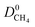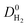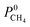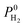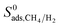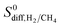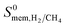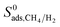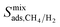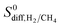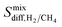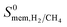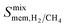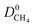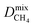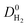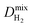Open Access Article
Open Access ArticleComputer simulations of 4240 MOF membranes for H2/CH4 separations: insights into structure–performance relations†
Cigdem Altintas‡
a,
Gokay Avci‡a,
Hilal Daglar‡a,
Ezgi Gulcay-Ozcan‡b,
Ilknur Erucar *c and
Seda Keskin
*c and
Seda Keskin *a
*a
aDepartment of Chemical and Biological Engineering, Koc University, Rumelifeneri Yolu, Sariyer, Istanbul, 34450, Turkey. E-mail: skeskin@ku.edu.tr; Tel: +90 212 338 1362
bDepartment of Mechanical Engineering, Faculty of Engineering, Ozyegin University, Cekmekoy, Istanbul, 34794, Turkey
cDepartment of Natural and Mathematical Sciences, Faculty of Engineering, Ozyegin University, Cekmekoy, Istanbul, 34794, Turkey. E-mail: ilknur.erucar@ozyegin.edu.tr; Tel: +90 216 564 9297
First published on 15th March 2018
Abstract
Design of new membranes having high H2/CH4 selectivity and high H2 permeability is strongly desired to reduce the energy demand for H2 production. Metal organic frameworks (MOFs) offer a great promise for membrane-based gas separations due to their tunable physical and chemical properties. We performed a high-throughput computational screening study to examine membrane-based H2/CH4 separation potentials of 4240 MOFs. Grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulations were used to compute adsorption and diffusion of H2 and CH4 in MOFs. Simulation results were then used to predict adsorption selectivity, diffusion selectivity, gas permeability and membrane selectivity of MOFs. A large number of MOF membranes was found to outperform traditional polymer and zeolite membranes by exceeding the Robeson's upper bound for selective separation of H2 from CH4. Structure–performance analysis was carried out to understand the relations between MOF membranes' selectivities and their pore sizes, surface areas, porosities, densities, lattice systems, and metal types. Results showed that MOFs with pore limiting diameters between 3.8 and 6 Å, the largest cavity diameters between 6 and 12 Å, surface areas less than 1000 m2 g−1, porosities between 0.5 and 0.75, and densities between 1 and 1.5 g cm−3 are the most promising membranes leading to H2 selectivities >10 and H2 permeabilities >104 Barrer. Our results suggest that monoclinic MOFs having copper metals are the best membrane candidates for H2/CH4 separations. This study represents the first high-throughput computational screening of the most recent MOF database for membrane-based H2/CH4 separation and microscopic insight provided from molecular simulations will be highly useful for the future design of new MOFs having extraordinarily high H2 selectivities.
1. Introduction
Metal organic frameworks (MOFs) are composed of metal complexes that are linked by organic ligands to create highly porous frameworks.1,2 They have been recently considered as strong alternatives to traditional porous materials such as zeolites and activated carbons due to their attractive physical and chemical properties. MOFs offer very large surface areas (500–6500 m2 g−1), high pore volumes (1–4 cm3 g−1), wide range of pore sizes (1–98 Å), reasonable chemical and mechanical stabilities. The greatest advantage of MOFs over conventional porous materials is the ability to change the metal and organic linker combination during synthesis in order to create a large diversity of materials with different geometry, pore size and chemical functionality.3,4 MOFs have been widely examined for a variety of physical, chemical and biological applications including gas storage,5 gas separation,6,7 drug storage and drug delivery,8 catalysis,9 optical and luminescent applications.10 Among these, MOFs have received significant interest for adsorption-based and membrane-based gas separations due to their permanent porosities and tunable pore sizes.Continuous operation of membranes generates significant advantages compared to the more complex modes of operation needed for adsorption-based gas separation methods. Although a large number of experimental studies investigated adsorption-based gas separations with MOFs,11 the number of studies on membrane-based gas separations using MOFs is limited due to the experimental challenges in making defect-free membranes from new crystalline materials.12 We reviewed the literature on MOF membranes and showed that only 29 different types of MOF membranes were experimentally fabricated and tested for gas separations.13 These MOF membranes were reported to have very good gas separation performances, higher selectivities and higher permeabilities, compared to the traditional polymer and zeolite membranes.14 The number of synthesized MOF membranes has been increasing but most of the membranes were fabricated using the same materials, generally prototype MOFs, such as MOF-5,15–19 CuBTC,20–22 ZIF-8.23–25 Considering the fact that the number of available MOFs has already reached to several thousands,26 it is highly possible that there are many other materials with better gas separation performances but these MOFs have not been fabricated and tested as membranes yet. The large materials space creates an opportunity for the discovery of highly useful membrane candidates for target gas separations. On the other hand, it is not possible to fabricate and test thousands of MOF membranes using purely experimental manners at the lab scale.
Computational methods, specifically molecular simulations, play an important role in screening large number of MOFs for a target application in a time effective manner. Potential of molecular simulations in choosing the best MOFs for a given gas separation has been shown in several studies.27 These simulations also provide molecular-level insights that can guide the experiments for design of new materials with better separation performances. Although molecular simulations have been widely used to examine CO2/CH4 and CO2/N2 separation performances of MOFs,27–30 H2/CH4 separation is rarely investigated as we will discuss below. H2/CH4 separation is industrially important in the process of purification of synthesis gas obtained from steam reforming of natural gas. Hydrogen is an important chemical in many applications and its production depends on the decomposition of CH4. Therefore, separation of H2 from CH4 with high selectivity and high permeability is strongly desired. Several different types of membrane materials such as zeolites31 and polymers32 have been studied for membrane-based separation of H2/CH4 mixtures in the past. However, these membranes could not meet the requirements of high H2 selectivity and high H2 permeability.33
Due to the tunability of pore sizes and shapes by judicious selection of metal clusters and organic linkers, MOFs offer great promise for efficient H2/CH4 separations. A small number of MOFs, maximum of 20, was studied using molecular simulations for membrane-based H2/CH4 separations in the literature.27,34–38 Haldoupis et al.39 performed the first large-scale molecular simulation study and used a geometric approach to calculate the ideal H2/CH4 selectivity of 143 MOF membranes at infinite dilution condition. They showed that many MOFs have high H2 permeabilities (>105 Barrer) relative to the well-known polymers in addition to high H2/CH4 selectivities in the range of 1–100. Our research group performed molecular simulations for equimolar H2/CH4 mixtures to examine the membrane-based separation performances of 172 MOFs under industrial operating conditions, 10 bar and 298 K.40 Adsorption data obtained from grand canonical Monte Carlo (GCMC) and diffusion data obtained from molecular dynamics (MD) simulations were used to predict gas permeabilities and selectivities of MOF membranes. This was the largest number of MOFs for which MD simulations were performed for H2/CH4 mixtures in the literature. Results showed that only a small number of MOF membranes is H2 selective and diffusion selectivity for H2 dominates the adsorption selectivity for CH4 in these MOFs. As can be seen from this literature review, there is no high-throughput computational study that examines the membrane-based H2/CH4 separation potential of all existing MOFs in the literature.
