Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-induced degradation of fullerenes in organic solar cells: a case study on TQ1:PC71BM†
Yuming
Wang
ab,
Mohammad Javad
Jafari
 b,
Nana
Wang
a,
Deping
Qian
b,
Fengling
Zhang
b,
Thomas
Ederth
b,
Nana
Wang
a,
Deping
Qian
b,
Fengling
Zhang
b,
Thomas
Ederth
 b,
Ellen
Moons
b,
Ellen
Moons
 c,
Jianpu
Wang
*a,
Olle
Inganäs
b,
Wei
Huang
a and
Feng
Gao
c,
Jianpu
Wang
*a,
Olle
Inganäs
b,
Wei
Huang
a and
Feng
Gao
 *b
*b
aKey Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials (IAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211800, China. E-mail: iamjpwang@njtech.edu.cn
bDepartment of Physics, Chemistry and Biology (IFM), Linköping University, Linköping, SE-58183, Sweden. E-mail: feng.gao@liu.se
cDepartment of Engineering and Physics, Karlstad University, 65188 Karlstad, Sweden
First published on 18th May 2018
Abstract
The stability of organic solar cells (OSCs) is critical for practical applications of this emerging technology. Unfortunately, in spite of intensive investigations, the degradation mechanisms in OSCs have not been clearly understood yet. In this report, we employ a range of spectroscopic and transport measurements, coupled with drift-diffusion modelling, to investigate the light-induced degradation mechanisms of fullerene-based OSCs. We find that trap states formed in the fullerene phase under illumination play a critical role in the degradation of the open-circuit voltage (VOC) in OSCs. Our results indicate that the degradation is intrinsic to the fullerenes in OSCs and that alternative acceptor materials are desired for the development of stable OSCs.
Introduction
Organic solar cells (OSCs) have attracted intensive attention during the past few decades. Very recently, the power conversion efficiency (PCE) of OSCs has reached a high value of above 14%,1,2 making this technology commercially relevant. However, the stability of OSCs remains an issue for the practical applications of OSCs.The degradation of OSCs can be caused by different factors, including moisture, oxygen,3–6 heat,7–10 and light.11–14 Among these different factors, to enhance the stability of OSCs under light is critically important for OSCs, as OSCs are inevitably illuminated during the operation. Light exposure can affect the photovoltaic parameters of OSCs in different ways. For instance, in some material systems mainly the short-circuit current (JSC) and fill factor (FF) were affected by light exposure,15 while in some other systems mainly the open-circuit voltage (VOC) was affected.16,17
Different mechanisms were found in light-induced degradation processes. For instance, new sub-band gap trap states were identified in polymer donor materials after exposure to light, and the light-induced new traps can broaden the density of states, decreasing the VOC.16,17 In addition, the ultraviolet (UV) region of the spectrum can trigger the photochemical reaction between the polymer donor materials and the fullerene, resulting in irreversible degradation of the active layer.18 For fullerene based OSCs, the fullerene can be dimerized when exposed to light in an inert atmosphere.19–21 The dimerization of the fullerene could result in a significantly reduced carrier mobility or morphological changes of the active layer, leading to decreased JSC and FF.19–21
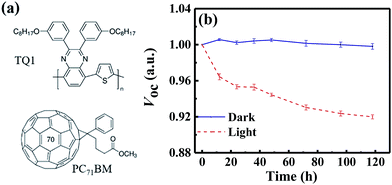
In this paper, we investigate the light-induced degradation mechanisms in a classical fullerene-based OSC blend solar cell, i.e. poly[2,3-bis-(3-octyloxyphenyl)quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl] (TQ1):[6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) (as shown in Fig. 1a). We find that the VOC significantly decreases during the light-induced degradation process in both conventional and inverted device structures. We attribute the decrease of VOC to non-radiative recombination, as identified by highly sensitive Fourier Transform Photocurrent Spectroscopy (FTPS), electroluminescence (EL) and external quantum efficiency of EL (EQEEL) measurements. We combine temperature-dependent charge transport measurements and Extended Gaussian Disorder Modelling (EGDM) and find that the Gaussian disorder width of the electron states increases during the photo-degradation, while that of the holes remains the same. Fourier transform infrared spectroscopy (FTIR) measurements reveal that the fullerenes are oxidized during the degradation process, resulting in electron traps. These different measurements present a consistent picture that the photo-induced oxidation of fullerenes plays a critical role in the degradation of this system, resulting in electron traps and additional non-radiative recombination, which decreases the VOC.
