Understanding the critical chemistry to inhibit lithium consumption in lean lithium metal composite anodes†
Abstract
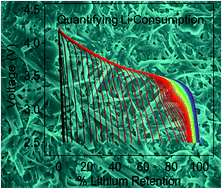
Lithium metal has been deemed by many as the “Holy Grail” of anode materials due to its high theoretical capacity (∼3860 mA h g−1), redox potential of −3.040 V vs. SHE, and light weight. The goal of this work is to investigate the relationship between a lean lithium metal anode and its consumption as a function of host materials, electrochemical protocol and electrolyte composition. With the use of carbon nanofibers, lithium metal has been electrodeposited onto the host matrix and used in a battery with a LiNi0.6Mn0.2Co0.2O2 cathode. The mass-loading of lithium can be easily controlled and utilized to investigate the practicality of an anode limited battery (i.e., limited lithium with an effective thickness <15 μm) in a high surface area matrix. We quantify the consumption rate of active lithium using different electrolyte additives and current rates in full cells and observe that the lithium consumption behavior in an anode-limited cell is different from that in an anode-excess cell. Our results highlight the necessity of applying truly lithium-limited cells when evaluating the electrochemical properties of lithium anodes and electrolyte additives. By extending this method to a standard graphite host, full cells can retain 75% of their initial capacities after 1000 cycles. The present study demonstrates the importance of graphitic carbon in increasing the lifespan of limited lithium (<15 μm) for practical lithium metal batteries.


 Please wait while we load your content...
Please wait while we load your content...