The most complete collection of MOFs maintained by the Cambridge Structural Database (CSD) was recently reported.41 We recently screened this database for adsorption-based separation of CH4/H2 mixtures and reported several adsorbent evaluation metrics of MOFs such as adsorption selectivity, working capacity, adsorbent performance score, sorbent selection parameter, and regenerability.42 However, this MOF database has not been screened for any membrane-based gas separation application to date to the best of our knowledge. This type of study is strongly needed not only to see if MOF membranes can replace traditional membranes but also to provide insights into the influence of pore size, pore topology and chemistry of MOFs on the membranes' performances. In this work, we performed the first high-throughput molecular simulation study in the literature to assess the potential of the most complete collection of MOFs for membrane-based H2/CH4 separation. Combining GCMC and MD simulations, we computed adsorption and diffusion coefficients of H2 and CH4 in all MOFs. This data was then used to estimate adsorption selectivity, diffusion selectivity and membrane selectivity of MOFs and relations between these selectivities were discussed to understand the individual effects of adsorption and diffusion on the membranes' performances. H2/CH4 selectivities and H2 permeabilities of 4240 different MOF membranes were calculated and compared with traditional membranes, such as zeolites and polymers, to reveal the membrane-based H2/CH4 separation potential of the MOF structures. The most promising MOF membranes that are above the Robeson's upper bound43 were identified. Mixture GCMC and MD simulations were also performed for the top ten most promising MOF membranes to evaluate their performances for separation of equimolar H2/CH4 mixtures under practical operating conditions. Results were compared with the predictions of molecular simulations performed at infinite dilution loadings of single-component gases. The accuracy of the high-throughput screening of MOFs based on single-component adsorption and diffusion data was discussed in detail. We finally examined the relations between structural properties of MOFs such as pore sizes, porosities, surface areas, densities, lattice structure types, metal types and their membrane selectivities and gas permeabilities to provide quantitative structure–performance relationships that can serve as a map for the development of new MOF membranes with better H2/CH4 separation performances. This type of fundamental understanding into structure–performance relations of MOF membranes that are capable of achieving energy efficient H2/CH4 separations has the potential to allow revolutionary changes in practical gas separation applications. Therefore, results presented in this work, identification of the high performance MOF membranes and microscopic insights provided for the structure–performance relations, will motivate a large number of researchers working on design and development of MOF membranes for various gas separations.
2. Computational methods
2.1 MOFs
The most complete collection of MOFs and the only collection integrated within the CSD database generated by Jimenez's group41 was used in this work. This collection is comprised of 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 non-disordered MOFs with a wide range of chemical and structural properties. We first removed the bound and unbound solvents in MOFs using a Python script available in the literature41 and then computed structural properties of MOFs such as pore limiting diameter (PLD), the largest cavity diameter (LCD), accessible gravimetric surface area (SA), porosity (ϕ) and density (ρ) using Zeo++ software.44 We refined the MOF database to remove the materials that have zero accessible gravimetric SAs. In order to compute gas permeabilities through the MOF membranes, gas diffusivities should be computable within the pores of MOFs. The kinetic diameters of H2 (2.96 Å) and CH4 (3.73 Å) are different leading to different diffusion rates. We focused on the MOFs that have PLDs greater than 3.8 Å so that both CH4 and H2 molecules can pass through the membranes' pores. As a result of these refinements, we ended up with 4240 different MOFs that span a wide range of chemical and structural functionalities. In order to investigate structural similarities of MOFs, a similarity matrix was built following the algorithms described in the literature.45,46 Each MOF structure was used as a seed one by one and compared to all MOFs using constrained ray trace method. In order to provide high statistical accuracy, 100
808 non-disordered MOFs with a wide range of chemical and structural properties. We first removed the bound and unbound solvents in MOFs using a Python script available in the literature41 and then computed structural properties of MOFs such as pore limiting diameter (PLD), the largest cavity diameter (LCD), accessible gravimetric surface area (SA), porosity (ϕ) and density (ρ) using Zeo++ software.44 We refined the MOF database to remove the materials that have zero accessible gravimetric SAs. In order to compute gas permeabilities through the MOF membranes, gas diffusivities should be computable within the pores of MOFs. The kinetic diameters of H2 (2.96 Å) and CH4 (3.73 Å) are different leading to different diffusion rates. We focused on the MOFs that have PLDs greater than 3.8 Å so that both CH4 and H2 molecules can pass through the membranes' pores. As a result of these refinements, we ended up with 4240 different MOFs that span a wide range of chemical and structural functionalities. In order to investigate structural similarities of MOFs, a similarity matrix was built following the algorithms described in the literature.45,46 Each MOF structure was used as a seed one by one and compared to all MOFs using constrained ray trace method. In order to provide high statistical accuracy, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 sample points with 0.5 Å probe size was provided into the Zeo++ software. Peak height ratios between different ray trace intensities were summed and normalized with the total data points to create a similarity index. Similarity index between 0 and 1 provides a range of similarity measure for MOF structures, 0 as the least similar and 1 as the most similar.
000 sample points with 0.5 Å probe size was provided into the Zeo++ software. Peak height ratios between different ray trace intensities were summed and normalized with the total data points to create a similarity index. Similarity index between 0 and 1 provides a range of similarity measure for MOF structures, 0 as the least similar and 1 as the most similar.
2.2 Molecular simulations
GCMC and MD simulations, which have been widely used to compute gas adsorption isotherms and diffusivities in porous materials,47 were performed in this work as implemented in the RASPA simulation code.48 The gas–gas and gas-MOF interactions were defined using Lennard-Jones (LJ) potential. Single-site spherical LJ 12–6 potential was used to model H2 (ref. 49) and CH4 (ref. 50) molecules. The potential parameters of MOF atoms were taken from the Universal Force Field (UFF).51 These force fields were selected based on the results of our previous simulation studies in which very good agreements between our simulations and experimental measurements were shown for CH4 and H2 uptakes38,52,53 and diffusivities54 in many MOFs. The validity of our computational approach to predict the membrane performances of various MOFs (such as IRMOF-1, ZIF-8, ZIF-69, ZIF-78, ZIF-90, ZIF-95, Ni-MOF-74, Zn(bdc)(ted)0.5) was also shown in several of our previous studies40,55,56 in which good agreements between our simulations and experiments for single-component H2 and CH4 permeabilities and for equimolar H2/CH4 mixture permeabilities were reported.The Henry's constants (K0) of gas molecules were initially calculated at the limit of zero-coverage (infinite dilution) using 105 moves of the Widom particle insertion method47 by performing Monte Carlo simulations. We also performed GCMC simulations for equimolar H2/CH4 mixtures at 1 bar and 298 K. In mixture GCMC simulations, four different types of moves including translation, reinsertion, swap of a molecule and identity exchange of molecules were considered. The Lorentz–Berthelot mixing rules were employed and the Peng–Robinson equation of state was used to convert the pressure to the corresponding fugacity. The cut-off distance for truncation of the intermolecular interactions was set to 13 Å. The simulation cell lengths were increased to at least 26 Å along each dimension and periodic boundary conditions were applied in all simulations. For each MOF, simulations were carried out for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with the first 5000 cycles for initialization and the last 5000 cycles for taking ensemble averages.
000 cycles with the first 5000 cycles for initialization and the last 5000 cycles for taking ensemble averages.
We then performed MD simulations to compute single component self-diffusivities (D0) of H2 and CH4 in MOFs' pores at 298 K and infinite dilution. Following the literature,29 we switched off the gas–gas intermolecular interactions and inserted 30 gas molecules into each MOF to represent the infinite dilution condition and to obtain high accuracy in the simulations. The self-diffusion coefficients were calculated by using the slope of the mean square displacement of gas molecules. For each MOF, MD simulations were carried out for 106 cycles in the NVT ensemble using a time step of 1 fs. We used 1000 initialization cycles and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 equilibration cycles. The Nosé–Hoover thermostat47 was used in NVT-MD simulations. We also performed mixture MD simulations and the initial states of these simulations were created with the appropriate loadings determined from the GCMC simulations performed at 1 bar and 298 K. Both gas–gas and gas–framework interactions were considered. Mixture MD simulations were carried out using 106 cycles in the NVT ensemble with a time step of 1 fs. At least 10 trajectories were used to compute self-diffusivities of each component. More details of these simulations can be found in the literature.47,57
000 equilibration cycles. The Nosé–Hoover thermostat47 was used in NVT-MD simulations. We also performed mixture MD simulations and the initial states of these simulations were created with the appropriate loadings determined from the GCMC simulations performed at 1 bar and 298 K. Both gas–gas and gas–framework interactions were considered. Mixture MD simulations were carried out using 106 cycles in the NVT ensemble with a time step of 1 fs. At least 10 trajectories were used to compute self-diffusivities of each component. More details of these simulations can be found in the literature.47,57
MOFs were assumed to be rigid in their reported crystallographic structures in simulations. This assumption has been used in all large-scale molecular simulation studies of MOFs to save significant computational time. We recently showed that if the MOF has large pore sizes, framework flexibility has a negligible effect on the gas permeability and H2/CH4 selectivity of MOF membranes.40 Flexibility was found to affect only gas permeability without changing the selectivity of the MOF membranes which have narrow pore sizes. In this work, we only considered MOFs with pore sizes larger than the kinetic diameters of both gas molecules, therefore flexibility is expected to have a negligible effect on the results.