Results and discussion
We employ a conventional device structure, where the active layer is sandwiched between ITO/PEDOT:PSS and LiF/Al. As shown in Fig. 1b, the VOC of the device stored under illumination (without load) shows a significant decrease, while the one of the device stored in the dark stays constant. The VOC degradation can be due to either the interlayers or the active layer.22,23 In order to distinguish between the contribution from the interlayers and that from the active layer, we perform a control experiment on an inverted device, where the active layer is sandwiched between two different interlayers (ITO/ZnO and MoOx/Al). Similar to that observed in the conventional structure, the VOC in the inverted structure has also decreased upon light exposure for 72 h (Fig. S1d†). In terms of the JSC and FF, their degradation behaviour is obviously affected by the device structures (and hence the interlayers) (Fig. S1 and S2†), meaning that the effects of the interlayers and active layer cannot be easily separated in this case.23 The change in VOC, on the other hand, is the parameter that contains unique information about the light-induced degradation of the active layer, which is the focus of this work.In order to understand the mechanisms of light-induced VOC degradation, we quantify the VOC. In the bulk-heterojunction (BHJ) structured OSCs, the VOC can be described using the following equations:24
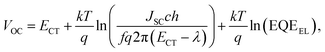 | (1) |
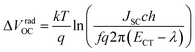 | (2) |
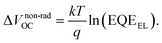 | (3) |
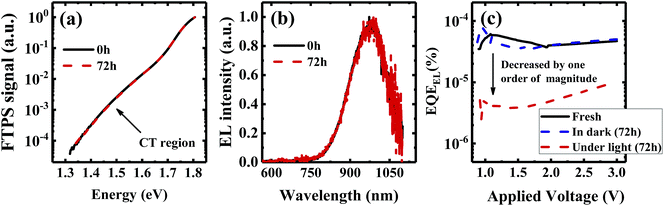
We investigate all three items in eqn (1) to determine the origin of the VOC degradation under illumination. The absorption of the CT states is very weak because of the weak overlap between the highest occupied molecular orbital (HOMO) of the donor and the lowest unoccupied molecular orbital (LUMO) of the acceptor. Herein we employ a sensitive photocurrent spectroscopy method FTPS to measure the EQEPV in the CT state region. As shown in Fig. 2a, there is a region with a swollen FTPS signal at low energy, corresponding to the photocurrent due to CT state absorption.24–26 The shape of the EQEPV curve in the CT state region shows no change after 72 h illumination, indicating that there is no change in either the ECT or ΔVradOC. In order to further confirm the information we obtained from the FTPS measurements, we measure the EL spectrum of the devices as well. As shown in Fig. 2b, the EL spectra of CT states (Fig. S3†) show no changes after 72 h illumination, consistent with the FTPS results.
The EQEEL results are depicted in Fig. 2c. Compared with the device stored in the dark, the EQEEL of the device aged under light decreases by one order of magnitude, corresponding to an increase of a non-radiative VOC loss by ∼60 meV according to eqn (3). This result matches well with the overall VOC decrease after 72 h illumination (Table S2†). We also perform EQEEL measurements on the inverted devices and obtain similar results (Fig. S4 and Table S4†), further confirming that the degradation originates from the active layer, rather than the interlayers. From this quantification of the light-induced VOC losses, we can conclude that the light-induced VOC loss stems from the increased non-radiative recombination losses in the active layer.
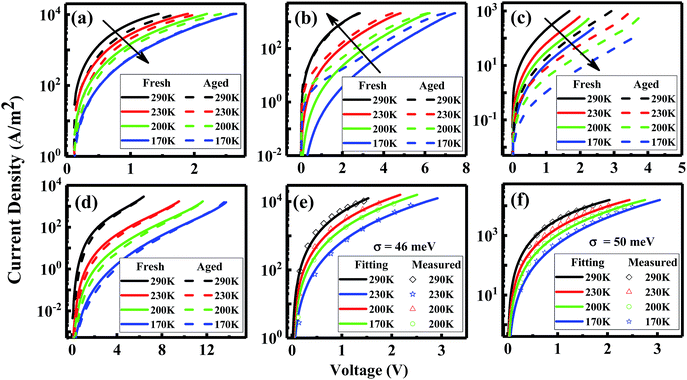
A possible reason for the increased non-radiative VOC losses is the formation of additional trap states induced by illumination, leading to trap-assisted non-radiative recombination. A direct approach to investigate the traps is to perform temperature-dependent mobility measurements.27–29 Therefore, we measure the J–V curves of the hole-only (HO) and electron-only (EO) devices based on blends at different temperatures. As shown in Fig. 3a, the electron transport of the blend devices decreases after 72 h illumination, meaning that degradation generates an increasing amount of electron traps. We can further quantify the disorder values in the devices before and after degradation by using EGDM. The details of this method are shown in the ESI of ref. 12. We find that the disorder value, which is denoted as σ, increases from 46 meV to 50 meV upon degradation (Fig. 3e and f). Surprisingly, the hole transport shows an enhancement after 72 h of illumination (Fig. 3d), the reason of which will be discussed later on.
