Formation of Ti–Fe mixed sulfide nanoboxes for enhanced electrocatalytic oxygen evolution†
Abstract
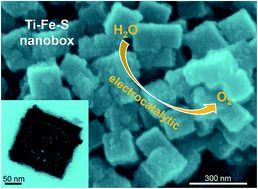
Exploring effective electrocatalysts is always required to boost the efficiency of water splitting. In this work, we report a self-template strategy to synthesize Ti–Fe mixed sulfide nanoboxes for the electrocatalytic oxygen evolution reaction (OER). The mixed sulfide nanoboxes are derived from corresponding Prussian blue analog nanoboxes, which are first obtained by hollowing the nanocube precursor with a solvothermal-engaged etching process. The resulting Ti–Fe mixed sulfide nanoboxes perform as a promising OER electrocatalyst with enhanced activity, affording a current density of 10 mA cm−2 and a high mass activity of 100 A g−1 at a small overpotential of 350 mV, a Tafel slope as small as 55 mV dec−1, and excellent stability in alkaline medium.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...