2.3 High-throughput screening methodology
It is important to differentiate the methodology of our current work from our previous work. In our previous work,42 we performed GCMC simulations for equimolar CH4/H2 mixtures and reported adsorption selectivities of MOFs at 1 and 10 bar. In this work, we used a different approach to model MOF membranes: we first computed the Henry's constants (K0) of gas molecules at infinite dilution using the Widom particle insertion method and then performed MD simulations to compute single component self-diffusivities (D0) again at infinite dilution for all 4240 MOFs. Using this information, adsorption selectivities, diffusion selectivities and membrane selectivities of MOFs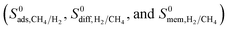 were computed in addition to the gas permeabilities
were computed in addition to the gas permeabilities 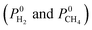 as described in the equations given in Table 1. We then identified the promising MOF membranes, which have membrane selectivities,
as described in the equations given in Table 1. We then identified the promising MOF membranes, which have membrane selectivities, 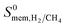 greater than 10 and H2 permeabilities,
greater than 10 and H2 permeabilities, 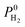 greater than 104 Barrer. Mixture GCMC and MD simulations were only performed for the top ten most promising MOF membranes that have the highest
greater than 104 Barrer. Mixture GCMC and MD simulations were only performed for the top ten most promising MOF membranes that have the highest 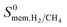 because performing MD simulations for gas mixtures is computationally very demanding due to the very large number of gas molecules interacting with each other and with the MOF atoms. These MD simulations were performed considering equimolar H2/CH4 mixtures at 1 bar and 298 K. Calculations of mixture membrane selectivities
because performing MD simulations for gas mixtures is computationally very demanding due to the very large number of gas molecules interacting with each other and with the MOF atoms. These MD simulations were performed considering equimolar H2/CH4 mixtures at 1 bar and 298 K. Calculations of mixture membrane selectivities 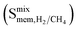 and mixture gas permeabilities
and mixture gas permeabilities 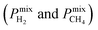 are also shown in Table 1. It is important to note that our suggested computational approach makes accurate predictions for the separation performances of MOF membranes. Fig. 1 shows the comparison of our results with the experimentally reported data for single-component CH4 and H2 permeances and H2/CH4 mixture permeances of several fabricated MOF membranes including IRMOF-1, Ni-MOF-74, ZIF-8, ZIF-69, ZIF-78, ZIF-90, ZIF-95. We also presented the good agreement between our calculations and experiments for the gas permeabilities of CH4 and H2 through mixed matrix membranes having MOFs (IRMOF-1 and CuBTC) as fillers in Fig. S1.† Gas permeance measurement conditions and related experimental references of MOF membranes and MOF-based mixed matrix membranes can be seen in Table S1.† All these results validate the accuracy of our computational approach.
are also shown in Table 1. It is important to note that our suggested computational approach makes accurate predictions for the separation performances of MOF membranes. Fig. 1 shows the comparison of our results with the experimentally reported data for single-component CH4 and H2 permeances and H2/CH4 mixture permeances of several fabricated MOF membranes including IRMOF-1, Ni-MOF-74, ZIF-8, ZIF-69, ZIF-78, ZIF-90, ZIF-95. We also presented the good agreement between our calculations and experiments for the gas permeabilities of CH4 and H2 through mixed matrix membranes having MOFs (IRMOF-1 and CuBTC) as fillers in Fig. S1.† Gas permeance measurement conditions and related experimental references of MOF membranes and MOF-based mixed matrix membranes can be seen in Table S1.† All these results validate the accuracy of our computational approach.
| Formula | |
|---|---|
| a K 0: Henry's constant at infinite dilution. D0: self-diffusivity at infinite dilution. C: loading of the gas species in the mixture. y: composition of the gas species in the bulk phase. Dmix: self-diffusivity of gas species in the mixture. i, j: gas species and i/j represents the selectivity of i over j. | |
| Permeability | P 0i = K0i × D0i |
| Adsorption selectivity | 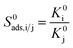 |
| Diffusion selectivity | 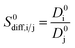 |
| Membrane selectivity | S 0mem,i/j = S0ads,i/j × S0diff,i/j |
| Mixture adsorption selectivity | 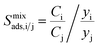 |
| Mixture diffusion selectivity | 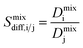 |
| Mixture membrane selectivity | S mixmem,i/j = Smixads,i/j × Smixdiff,i/j |
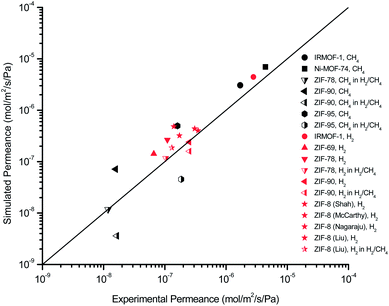 | ||
| Fig. 1 Comparison of our results and experiments for single-component and mixture gas permeances through IRMOF-1, Ni-MOF-74, ZIF-8, ZIF-69, ZIF-78, ZIF-90 and ZIF-95 membranes. Gas permeance measurement conditions of MOF membranes and related experimental references can be seen in Table S1.† | ||
3. Result and discussions
3.1 Performances of MOF membranes
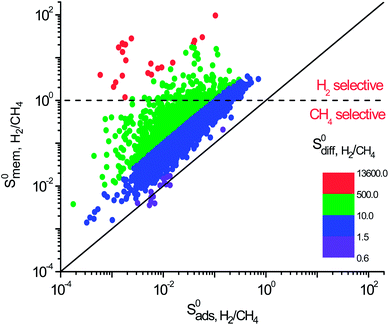
Selectivity has been considered as the most critical factor to assess both equilibrium and kinetic-based separation potentials of materials. Adsorption selectivity is used to evaluate MOF adsorbents and membrane selectivity is used to assess MOF membranes, which is calculated as the multiplication of adsorption and diffusion selectivities as shown in Table 1. We first compared these three selectivities to understand the influence of adsorption and diffusion on the performance of MOF membranes in Fig. 2. CH4 is energetically preferred over H2 and it is more strongly adsorbed compared to H2 in all MOFs. The K0 values of CH4 and H2 were calculated to be in the range of 1.36 × 10−7–8.27 × 10−4 mol kg−1 Pa−1 and 1.97 × 10−8–1.89 × 10−6 mol kg−1 Pa−1, respectively. Since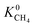 is larger than
is larger than 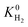 in all MOFs, adsorption selectivity favors CH4 over H2. Adsorption selectivities of MOFs for CH4 over H2 computed at infinite dilution loading
in all MOFs, adsorption selectivity favors CH4 over H2. Adsorption selectivities of MOFs for CH4 over H2 computed at infinite dilution loading 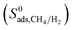 range from 1.40 to 5.68 × 103. Since the aim of this work is to identify the MOF membranes that are H2 selective, we defined all three selectivities as H2/CH4. Therefore,
range from 1.40 to 5.68 × 103. Since the aim of this work is to identify the MOF membranes that are H2 selective, we defined all three selectivities as H2/CH4. Therefore, 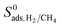 values are less than 1 on the x axis of Fig. 2. Diffusion favors H2 over CH4 because lighter, smaller and weakly adsorbed H2 molecules diffuse faster than the heavier, bulkier and strongly adsorbed CH4 molecules. The self-diffusivities of CH4 and H2
values are less than 1 on the x axis of Fig. 2. Diffusion favors H2 over CH4 because lighter, smaller and weakly adsorbed H2 molecules diffuse faster than the heavier, bulkier and strongly adsorbed CH4 molecules. The self-diffusivities of CH4 and H2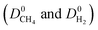 in MOFs were computed to be between 1.36 × 10−8–1.5 × 10−3 cm2 s−1 and 1.8 × 10−8–1.35 × 10−2 cm2 s−1, respectively. As a result, diffusion selectivities of MOFs for H2 over CH4 computed at infinite dilution
in MOFs were computed to be between 1.36 × 10−8–1.5 × 10−3 cm2 s−1 and 1.8 × 10−8–1.35 × 10−2 cm2 s−1, respectively. As a result, diffusion selectivities of MOFs for H2 over CH4 computed at infinite dilution 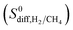 range from 0.58 to 1.36 × 104. The colored dots in Fig. 2 show the distribution of
range from 0.58 to 1.36 × 104. The colored dots in Fig. 2 show the distribution of 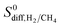 . There are 16 MOFs in which
. There are 16 MOFs in which 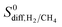 ranges from 0.6 to 1.5 (purple points), indicating that CH4 diffusion is almost at the same order with the H2 diffusion in these MOFs. A large number of MOFs (2749) has
ranges from 0.6 to 1.5 (purple points), indicating that CH4 diffusion is almost at the same order with the H2 diffusion in these MOFs. A large number of MOFs (2749) has 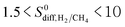 (blue points) and 1453 MOFs have high diffusion selectivities between 10 and 500 (green points). Those are the MOFs in which H2 diffuses faster than CH4. A smaller number of MOFs (22) exhibits very high diffusion selectivity, >500, for H2 as shown by red points in Fig. 2.