In order to further confirm the light-induced electron traps and understand the unusual enhancement of the hole transport, we measure the single-carrier devices of the pristine materials (Fig. 3e and f). Indeed, consistent with the EO device of the blend, the EO device of PC71BM shows decreased transport, confirming light-induced electron traps. In contrast to the enhancement of the hole transport in the blend, the hole transport in the pristine TQ1 device shows no difference after 72 h illumination.
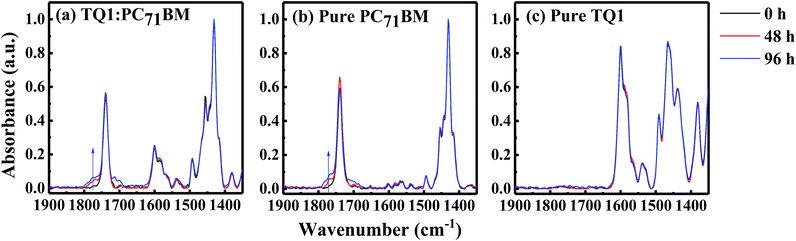
We further investigate the degradation processes at the molecular level with polarization-modulation infrared reflection-absorption spectroscopy (PM-IRRAS). As shown in Fig. 4a, a new band appears at ∼1776 cm−1, near the PC71BM methyl ester carbonyl peak at 1732 cm−1, during the illumination in the TQ1:PC71BM blend film. A similar band at ∼1776 cm−1 also appears in the pure PC71BM film (Fig. 4b), but not in the pure TQ1 film (Fig. 4c), indicating that the new vibrational peak is due to the oxidation of PC71BM. The fact that pure TQ1 shows no light-induced vibrational band is consistent with the charge transport result in Fig. 3f, where no change of the hole transport is observed in the HO device of TQ1. We propose that the formation of the band at ∼1776 cm−1 is due to the oxidation of carbon elements resulting in the formation of carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in PC71BM. The origin of oxygen could be from the oxygen in the glovebox (5–10 ppm oxygen level) and/or small amounts of oxygen remaining in the materials before they are transferred into the glovebox (the solid materials are stored outside in a fridge). It is now becoming clear why we observe abnormal enhancement of the hole transport in the blend film, as shown in Fig. 3d. We suppose that in the blend film, the oxygen in TQ1 tends to react with the PC71BM due to the scavenger properties of fullerene,30,31 helping to de-dope the polymer and thus improving the hole transport.32,33 This process was previously demonstrated to be facilitated by illumination.20
O) in PC71BM. The origin of oxygen could be from the oxygen in the glovebox (5–10 ppm oxygen level) and/or small amounts of oxygen remaining in the materials before they are transferred into the glovebox (the solid materials are stored outside in a fridge). It is now becoming clear why we observe abnormal enhancement of the hole transport in the blend film, as shown in Fig. 3d. We suppose that in the blend film, the oxygen in TQ1 tends to react with the PC71BM due to the scavenger properties of fullerene,30,31 helping to de-dope the polymer and thus improving the hole transport.32,33 This process was previously demonstrated to be facilitated by illumination.20
In order to further demonstrate the oxidization of the materials, we intentionally expose the films in air under light exposure, and then perform the FTIR measurements. As shown in Fig. S5a–c,† we observe that all the films exposed to air show a new vibration signal, corresponding to the carbonyl group. This vibration signal now emerges much more quickly, compared with the experiments performed in the glovebox, as a result of the large amounts of oxygen available in air. We notice that TQ1 in this case also shows an oxidation signal. The lack of the oxidation signal of the TQ1 film in the glove-box might be due to the fact that compared with PC71BM, the TQ1 is less reactive with oxygen.34 Based on these results, we conclude that the newly appearing carbonyl vibrational band in Fig. 4a comes from the oxidation of PC71BM during the illumination.
