Superhydrophilic amorphous Co–B–P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction†
Abstract
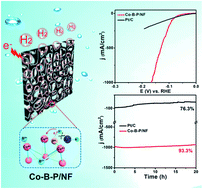
Developing durable, nonprecious hydrogen evolution reaction (HER) electrocatalysts with Pt-like performance is desirable but remains challenging. Here we report a one-step electroless synthesis of an amorphous Co2.90B0.73P0.27 ternary alloy and its application as a new highly active and robust HER catalyst. The synthesized Co2.90B0.73P0.27 nanosheets supported on Ni foam exhibit remarkable catalytic performance in alkaline media, delivering a low onset overpotential of 12 mV and a low Tafel slope of 42.1 mV dec−1. Moreover, this self-supported monolithic electrode sustains a high current density of 1000 mA cm−2 and extended polarization over 20 h, outperforming the Pt/C benchmark. The superior performance is attributed to the superhydrophilic properties of the nanosheet microstructure and the synergistic effect of elements P and B, which favors dissociation of H2O, weakens surface H absorption, and suppresses Co oxidation. This work provides a new avenue for the design and optimization of transition metal boron phosphides for HER electrocatalysis.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...