(blue points) and 1453 MOFs have high diffusion selectivities between 10 and 500 (green points). Those are the MOFs in which H2 diffuses faster than CH4. A smaller number of MOFs (22) exhibits very high diffusion selectivity, >500, for H2 as shown by red points in Fig. 2.
The membrane selectivities of MOFs computed at infinite dilution 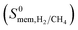 change from 1.4 × 10−3 to 97. The majority of the MOFs (3777) was identified to be CH4 selective as shown in Fig. 2. The maximum CH4 selectivity of MOF membranes was predicted to be 713. In these MOFs, high adsorption selectivity towards CH4 dominates the diffusion selectivity towards H2. We calculated selectivity of 68 MOFs to be around unity,
change from 1.4 × 10−3 to 97. The majority of the MOFs (3777) was identified to be CH4 selective as shown in Fig. 2. The maximum CH4 selectivity of MOF membranes was predicted to be 713. In these MOFs, high adsorption selectivity towards CH4 dominates the diffusion selectivity towards H2. We calculated selectivity of 68 MOFs to be around unity, 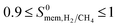 , which means they do not have a preference for CH4 or H2, therefore they cannot be used as selective membranes. A smaller number of MOFs, 395, was found to be H2 selective. In these MOFs, high diffusion selectivity towards H2 overcompensates the adsorption selectivity towards CH4 and the highest H2 selectivity of the MOF membrane was estimated to be 97. Overall, Fig. 2 represents that the most H2 selective MOF membranes are the ones that have high diffusion selectivities as shown in red colors.
, which means they do not have a preference for CH4 or H2, therefore they cannot be used as selective membranes. A smaller number of MOFs, 395, was found to be H2 selective. In these MOFs, high diffusion selectivity towards H2 overcompensates the adsorption selectivity towards CH4 and the highest H2 selectivity of the MOF membrane was estimated to be 97. Overall, Fig. 2 represents that the most H2 selective MOF membranes are the ones that have high diffusion selectivities as shown in red colors.
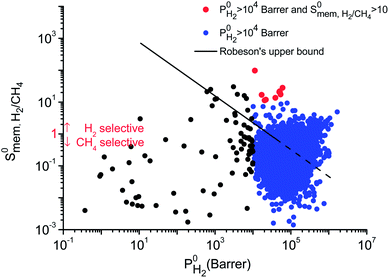
Separation performances of MOF membranes were compared with those of traditional polymer and zeolite membranes to assess the potential of MOFs in H2/CH4 separations. Polymeric membranes have been reported to be selective for H2 over CH4 and Robeson43 described an upper bound for these membranes. Several polyimide membranes establish the upper bound at the low H2 permeability end whereas poly(trimethylsilylpropyne) membranes are located at the high H2 permeability end of the upper bound. Fig. 3 shows H2 permeability and H2/CH4 selectivities of MOF membranes together with the Robeson's upper bound. The H2 permeabilities of MOF membranes were calculated to have a very wide range from 0.372 to 1.67 × 106 Barrer. Most of MOFs (4162) exhibit H2 permeabilities in the range of 104–106 Barrer as shown with blue points in Fig. 3. At that point it is important to highlight the importance of studying the entire MOF database rather than focusing on a small number of MOFs having similar physical and chemical properties. In our previous work,40 by studying 172 MOFs, we concluded that H2 permeabilities of MOFs are generally in the range of 103–105 Barrer. In this work, by examining the most recent and complete collection of the MOF database that spans a large variety in pore sizes and chemical topologies, we showed that H2 permeabilities of many MOFs are actually very large, >105 Barrer. Since these H2 permeabilities are significantly higher than the permeabilities of polymeric membranes, we extrapolated the Robeson's upper bound with a dashed line in Fig. 3. High gas permeabilities of MOFs can be attributed to their high porosities as we will discuss below. 1545 MOFs were found to exceed the upper bound either due to their high H2 permeabilities or high H2 selectivities or a combination of these two. In order to describe the most promising membrane materials, we specifically focused on the MOFs that have 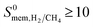 and
and 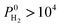 Barrer, which are shown by red points in Fig. 3. Gas diffusivities, gas permeabilities, adsorption, diffusion and membrane selectivities of the top ten MOF membranes are all listed in Table 2 in addition to their structural properties.
Barrer, which are shown by red points in Fig. 3. Gas diffusivities, gas permeabilities, adsorption, diffusion and membrane selectivities of the top ten MOF membranes are all listed in Table 2 in addition to their structural properties.
| MOF | LCD (Å) | PLD (Å) | ϕ | (cm2 s−1) | (cm2 s−1) | (Barrer) | (Barrer) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MEFMEQ | 5.24 | 4.26 | 0.52 | 2.98 × 10−7 | 2.77 × 10−4 | 1.16 × 102 | 1.13 × 104 | 9.60 | 930.57 | 96.95 |
| OGAJEN | 6.47 | 3.96 | 0.55 | 3.74 × 10−8 | 4.36 × 10−4 | 2.15 × 103 | 6.02 × 104 | 416.16 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.87 650.87 |
28.00 |
| OGAMOA | 6.37 | 3.80 | 0.54 | 2.58 × 10−8 | 3.51 × 10−4 | 2.33 × 103 | 5.06 × 104 | 626.72 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599.93 599.93 |
21.70 |
| OGALEP | 6.38 | 3.80 | 0.54 | 2.78 × 10−8 | 3.49 × 10−4 | 2.38 × 103 | 4.96 × 104 | 603.51 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558.17 558.17 |
20.81 |
| OGALUF | 6.38 | 3.84 | 0.54 | 3.35 × 10−8 | 3.68 × 10−4 | 2.56 × 103 | 5.17 × 104 | 545.05 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995.69 995.69 |
20.17 |
| OGAKUE | 6.32 | 3.81 | 0.54 | 2.82 × 10−8 | 3.63 × 10−4 | 3.09 × 103 | 5.37 × 104 | 743.25 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.03 896.03 |
17.35 |
| PIZHOX | 6.01 | 4.81 | 0.65 | 5.58 × 10−7 | 2.36 × 10−4 | 1.01 × 103 | 1.70 × 104 | 24.99 | 422.00 | 16.88 |
| OGAJAJ | 6.24 | 3.82 | 0.54 | 3.22 × 10−8 | 2.72 × 10−4 | 2.87 × 103 | 3.89 × 104 | 622.61 | 8438.47 | 13.55 |
| HIFVUO | 7.50 | 5.98 | 0.71 | 4.50 × 10−6 | 4.65 × 10−4 | 1.84 × 103 | 2.18 × 104 | 8.67 | 103.15 | 11.90 |
| QONKOV | 5.98 | 4.81 | 0.64 | 1.01 × 10−6 | 2.74 × 10−4 | 1.80 × 103 | 2.04 × 104 | 24.09 | 272.50 | 11.31 |
The most promising MOF membrane in Table 2 is MEFMEQ. This MOF offers both high 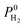 , 1.1 × 104 Barrer and high
, 1.1 × 104 Barrer and high 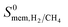 , 97. The high membrane selectivity is due to its large diffusion selectivity towards H2 (931) that dominates the weak adsorption selectivity towards CH4 (9.6). Similarly, the second most promising MOF, OGAJEN, has a high
, 97. The high membrane selectivity is due to its large diffusion selectivity towards H2 (931) that dominates the weak adsorption selectivity towards CH4 (9.6). Similarly, the second most promising MOF, OGAJEN, has a high 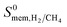 (28) due to its high diffusion selectivity for H2 (11
(28) due to its high diffusion selectivity for H2 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651). Although adsorption strongly favored CH4 in this MOF with a selectivity of 416, very fast diffusion of H2 (4.4 × 10−4 cm2 s−1) compared to CH4 (3.7 × 10−8 cm2 s−1) dominated the adsorption selectivity. In fact, Table 2 shows that all promising MOF membranes have very high
651). Although adsorption strongly favored CH4 in this MOF with a selectivity of 416, very fast diffusion of H2 (4.4 × 10−4 cm2 s−1) compared to CH4 (3.7 × 10−8 cm2 s−1) dominated the adsorption selectivity. In fact, Table 2 shows that all promising MOF membranes have very high 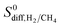 (>100). These MOFs have narrow PLDs, generally around 3.8–4.8 Å, close to the kinetic diameter of CH4. Strongly confined CH4 molecules diffuse slowly, in the order of 10−6–10−8 cm2 s−1, in these MOFs whereas H2 diffusion is at least two orders of magnitude higher, 10−4 cm2 s−1. As a result of this difference in diffusivities, MOFs become H2 selective membranes. Relations between selectivities and structural properties of MOFs will be discussed in detail below.