Conclusion
We investigate the light-induced degradation of the devices based on TQ1:PC71BM and find that the oxidation of PC71BM (as revealed by FTIR results) plays a key role in the degradation of the device parameters, especially the VOC. The oxidation of PC71BM results in electron traps, leading to enhanced non-radiative recombination loss of the VOC, which we have quantified by measuring the electroluminescence quantum efficiency of the devices. We also quantified the energetic disorder induced by the electron trap states due to the oxidation of PC71BM. Our work indicates that fullerene acceptors are detrimental to the light stability of devices, and alternative acceptor materials are required to develop stable OSCs. Our conclusion is in line with the recent trend towards the development of OSCs based on non-fullerene acceptors.35–38Experimental section
Solution preparation
TQ1 is provided by Ergang Wang's group at Chalmers University of Technology and PC71BM is purchased from Solenne b.v. The polymer and fullerene mixtures are dissolved at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 w/w ratio in 1,2-dichlorobenzene (o-DCB). The total concentration of the TQ1:PC71BM blend in o-DCB is 25 mg ml−1. The TQ1 is dissolved in o-DCB with a concentration of 15 mg ml−1 and the PC71BM is dissolved in chloroform (CF) with a concentration of 15 mg ml−1. All the solutions are stirred overnight at 60 °C before use. PEDOT:PSS Al 4083 is purchased from Heraeus and zinc oxide (ZnO) nanoparticle solution is purchased from Suprapur.
2.5 w/w ratio in 1,2-dichlorobenzene (o-DCB). The total concentration of the TQ1:PC71BM blend in o-DCB is 25 mg ml−1. The TQ1 is dissolved in o-DCB with a concentration of 15 mg ml−1 and the PC71BM is dissolved in chloroform (CF) with a concentration of 15 mg ml−1. All the solutions are stirred overnight at 60 °C before use. PEDOT:PSS Al 4083 is purchased from Heraeus and zinc oxide (ZnO) nanoparticle solution is purchased from Suprapur.
Device preparation
All the solar cells are fabricated on an indium tin oxide (ITO) coated glass substrate and a 90 nm aluminium layer is used as the top contact. The ITO glass is cleaned in the TL1 process (boiled for 20 min in the mixed solution of hydrogen peroxide (H2O2) and ammonia (NH3)). A structure of ITO/PEDOT:PSS/active layer/LiF/Al is employed for the conventional structure device and a structure of ITO/ZnO/active layer/MoOx/Al is employed for the inverted structure device. A structure of ITO/PEDOT:PSS/active layer/MoOx/Al is employed for the HO devices and a structure of ITO/ZnO/active layer/LiF/Al is employed for the EO devices. The thickness of the TQ1:PC71BM blend active layer is ∼90 nm. The thickness of the pure TQ1 and PC71BM active layers is ∼80 nm and ∼110 nm, respectively.Stability measurements
All the devices studied in our experiments are encapsulated with a coverslip which is UV-glued on the surface of the device. The devices in the glovebox (filled with nitrogen, ≤1% ppm moisture and 5–10% ppm oxygen) are divided into two groups, with one group stored in the dark and the other group stored under the illumination of a white LED. The LED spectrum is shown in Fig. S6† and the intensity is calibrated to be ∼50 mW cm−2 with a standard Si solar cell. J–V measurements of the devices are performed outside the glovebox. An AM1.5G sun simulator purchased from Oriel is used for the J–V measurements.FTPS measurements
FTPS measurements are performed on a Vertex 70 from Bruker optics, equipped with a QTH lamp, quartz beam-splitter and external detector option. A low noise current amplifier (SR570) is used to amplify the photocurrent produced by the illumination of FTIR on the devices. The output voltage of the amplifier is fed back to the external port of the FTIR, in order to be able to employ the FTIR software to collect the photocurrent spectrum.EL measurements
