs+/FA+ (cesium and formamidinium) cations. The irreversible reaction (CH3I/NH3 formation and release route) suffered by MA+ can be addressed in principle by replacing it with a mixture of Cs+/FA+ (cesium and formamidinium cations) in the A site of perovskite without large efficiency losses. Currently, it may be difficult if not impossible to find an atomic or molecular replacement for iodide ion while maintaining excellent light harvesting properties.
(2) Encapsulation of devices is necessary not only to avoid contact with external agents, but also to prevent leakage of volatile decomposition products (I2 for all iodine-based perovskites and CH3I/NH3/CH3NH2/HI for the specific case of MA+-based perovskites). Encapsulation ensures that the perovskite is inside a thermodynamically closed system, allowing only energy exchange and chemical equilibrium concentrations of solid and gas products according to the law of mass action at the perceived temperature.
(3) Selective contacts in solar cells, especially organic molecules or polymer-based HTM, must be as chemically inert as possible to be unaffected by an environment rich in highly acidic molecules (HI), good methylation reagents (CH3I), oxidizing agents (I2), and weak bases (CH3NH2, NH3).
(4) In relation to point no. 2 above, a hybrid halide perovskite material is assumed to undergo cycles of dynamic formation and decomposition processes; this can gradually decrease its crystalline grain size over time. Therefore, efforts to deposit large crystalline perovskite domains may not be beneficial to the long term stability of devices.
Conclusions
In summary, hybrid lead iodide perovskite has been demonstrated as a semiconductor material that exhibits dynamic processes of continuous decomposition and formation under visible light and/or mild temperature stimulus compatible with solar cell operation conditions. I2 gas is released from MAPbI3 even in dark conditions during mild heating at temperatures as low as 40 °C to 80 °C, which correspond to solar cell working temperatures. Fortunately, this photodecomposition reaction is reversible at least for PbI2, because back formation of Pb0 + X2 ⇌ PbX2 is observed. Because MAPbI3 also decomposes to CH3I + NH3, which corresponds to the irreversible degradation pathway, strategies such as replacement of MA+ cations by more stable Cs/FA combinations are suggested. MAPbBr3 shows enhanced stability compared to MAPbI3 because the former decomposes only into CH3NH2 + HBr at ambient temperature, which allows a clean self-healing process. Therefore, to further improve the operational stability of hybrid perovskite solar cells, detailed understanding of how to control of all these photodegradation and thermal degradation processes is required. Four guidelines/recommendations based on the outputs of the above degradation study may aid the design of better devices with increased operational stability. First, encapsulation of the device is necessary not only to avoid contact with ambient air, but also to prevent leakage of volatile released products. Second, careful selection of the organic cations in the A site of the compositional perovskite formula is necessary to avoid irreversible reactions. Third, selective contacts must be as chemically inert as possible toward volatile released products which can provoke undesired side-reactions. Finally, a hybrid halide perovskite material is assumed to undergo a dynamic formation and decomposition process; this can gradually decrease its crystalline grain size with time. Therefore, efforts to deposit large, highly crystalline perovskite may not be beneficial to the long term stability of devices.Methods
Materials
Lead(II) iodide (PbI2, 99.9%) was purchased from Tokyo Chemical Industry Co., Ltd, lead(II) bromide (PbBr2, 99.999%) was purchased from Sigma-Aldrich, and methylammonium iodide (MAI) and methylammonium bromide (MABr) were purchased from Dyesol Limited. All chemicals were used as received without any further purification. Hybrid perovskites in powdered polycrystalline material form were obtained by mimicking the procedure to deposit perovskite thin films on substrates. Briefly, 1 mL of DMF (Wako Pure Chemical Industries) solution (∼1 M) containing the desired stoichiometric precursor quantities to synthesize MAPbI3 or MAPbBr3 was poured on a mortar 10 cm in diameter and maintained at 100 °C inside a fume hood. The precursor solution was slowly spread on the mortar surface with the aid of the pestle. The DMF solvent was evaporated within 1 to 2 minutes, leaving a solid crystalline material on the mortar. The crystal was then carefully collected. The perovskite phase purity (i.e. absence of PbI2) and crystalline parameters were verified by powder XRD measurements (ESI Fig. S1†). Powder XRD data were recorded in glazing incidence XRD (GIXRD) mode (detector scan, omega = 0.5°) using a D8 Bruker Discover (Cu-Kα1 radiation) with 2θ degrees varying from 10° to 55° using 0.5 s acquisition times for every 0.02° 2θ interval. Quantitative analysis of the powder samples was performed by fitting the entire XRD pattern with the MAUD 2.71 software package.23Photodecomposition experiments
An extension of the Experimental section with full details is deposited in the ESI,† including further details on the calibration of light sources used in this work and the MS calibration.
Comment from authors
During the course of the review of this manuscript, an article (DOI: 10.1038/s41563-018-0038-0) appeared describing the enhancement of ion conduction in perovskite by the effect of light. This is a surprising effect that is assumed to be due to the generation of vacancies on perovskite; this effect can be better understood based on our MS measurements during the degradation tests under mild temperature and light conditions.Author contributions
Y. B. Q. conceived the idea and supervised the work. E. J. J. P. and Y. B. Q. designed the experiments. E. J. J. P. carried out all measurements (except XPS), data analysis, and results interpretation and wrote the first version of the manuscript. L. K. O., M. M. and Z. H. performed XPS measurements and assisted with XPS data analysis and interpretation. E. J. J. P. recorded and edited the “bubbling” perovskite video deposited in the ESI† file. All authors assisted with interpretation of the results and contributed to writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by funding from the Energy Materials and Surface Sciences Unit of the Okinawa Institute of Science and Technology Graduate University, the OIST Proof of Concept (POC) Program, the OIST R&D Cluster Research Program, and JSPS KAKENHI Grant Number 15K17925 and JP18K05266. We thank Steven D. Aird, the Technical Editor at Okinawa Institute of Science and Technology Graduate University, for valuable suggestions in revising the manuscript.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- Y. Yang and J. You, Nature, 2017, 544, 155–156 CrossRef CAS PubMed.
