Ultrathin two-dimensional cobalt–organic framework nanosheets for high-performance electrocatalytic oxygen evolution†
Abstract
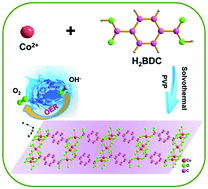
Ultrathin two-dimensional cobalt–organic framework (Co–MOF) nanosheets [Co2(OH)2BDC, BDC = 1,4-benzenedicarboxylate] with good catalytic activity for the oxygen evolution reaction were synthesized by a simple surfactant-assisted hydrothermal method. The resulting material shows a low overpotential of 263 mV at 10 mA cm−2 and a Tafel slope of 74 mV dec−1 in 1.0 M KOH. The morphology and properties of the material before and after electrocatalysis did not change significantly over a long period of time and it maintained electrochemical stability for 12 000 s. The unsaturated CoII active center on the surface of the ultrathin two-dimensional cobalt–organic framework nanosheets enhances their oxygen evolution reaction performance. These results are significantly superior to most of the previously reported transition metal–organic framework or metal oxide electrocatalysts. We believe that the ultrathin two-dimensional metal–organic framework nanosheets synthesized by this simple process will be widely used in future energy conversion devices.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...