A Keithley 2400 is used for supplying voltage/current to the devices. An Andor spectrometer (Shamrock sr-303i-B, coupled to a Newton EMCCD detector) is used to record the EL spectra.EQEEL measurements
The EQEEL measurements are performed on a home built setup comprising a Hamamatsu silicon photodiode 1010B, a Keithley 2400 for supplying voltages and recording the injected current, and a Keithley 485 for measuring the emitted light intensity.FTIR measurements
Polarization-modulation infrared reflection-absorption spectroscopy (PM-IRRAS) measurements are carried out by means of a Bruker PMA 50 with a LN2-cooled mercury cadmium telluride (MCT) detector at 86° grazing angle, using a Bruker VERTEX70 as a light source. A photo-elastic modulator (Hinds: ZnSe 50 kHz) is used to modulate the polarization of light. Spectra containing PC71BM are normalized to the fullerene peak near 1430 cm−1, and the TQ1 spectra normalized to the ring vibration at 1465 cm−1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Jonas Bergqvist for the supply of the LED. The authors are grateful to the National Basic Research Program of China (Grant No. 2015CB932200), Natural Science Foundation of Jiangsu (Grant No. BK20140952, BK20150043), National Natural Science Foundation of China (Grant No. 11474164, 61405091, 61634001), Joint Research Program between China and European Union (Grant No. 2016YFE0112000), Synergetic Innovation Center for Organic Electronics and Information Displays, National High Technology Research and Development Program of China (Grant No. 2011AA050520), Jiangsu Specially-Appointed Professor program, Chinese Scholarship Council (CSC), Swedish Research Council (VR), European Commission SOLAR-ERA-NET, Swedish Energy Agency (Energimyndigheten), Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant No. SFO-Mat-LiU #2009-00971), and Knut and Alice Wallenberg foundation (KAW) for financial support.Notes and references
- Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef.
- S. Zhang, Y. Qin, J. Zhu and J. Hou, Adv. Mater., 2018 DOI:10.1002/adma.201800868.
- M. P. Nikiforov, J. Strzalka and S. B. Darling, Sol. Energy Mater. Sol. Cells, 2013, 110, 36 CrossRef.
- Q. Bao, X. Liu, S. Braun and M. Fahlman, Adv. Energy Mater., 2014, 4, 1301272 CrossRef.
- Y. W. Soon, H. Cho, J. Low, H. Bronstein, I. McCulloch and J. R. Durrant, Chem. Commun., 2013, 49, 1291 RSC.
- S. Karuthedath, T. Sauermann, H.-J. Egelhaaf, R. Wannemacher, C. J. Brabec and L. Lüer, J. Mater. Chem. A, 2015, 3, 3399 Search PubMed.
- E. Vitoratos, S. Sakkopoulos, E. Dalas, N. Paliatsas, D. Karageorgopoulos, F. Petraki, S. Kennou and S. Choulis, Org. Electron., 2009, 10, 61 CrossRef.
- T. Sachs-Quintana, T. Heumüller, W. R. Mateker, D. E. Orozco, R. Cheacharoen, S. Sweetnam, C. J. Brabec and M. D. McGehee, Adv. Funct. Mater., 2014, 24, 3978 CrossRef.
- Z. Yi, W. Ni, Q. Zhang, M. Li, B. Kan, X. Wan and Y. Chen, J. Mater. Chem. C, 2014, 2, 7247 RSC.
- Z. Li, H. C. Wong, Z. Huang, H. Zhong, C. H. Tan, W. C. Tsoi, J. S. Kim, J. R. Durrant and J. T. Cabral, Nat. Commun., 2013, 4, 2227 CrossRef PubMed.
- E. A. Lukina, M. N. Uvarov and L. V. Kulik, J. Phys. Chem. C, 2014, 118, 18307 Search PubMed.
- M. O. Reese, A. M. Nardes, B. L. Rupert, R. E. Larsen, D. C. Olson, M. T. Lloyd, S. E. Shaheen, D. S. Ginley, G. Rumbles and N. Kopidakis, Adv. Funct. Mater., 2010, 20, 3476 CrossRef.
- T. Tromholt, M. V. Madsen, J. E. Carlé, M. Helgesen and F. C. Krebs, J. Mater. Chem., 2012, 22, 7592 RSC.
- H. K. H. Lee, A. M. Telford, J. A. Röhr, M. F. Wyatt, B. Rice, J. Wu, A. de Castro Maciel, S. M. Tuladhar, E. Speller, J. McGettrick, J. R. Searle, S. Pont, T. Watson, T. Kirchartz, J. R. Durrant, W. C. Tsoi, J. Nelson and Z. Li, Energy Environ. Sci., 2018, 11, 417 Search PubMed.