(>100). These MOFs have narrow PLDs, generally around 3.8–4.8 Å, close to the kinetic diameter of CH4. Strongly confined CH4 molecules diffuse slowly, in the order of 10−6–10−8 cm2 s−1, in these MOFs whereas H2 diffusion is at least two orders of magnitude higher, 10−4 cm2 s−1. As a result of this difference in diffusivities, MOFs become H2 selective membranes. Relations between selectivities and structural properties of MOFs will be discussed in detail below.
We also compared the MOF membranes with zeolite membranes. Similar to polymeric membranes, zeolites CHA, LTA and ITQ-29 (which is the pure silica version of LTA, in other words, the same structure) are H2 selective membranes because they have narrow windows which are not accessible for CH4 molecules. H2 selectivities of these zeolite membranes were reported as ∼89, 17, 125 and their H2 permeabilities were reported as 4.57 × 104, 5.8 × 103 and 8.21 × 103 Barrer, respectively.58 MOFs offer similar H2 selectivities and higher H2 permeabilities than these zeolites. Zeolite MFI, which has larger pore windows compared to other zeolites, was reported to exhibit high CH4 permeability (∼105 Barrer) and it is a CH4 selective membrane with a selectivity of 13. We identified 795 MOF membranes that are much more CH4 selective (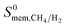 in the range of 13–713) and much more CH4 permeable (
in the range of 13–713) and much more CH4 permeable (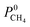 in the range of 1.0 × 105–1.2 × 108 Barrer) than MFI membrane. Carbon nanotube membranes (CNT) were shown to exhibit very large CH4 permeabilities (2 × 107 Barrer) and good CH4 selectivities (28) due to the very fast diffusion of gas molecules (0.1–1 cm2 s−1) which was attributed to the smoothness of the potential energy surface.59 17 MOFs were identified to outperform CNT membranes in terms of CH4 selectivities (69–713) and CH4 permeabilities (∼107–108 Barrer) in this work. This comparison shows that several MOFs can replace or even outperform zeolite and polymer membranes in H2/CH4 separations.
in the range of 1.0 × 105–1.2 × 108 Barrer) than MFI membrane. Carbon nanotube membranes (CNT) were shown to exhibit very large CH4 permeabilities (2 × 107 Barrer) and good CH4 selectivities (28) due to the very fast diffusion of gas molecules (0.1–1 cm2 s−1) which was attributed to the smoothness of the potential energy surface.59 17 MOFs were identified to outperform CNT membranes in terms of CH4 selectivities (69–713) and CH4 permeabilities (∼107–108 Barrer) in this work. This comparison shows that several MOFs can replace or even outperform zeolite and polymer membranes in H2/CH4 separations.
Results obtained so far showed that there are many promising MOFs that offer high permeability and selectivity for efficient separation of H2 from CH4. However, these MOFs have not been fabricated as membranes so far. In other words, MOFs that we identified as top promising membranes have not been fabricated as membranes yet, therefore it is not possible to make a direct comparison between experiments and simulations for the top promising MOF membranes. We already showed the good agreement between our simulations and experimental measurements for the performance of several different types of fabricated, prototype MOF membranes in Fig. 1 and we believe that these are the direct evidence of the good predicting power of our calculation methodology. Membrane studies in the literature generally focused on well-known MOFs. For example, several different research groups18,19 fabricated IRMOF-1 (also known as MOF-5) membranes and reported its H2/CH4 selectivity as 2–3 and H2 permeance (permeability divided by membrane thickness) as 8–47 × 10−7 mol m−2 s−1 Pa−1 based on single-component gas measurements. By studying the entire MOF database, we found that there are 21 MOFs that possess H2/CH4 selectivity >2 and H2 permeance >50 × 10−7 mol m−2 s−1 Pa−1 assuming 25 μm membrane thickness. There are 39 MOFs that exhibit H2/CH4 selectivity around 2, similar to MOF-5. It is important to note that selectivity and permeance of the MOF-5, SAHYIK in the CSD, was predicted as 1.39 and 44 × 10−7 mol m−2 s−1 Pa−1 in this work which agree well with the experimentally reported values.40,56 These results highlight the importance of computational screening of the entire MOF database to direct the experimental studies to more promising membrane materials. At that point, we would like to note that one of the most widely fabricated MOF membrane, ZIF-8, did not appear in our discussion because we limited our focus with MOFs having limiting pore sizes greater than 3.8 Å so that both CH4 and H2 molecules can pass through the membranes' pores. Since the small pore size of ZIF-8 was computed as 3.4 Å, this MOF was not included in the 4240 MOFs that we examined.
3.2 Structure–performance relations
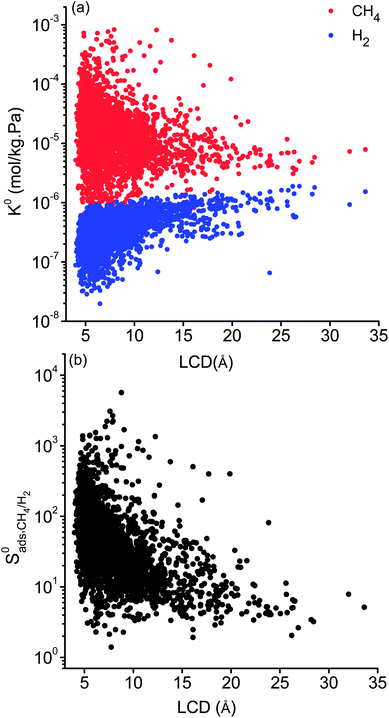
Understanding the relations between structural properties and performances of MOF membranes for a target gas separation is useful not only to easily identify the promising candidates but also to guide the design and synthesis of new MOFs with exceptionally high membrane-based gas separation performances. We examined the relations between K0 of gases, D0 of gases, three different selectivities and structural properties of MOFs, pore sizes (PLD and LCD), surface areas (SA), porosities (ϕ) and densities (ρ). Fig. 4(a) shows that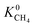 generally decreases with increasing LCD since small cavities are more favorable adsorption sites for large CH4 molecules due to the strong confinement. The degree of confinement of H2 molecules in MOFs with small pores and MOFs with large pores can be thought as similar, because in both cases H2 molecule is small relative to the pore size giving similar, low
generally decreases with increasing LCD since small cavities are more favorable adsorption sites for large CH4 molecules due to the strong confinement. The degree of confinement of H2 molecules in MOFs with small pores and MOFs with large pores can be thought as similar, because in both cases H2 molecule is small relative to the pore size giving similar, low 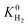 values. As a result of this,
values. As a result of this, 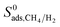 of MOFs generally decreases with LCD as shown in Fig. 4(b). Although this relation is not perfect, it is obvious that MOFs with LCDs > 12 Å exhibit lower
of MOFs generally decreases with LCD as shown in Fig. 4(b). Although this relation is not perfect, it is obvious that MOFs with LCDs > 12 Å exhibit lower 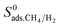 . For example, the average
. For example, the average 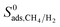 is about 26 for MOFs having LCD > 12 Å. Relations between K0 of gases and other structural properties, PLD, SA, ϕ and ρ are given in Fig. S2.†
is about 26 for MOFs having LCD > 12 Å. Relations between K0 of gases and other structural properties, PLD, SA, ϕ and ρ are given in Fig. S2.†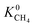 decreases with PLD and SA but there is no obvious relation between
decreases with PLD and SA but there is no obvious relation between 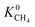 and ϕ, ρ. As the SA and ϕ (ρ) increase (decreases),
and ϕ, ρ. As the SA and ϕ (ρ) increase (decreases), 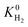 increases. Relations between
increases. Relations between 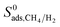 and PLD, SA, ϕ, ρ are also shown in Fig. S3† and results showed that S0ads decreases (increases) with increased pore sizes, SA and ϕ (ρ).
and PLD, SA, ϕ, ρ are also shown in Fig. S3† and results showed that S0ads decreases (increases) with increased pore sizes, SA and ϕ (ρ).