- G. Grancini, C. Roldan-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and others, Nature, 2017, 8, 1 Search PubMed.
- S. G. Hashmi, A. Tiihonen, D. Martineau, M. Ozkan, P. Vivo, K. Kaunisto, V. Ulla, S. M. Zakeeruddin and M. Grätzel, J. Mater. Chem. A, 2017, 5, 4797–4802 CAS.
- T. Matsui, J.-Y. Seo, M. Saliba, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2017, 1606258 CrossRef PubMed.
- W. Nie, J.-C. Blancon, A. J. Neukirch, K. Appavoo, H. Tsai, M. Chhowalla, M. A. Alam, M. Y. Sfeir, C. Katan, J. Even, S. Tretiak, J. J. Crochet, G. Gupta and A. D. Mohite, Nat. Commun., 2016, 7, 11574 CrossRef CAS PubMed.
- S. Wang, Y. Jiang, E. J. Juarez-Perez, L. K. Ono and Y. B. Qi, Nat. Energy, 2016, 2, 16195 CrossRef.
- R. G. Wilks and M. Bär, Nat. Energy, 2017, 2, 16204 CrossRef.
- E. J. Juarez-Perez, Z. Hawash, S. R. Raga, L. K. Ono and Y. B. Qi, Energy Environ. Sci., 2016, 9, 3406–3410 CAS.
- R. I. Dawood, A. J. Forty and M. R. Tubbs, Proc. R. Soc. London, Ser. A, 1965, 284, 272–288 CrossRef CAS.
- J. Schoonman, Chem. Phys. Lett., 2015, 619, 193–195 CrossRef CAS.
- K. Okhotnikov, T. Charpentier and S. Cadars, J. Cheminf., 2016, 8, 17 Search PubMed.
- R. K. Misra, S. Aharon, B. Li, D. Mogilyansky, I. Visoly-Fisher, L. Etgar and E. A. Katz, J. Phys. Chem. Lett., 2015, 6, 326–330 CrossRef CAS PubMed.
- R. K. Misra, L. Ciammaruchi, S. Aharon, D. Mogilyansky, L. Etgar, I. Visoly-Fisher and E. A. Katz, ChemSusChem, 2016, 9, 2572–2577 CrossRef CAS PubMed.
- A. F. Akbulatov, S. Y. Luchkin, L. A. Frolova, N. N. Dremova, K. L. Gerasimov, I. S. Zhidkov, D. V. Anokhin, E. Z. Kurmaev, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. Lett., 2017, 1211–1218 CrossRef CAS PubMed.
- A. E. Williams, P. J. Holliman, M. J. Carnie, M. L. Davies, D. A. Worsley and T. M. Watson, J. Mater. Chem. A, 2014, 2, 19338–19346 CAS.
- J. L. Minns, P. Zajdel, D. Chernyshov, W. van Beek and M. A. Green, Nat. Commun., 2017, 8, 15152 CrossRef CAS PubMed.
- J. Xie, Y. Liu, J. Liu, L. Lei, Q. Gao, J. Li and S. Yang, J. Power Sources, 2015, 285, 349–353 CrossRef CAS.
- A. Latini, G. Gigli and A. Ciccioli, Sustainable Energy Fuels, 2017, 1, 1351–1357 CAS.
- J. Chun-Ren Ke, A. S. Walton, D. J. Lewis, A. Tedstone, P. O'Brien, A. G. Thomas and W. R. Flavell, Chem. Commun., 2017, 53, 5231–5234 RSC.
- S. R. Raga, L. K. Ono and Y. B. Qi, J. Mater. Chem. A, 2016, 4, 2494–2500 CAS.
- S. Pang, Y. Zhou, Z. Wang, M. Yang, A. R. Krause, Z. Zhou, K. Zhu, N. P. Padture and G. Cui, J. Am. Chem. Soc., 2016, 138, 750–753 CrossRef CAS PubMed.
- L. Lutterotti, R. Vasin and H.-R. Wenk, Powder Diffr., 2014, 29, 76–84 CrossRef CAS.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D'Haen, L. D'Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca and E. Mosconi, et al. , Adv. Energy Mater., 2015, 5, 1500477 CrossRef.
- B. Philippe, B.-W. Park, R. Lindblad, J. Oscarsson, S. Ahmadi, E. M. J. Johansson and H. Rensmo, Chem. Mater., 2015, 27, 1720–1731 CrossRef CAS.
- D. A. Shirley, Phys. Rev. B: Solid State, 1972, 5, 4709 CrossRef.
- J. Scofield, J. Electron Spectrosc. Relat. Phenom., 1976, 8, 129–137 CrossRef CAS.
- S. Olthof and K. Meerholz, Sci. Rep., 2017, 7, 40267 CrossRef CAS PubMed.
- S. Nakayashiki, H. Daisuke, Y. Ogomi and S. Hayase, J. Photonics Energy, 2015, 5, 057410 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ta03501f |
| This journal is © The Royal Society of Chemistry 2018 |