- C. J. Schaffer, C. M. Palumbiny, M. A. Niedermeier, C. Jendrzejewski, G. Santoro, S. V. Roth and P. Muller-Buschbaum, Adv. Mater., 2013, 25, 6760 CrossRef PubMed.
- T. Heumueller, T. M. Burke, W. R. Mateker, I. T. Sachs-Quintana, K. Vandewal, C. J. Brabec and M. D. McGehee, Adv. Energy Mater., 2015, 5, 1500111 CrossRef.
- T. Heumueller, W. R. Mateker, I. T. Sachs-Quintana, K. Vandewal, J. A. Bartelt, T. M. Burke, T. Ameri, C. J. Brabec and M. D. McGehee, Energy Environ. Sci., 2014, 7, 2974 Search PubMed.
- D. Bartesaghi, G. Ye, R. C. Chiechi and L. J. A. Koster, Adv. Energy Mater., 2016, 6, 1502338 CrossRef.
- N. Wang, X. Tong, Q. Burlingame, J. Yu and S. R. Forrest, Sol. Energy Mater. Sol. Cells, 2014, 125, 170 CrossRef.
- T. Heumueller, W. R. Mateker, A. Distler, U. F. Fritze, R. Cheacharoen, W. H. Nguyen, M. Biele, M. Salvador, M. von Delius, H.-J. Egelhaaf, M. D. McGehee and C. J. Brabec, Energy Environ. Sci., 2016, 9, 247 Search PubMed.
- F. Piersimoni, G. Degutis, S. Bertho, K. Vandewal, D. Spoltore, T. Vangerven, J. Drijkoningen, M. K. Van Bael, A. Hardy, J. D'Haen, W. Maes, D. Vanderzande, M. Nesladek and J. Manca, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1209 CrossRef.
- Y.-M. Chang and C.-Y. Leu, J. Mater. Chem. A, 2013, 1, 6446 Search PubMed.
- C. H. Peters, I. T. Sachs-Quintana, W. R. Mateker, T. Heumueller, J. Rivnay, R. Noriega, Z. M. Beiley, E. T. Hoke, A. Salleo and M. D. McGehee, Adv. Mater., 2012, 24, 663 CrossRef PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 125204 CrossRef.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganas and J. V. Manca, Nat. Mater., 2009, 8, 904 CrossRef PubMed.
- Z. Tang, L. M. Andersson, Z. George, K. Vandewal, K. Tvingstedt, P. Heriksson, R. Kroon, M. R. Andersson and O. Inganas, Adv. Mater., 2012, 24, 554 CrossRef PubMed.
- J. C. Blakesley, H. S. Clubb and N. C. Greenham, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 045210 CrossRef.
- F. Gao, S. Himmelberger, M. Andersson, D. Hanifi, Y. Xia, S. Zhang, J. Wang, J. Hou, A. Salleo and O. Inganas, Adv. Mater., 2015, 27, 3868 CrossRef PubMed.
- W. F. Pasveer, J. Cottaar, C. Tanase, R. Coehoorn, P. A. Bobbert, P. W. Blom, D. M. de Leeuw and M. A. Michels, Phys. Rev. Lett., 2005, 94, 206601 CrossRef PubMed.
- J. J. Yin, F. Lao, P. P. Fu, W. G. Wamer, Y. Zhao, P. C. Wang, Y. Qiu, B. Sun, G. Xing, J. Dong, X. J. Liang and C. Chen, Biomaterials, 2009, 30, 611 CrossRef PubMed.
- E. B. Zeynalov, N. S. Allen and N. I. Salmanova, Polym. Degrad. Stab., 2009, 94, 1183 CrossRef.
- J. Schafferhans, A. Baumann, A. Wagenpfahl, C. Deibel and V. Dyakonov, Org. Electron., 2010, 11, 1693 CrossRef.
- C.-K. Lu and H.-F. Meng, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 235206 CrossRef.
- R. Hansson, C. Lindqvist, L. K. Ericsson, A. Opitz, E. Wang and E. Moons, Phys. Chem. Chem. Phys., 2016, 18, 11132 RSC.
- Y. Cai, L. Huo and Y. Sun, Adv. Mater., 2017, 29, 1605437 CrossRef PubMed.
- H. Fu, Z. Wang and Y. Sun, Sol. RRL, 2018, 2, 1700158 CrossRef.
- P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131 CrossRef.
- J. Hou, O. Inganas, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ta03112f |
| This journal is © The Royal Society of Chemistry 2018 |