We then examined relations between gas diffusivities and PLDs of MOFs. Fig. 5(a) and (b) show that both 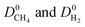 increase as the PLD and ϕ of MOFs increase.
increase as the PLD and ϕ of MOFs increase. 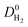 is generally at least one order of magnitude larger than
is generally at least one order of magnitude larger than 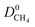 in MOFs having small PLDs (<5 Å) since smaller H2 molecules diffuse faster than the bulkier CH4 molecules in narrow pores of MOFs. Diffusivities get close to each other in MOFs having large pore sizes (>10 Å) since both gases can easily diffuse regardless of the molecular size. As a result of these, the highest
in MOFs having small PLDs (<5 Å) since smaller H2 molecules diffuse faster than the bulkier CH4 molecules in narrow pores of MOFs. Diffusivities get close to each other in MOFs having large pore sizes (>10 Å) since both gases can easily diffuse regardless of the molecular size. As a result of these, the highest 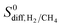 is located at PLDs < 5 Å and ϕ < 0.75 as shown in Fig. 5(c). As the PLD and ϕ increase,
is located at PLDs < 5 Å and ϕ < 0.75 as shown in Fig. 5(c). As the PLD and ϕ increase, 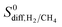 generally decreases since both molecules can readily diffuse regardless of their molecular sizes. At that point it is important to note that diffusion rates of gases and hence S0diff do not solely depend on the kinetic diameters of gas molecules and pore sizes of MOFs. At the narrow PLD region,
generally decreases since both molecules can readily diffuse regardless of their molecular sizes. At that point it is important to note that diffusion rates of gases and hence S0diff do not solely depend on the kinetic diameters of gas molecules and pore sizes of MOFs. At the narrow PLD region, 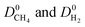 show a large variety of values from 10−8 to 10−3 cm2 s−1 indicating that diffusion is not only determined by the pore sizes but also interactions between gas molecules and MOFs play an important role. Finally, Fig. S4† shows that D0 of both gases generally increases (decreases) with increased SA and ϕ (ρ). This is expected because as the porosity increases, framework density decreases and gas molecules easily diffuse in less confined spaces. Since both diffusivities have similar trends with the SA and ρ,
show a large variety of values from 10−8 to 10−3 cm2 s−1 indicating that diffusion is not only determined by the pore sizes but also interactions between gas molecules and MOFs play an important role. Finally, Fig. S4† shows that D0 of both gases generally increases (decreases) with increased SA and ϕ (ρ). This is expected because as the porosity increases, framework density decreases and gas molecules easily diffuse in less confined spaces. Since both diffusivities have similar trends with the SA and ρ, 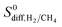 , which was calculated as the ratio of diffusivity of H2 to that of CH4, is almost independent from them. According to Fig. S5,†S0diff does not show an obvious dependency to SA, ϕ, and ρ. Relations between P0 of gases, PLD and ϕ of MOFs are shown in Fig. 6. The trend of P0 and PLD shown in Fig. 6(a) is very similar to the one between D0 and PLD given in Fig. 5(a), since
, which was calculated as the ratio of diffusivity of H2 to that of CH4, is almost independent from them. According to Fig. S5,†S0diff does not show an obvious dependency to SA, ϕ, and ρ. Relations between P0 of gases, PLD and ϕ of MOFs are shown in Fig. 6. The trend of P0 and PLD shown in Fig. 6(a) is very similar to the one between D0 and PLD given in Fig. 5(a), since 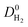 increases with ϕ,
increases with ϕ, 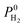 also increases. Fig. S6† represents that
also increases. Fig. S6† represents that 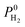 is positively (negatively) correlated with SA (ρ) due to the same reasons we discussed for
is positively (negatively) correlated with SA (ρ) due to the same reasons we discussed for 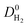 . As a result of these, narrow pore sizes and smaller porosities lead to higher
. As a result of these, narrow pore sizes and smaller porosities lead to higher 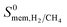 as shown in Fig. 6(c).
as shown in Fig. 6(c). 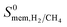 also shows an increasing (decreasing) trend with respect to SA and ϕ (ρ) as shown in Fig. S7.† We defined quantitative limits for the structural properties of MOFs that lead to high performance membranes in the next section. It is also important to note that these structure–performance relations have some similarities with the ones reported in a molecular simulation study which examined the CO2/CH4 separation performances of hypothetical MOF membranes that have PLDs between 3 and 4 Å.29 It was shown that diffusivities of CO2 and CH4 increase with increasing PLDs of hypothetical MOFs and the highest diffusion selectivities for CO2/CH4 were obtained at the low PLD region, between 3.0 and 3.2 Å. In our work, we considered real, synthesized MOF database with PLDs between 3.8 and 31 Å and showed that the highest diffusion selectivities for H2/CH4 separation was also found to be at the low PLD region, <5 Å.
also shows an increasing (decreasing) trend with respect to SA and ϕ (ρ) as shown in Fig. S7.† We defined quantitative limits for the structural properties of MOFs that lead to high performance membranes in the next section. It is also important to note that these structure–performance relations have some similarities with the ones reported in a molecular simulation study which examined the CO2/CH4 separation performances of hypothetical MOF membranes that have PLDs between 3 and 4 Å.29 It was shown that diffusivities of CO2 and CH4 increase with increasing PLDs of hypothetical MOFs and the highest diffusion selectivities for CO2/CH4 were obtained at the low PLD region, between 3.0 and 3.2 Å. In our work, we considered real, synthesized MOF database with PLDs between 3.8 and 31 Å and showed that the highest diffusion selectivities for H2/CH4 separation was also found to be at the low PLD region, <5 Å.
