Intercalating cation specific self-repairing of vermiculite nanofluidic membrane†
Abstract
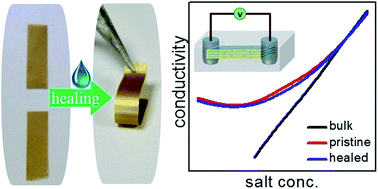
The balance between expanding and contracting forces of lamellar clay minerals was exploited for preparing self-repairable nanofluidic membranes. Application of a tiny drop of water (20 μL) not only healed physical damages of lamellar vermiculite membrane, such as punching a hole, scratching with a sharp object or breaking into multiple pieces, but also completely recovered its nanofluidic ionic conductivity. The extraordinary thermal stability of the clay layer was exploited to perform the healing experiment at elevated temperature, reducing the healing time to just 30 seconds at 140 °C. Experiments with different charge balancing cations and in situ monitoring of the healing process through X-ray diffraction studies suggest that cations with hydration energy higher than the energy of the clay-cation attraction, such as that of Li+, initiate water-assisted swelling of the layers, which leads to the re-assembling of the flakes in the damaged sites. The dependence of vermiculite-swelling on the nature of interlayer cations is also exploited for preparing highly responsive bilayer membranes by assembling clay flakes with different charge balancing cations. The water-assisted swelling of Li-vermiculite is also utilized to fuse them with lamellar membranes of other 2D materials such as graphene oxide and vanadium pentoxide. Even though vermiculite sheets are electrically insulators, air dried vermiculite membranes exhibit an interesting self-healable humidity dependent conductivity.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...