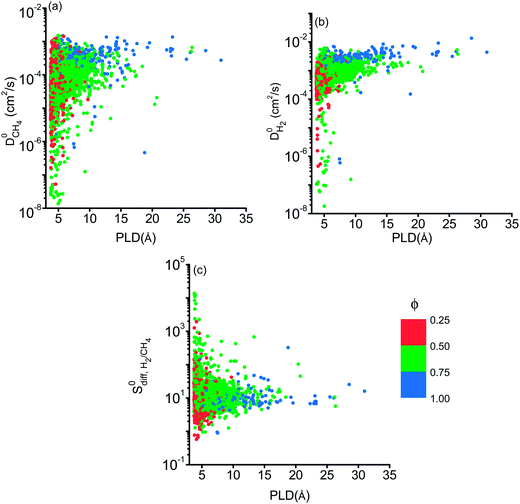 | ||
| Fig. 5 Self diffusivities of (a) CH4 (b) H2 as a function of PLDs of MOFs. (c) Diffusion selectivities of MOFs for H2/CH4 as a function of PLD and porosity. | ||
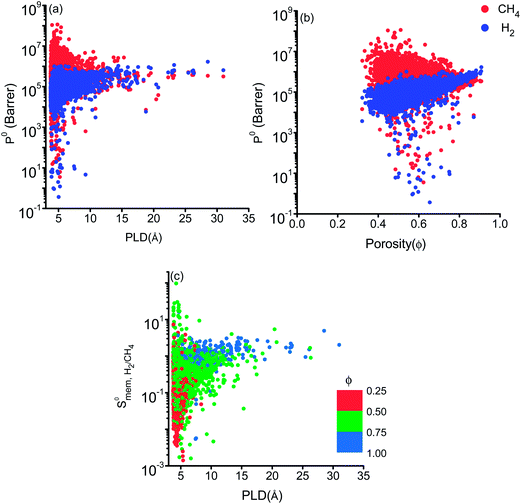 | ||
| Fig. 6 Gas permeabilities as a function of (a) PLD (b) porosity of MOFs. (c) Membrane selectivities of MOFs for H2/CH4 as a function of PLD and porosity. | ||
3.3 Top promising MOF membranes
So far, we examined the structure–performance relations for the entire MOF database. We now turn to the top ten promising MOFs that offer the highest . The aim is to provide better understandings of the fundamental physics behind the most promising MOF membranes at the molecular level in order to facilitate rational design of new materials. This structure–performance analysis will also guide the experimental studies to select MOFs with pre-determined structural characteristics in order to achieve high performance H2/CH4 separation. Fig. 7 shows the effects of PLD, LCD, the ratio of PLD to LCD, SA, ϕ, ρ, lattice system and metal types available in the MOFs on the H2 selectivities of the MOF membranes. The outer circle represents all MOFs considered in this study (4240 MOFs), the middle circle represents the top 100 H2 selective MOFs with
. The aim is to provide better understandings of the fundamental physics behind the most promising MOF membranes at the molecular level in order to facilitate rational design of new materials. This structure–performance analysis will also guide the experimental studies to select MOFs with pre-determined structural characteristics in order to achieve high performance H2/CH4 separation. Fig. 7 shows the effects of PLD, LCD, the ratio of PLD to LCD, SA, ϕ, ρ, lattice system and metal types available in the MOFs on the H2 selectivities of the MOF membranes. The outer circle represents all MOFs considered in this study (4240 MOFs), the middle circle represents the top 100 H2 selective MOFs with 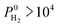 Barrer and the inner circle represents the top 10 most promising MOFs that we discussed in Table 2, the ones having
Barrer and the inner circle represents the top 10 most promising MOFs that we discussed in Table 2, the ones having 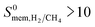 and
and 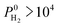 Barrer. This analysis suggests that MOFs with 3.8 Å < PLD < 6 Å, 6 Å < LCD < 12 Å, SA < 1000 m2 g−1, 0.5 < ϕ < 0.75, 1 < ρ < 1.5 g cm−3 are the most promising membranes. This analysis also suggests that triclinic and monoclinic MOFs are more promising for H2/CH4 separations compared to other lattice types. MOFs have a large variety of metals in their structures but most MOFs have Ag, Cd, Cu, Co, Zn. The top ten MOF membranes generally have Cd, Cu and Zn and more specifically, monoclinic MOFs having Cu metals were found to be promising as H2 selective membranes.
Barrer. This analysis suggests that MOFs with 3.8 Å < PLD < 6 Å, 6 Å < LCD < 12 Å, SA < 1000 m2 g−1, 0.5 < ϕ < 0.75, 1 < ρ < 1.5 g cm−3 are the most promising membranes. This analysis also suggests that triclinic and monoclinic MOFs are more promising for H2/CH4 separations compared to other lattice types. MOFs have a large variety of metals in their structures but most MOFs have Ag, Cd, Cu, Co, Zn. The top ten MOF membranes generally have Cd, Cu and Zn and more specifically, monoclinic MOFs having Cu metals were found to be promising as H2 selective membranes.
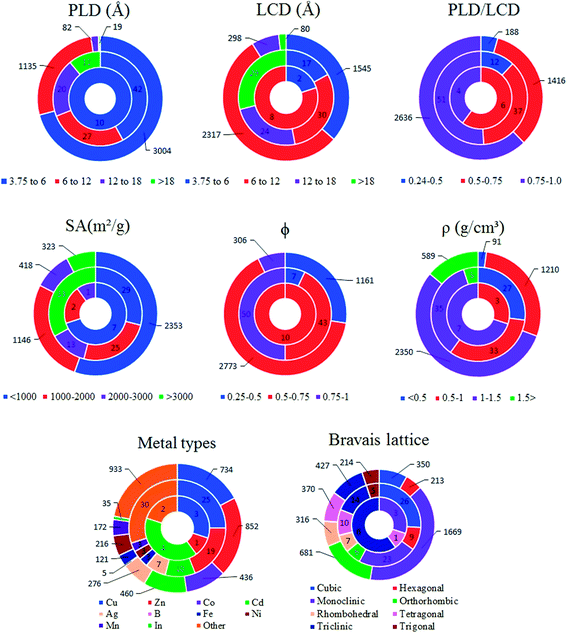 | ||
Fig. 7 Effects of structural properties on the performance of MOF membranes. Numbers on the circles represent the number of MOFs. The outer circle represents all MOFs considered in this study (4240 MOFs), the middle circle represents the top 100 H2 selective MOFs having 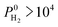 Barrer and the inner circle represents the top 10 most promising MOFs that we discussed in Table 2, the ones having Barrer and the inner circle represents the top 10 most promising MOFs that we discussed in Table 2, the ones having 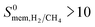 and and 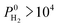 Barrer. Barrer. | ||
We also examined the structural similarities of the top ten most promising MOFs and results are shown in Fig. S8† together with the 2 × 2 × 2 unit cell representations of these MOFs. Structural similarity between two MOFs is represented with an index. Green colors correspond to the most similar materials, the ones having similarity index >0.8. Light green colors represent highly similar structures with indexes between 0.7 and 0.8, yellow colors show the slightly similar structures with indexes between 0.5 and 0.7 and orange colors correspond to least similar structures with indexes between 0.3 and 0.5. Red colors show the dissimilar MOFs in term of their analyzed ray trace representation resulting in an index of 0.0–0.3. Fig. S8† shows that MEFMEQ and 6 OGA-materials have high similarities with indexes greater than 0.8. PIZHOX and QONKOV also showed similarities with other top MOFs with an index of 0.65 whereas the index between those two structures was 0.83 indicating a high similarity. HIFVUO has the least similarity with other MOFs giving indexes around 0.5. There is no red color in Fig. S8† which means that the top ten MOF membranes have some common structural features which is also observable from the unit cell representations.
As explained in the screening strategy described in Section 2.3, the top ten most promising MOFs were identified by performing single-component simulations at infinite dilution. We also performed equimolar mixture GCMC and MD simulations at 1 bar to unlock the mixture separation performances of the top promising MOF membranes under realistic operating conditions. Gas diffusivities, permeabilities, and all three selectivities computed using single-component data at infinite dilution were compared with the ones computed for equimolar mixtures in Table 3. Results showed that mixture adsorption selectivities are similar to the ones computed at infinite dilution for 4 MOFs, HIFVUO, MEFMEQ, PIZHOX and QONKOV. These are the MOFs that have relatively low adsorption selectivities, <25. On the other hand, the MOFs (OGA-materials) which exhibit very high adsorption selectivities at infinite dilution, ∼500, were found to have significantly lower mixture adsorption selectivities, ∼90. Fig. S9† compares 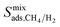 computed at 1 bar with
computed at 1 bar with 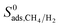 for the entire MOF database and results show that infinite dilution adsorption selectivities significantly overestimate mixture adsorption selectivities especially if the MOF is highly CH4 selective (>50). For 4 MOFs which have similar
for the entire MOF database and results show that infinite dilution adsorption selectivities significantly overestimate mixture adsorption selectivities especially if the MOF is highly CH4 selective (>50). For 4 MOFs which have similar 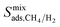 and
and 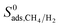 (MEFMEQ, PIZHOX, HIFVUO and QONKOV), mixture gas diffusivities were found to be very similar to the ones computed at infinite dilution. As a result, membrane selectivity computed for mixture
(MEFMEQ, PIZHOX, HIFVUO and QONKOV), mixture gas diffusivities were found to be very similar to the ones computed at infinite dilution. As a result, membrane selectivity computed for mixture 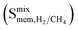 was found be close to the membrane selectivity computed at infinite dilution
was found be close to the membrane selectivity computed at infinite dilution 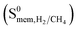 . On the other hand, for MOFs that have significant differences between
. On the other hand, for MOFs that have significant differences between 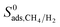 and
and 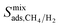 , mixture diffusivities were computed to be different than the single-component diffusivities. Table 3 shows that
, mixture diffusivities were computed to be different than the single-component diffusivities. Table 3 shows that 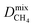 is almost one order of magnitude larger than
is almost one order of magnitude larger than 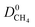 whereas
whereas 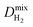 and
and 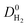 are almost same. The increase in the diffusion rate of CH4 can be attributed to the multi-component mixture effects60 and collaborative interactions between many adsorbed CH4 molecules, which are missing in the single-component case. It is known that fast-diffusing gas molecules (in our case H2) can fasten the slow-diffusing gas molecules (in our case CH4) in the MOFs' pores due to the multi-component mixture effects.36 As a result,
are almost same. The increase in the diffusion rate of CH4 can be attributed to the multi-component mixture effects60 and collaborative interactions between many adsorbed CH4 molecules, which are missing in the single-component case. It is known that fast-diffusing gas molecules (in our case H2) can fasten the slow-diffusing gas molecules (in our case CH4) in the MOFs' pores due to the multi-component mixture effects.36 As a result, 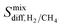 becomes much lower than
becomes much lower than 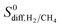 . Overall, simulations performed for equimolar mixtures at 1 bar give higher adsorption selectivities but lower diffusion selectivities for H2 than the simulations performed at infinite dilution. As a result, H2 selectivities of MOF membranes, which were computed as the multiplication of adsorption and diffusion selectivities, become similar. Comparison of the single-component and mixture calculations for the H2 permeability and H2/CH4 selectivities of the top promising MOF membranes is also given in Fig. S10.† 9 out of 10 top performing MOFs analyzed using mixture simulations still satisfy the two performance criteria,
. Overall, simulations performed for equimolar mixtures at 1 bar give higher adsorption selectivities but lower diffusion selectivities for H2 than the simulations performed at infinite dilution. As a result, H2 selectivities of MOF membranes, which were computed as the multiplication of adsorption and diffusion selectivities, become similar. Comparison of the single-component and mixture calculations for the H2 permeability and H2/CH4 selectivities of the top promising MOF membranes is also given in Fig. S10.† 9 out of 10 top performing MOFs analyzed using mixture simulations still satisfy the two performance criteria, 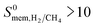 and
and 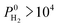 Barrer, used to identify the best MOF membranes. This result shows that the high-throughput computational screening strategy which utilizes gas loadings and diffusivities computed at infinite dilution can be used to make quick and accurate estimations for the mixture separation performances of MOF membranes to identify the most promising candidates.
Barrer, used to identify the best MOF membranes. This result shows that the high-throughput computational screening strategy which utilizes gas loadings and diffusivities computed at infinite dilution can be used to make quick and accurate estimations for the mixture separation performances of MOF membranes to identify the most promising candidates.
| MOF | (cm2 s−1) | (cm2 s−1) | (cm2 s−1) | (cm2 s−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEFMEQ | 9.60 | 10.01 | 930.57 | 526.66 | 96.95 | 52.60 | 2.98 × 10−7 | 3.86 × 10−7 | 2.77 × 10−4 | 2.04 × 10−4 |
| OGAJEN | 416.16 | 86.74 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.87 650.87 |
1700.71 | 28.00 | 19.61 | 3.74 × 10−8 | 2.63 × 10−7 | 4.36 × 10−4 | 4.47 × 10−4 |
| OGAMOA | 626.72 | 90.95 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599.93 599.93 |
1527.64 | 21.70 | 16.80 | 2.58 × 10−8 | 2.30 × 10−7 | 3.51 × 10−4 | 3.51 × 10−4 |
| OGALEP | 603.51 | 91.19 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558.17 558.17 |
1408.20 | 20.81 | 15.44 | 2.78 × 10−8 | 2.74 × 10−7 | 3.49 × 10−4 | 3.86 × 10−4 |
| OGALUF | 545.05 | 93.86 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 995.69 995.69 |
2068.35 | 20.17 | 22.04 | 3.35 × 10−8 | 2.30 × 10−7 | 3.68 × 10−4 | 4.76 × 10−4 |
| OGAKUE | 743.25 | 96.23 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.03 896.03 |
1969.58 | 17.35 | 20.47 | 2.82 × 10−8 | 1.96 × 10−7 | 3.63 × 10−4 | 3.85 × 10−4 |
| PIZHOX | 24.99 | 23.72 | 422.00 | 567.13 | 16.88 | 23.91 | 5.58 × 10−7 | 4.70 × 10−7 | 2.36 × 10−4 | 2.66 × 10−4 |
| OGAJAJ | 622.61 | 97.36 | 8438.47 | 1800.22 | 13.55 | 18.49 | 3.22 × 10−8 | 1.77 × 10−7 | 2.72 × 10−4 | 3.19 × 10−4 |
| HIFVUO | 8.67 | 8.31 | 103.15 | 171.90 | 11.90 | 20.68 | 4.50 × 10−6 | 3.76 × 10−6 | 4.65 × 10−4 | 6.44 × 10−4 |
| QONKOV | 24.09 | 22.93 | 272.50 | 251.49 | 11.31 | 10.97 | 1.01 × 10−6 | 9.49 × 10−7 | 2.74 × 10−4 | 2.39 × 10−4 |
Finally, it is important to discuss the advancement of this work compared to our previous study on MOF membranes.40 In our previous work, we performed mixture GCMC and MD simulations to predict membrane performances of 172 types of MOFs. In this work, we described a high-throughput computational screening strategy which utilizes gas loadings and diffusivities computed at infinite dilution to make accurate estimations for the mixture separation performances of MOF membranes. Since we screened a very large number of MOF membranes, we were able to show clear structure–performance results that were not present when we studied only hundreds of MOFs. Therefore, we advanced the methodology to save significant computational time and also provided solid insights into structure–performance relations of MOFs by studying the whole MOF database.
4. Conclusion
High-throughput computational screening of the MOF database was performed combining GCMC and MD simulations to examine membrane-based H2/CH4 separation performances of MOFs. Adsorption, diffusion and membrane selectivities of 4240 MOFs were computed at infinite dilution. Results showed that there are more than 1500 MOFs which exhibit high H2 selectivity or high H2 permeability or both. Many MOF membranes were identified to outperform polymer and zeolite membranes in H2/CH4 separations by exceeding the upper bound. The top ten most promising MOF membranes were identified and gas permeabilities and selectivities of these membranes were also calculated for separation of equimolar H2/CH4 mixtures using GCMC and MD simulations. Results obtained from the mixture simulations were found to be similar to the results obtained from the single-component gas simulations, indicating that high-throughput computational screening strategy which uses gas loadings and diffusivities computed at infinite dilution can be used to make rapid and accurate estimations for the mixture separation performances of MOF membranes. We performed structure–performance analysis for the MOF membranes and results showed that MOFs with 3.8 Å < PLD < 6 Å, 6 Å < LCD < 12 Å, SA < 1000 m2 g−1, 0.5 < ϕ < 0.75, 1 < ρ < 1.5 g cm−3 are the most promising MOF membranes exhibiting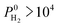 Barrer and
Barrer and 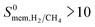 . These promising MOFs generally have Cd, Cu and Zn as their metals. The top ten most promising MOFs were found to have common structural features based on similarity index analysis. The MOFs identified as top promising membranes have not been fabricated as membranes yet, therefore it is not possible to make a direct comparison between experiments and simulations for these membranes. We already showed the good agreement between our simulations and experimental measurements for the performance of several different types of fabricated, prototype MOF membranes and we believe that these are the direct evidence of the good predicting power of our calculation methodology. Our results will be useful to guide the experimental synthesis of new MOF membranes to achieve high performance H2/CH4 separations and the computational methodology employed in this work will be used to identify the promising MOF membranes for various industrially important gas separations.
. These promising MOFs generally have Cd, Cu and Zn as their metals. The top ten most promising MOFs were found to have common structural features based on similarity index analysis. The MOFs identified as top promising membranes have not been fabricated as membranes yet, therefore it is not possible to make a direct comparison between experiments and simulations for these membranes. We already showed the good agreement between our simulations and experimental measurements for the performance of several different types of fabricated, prototype MOF membranes and we believe that these are the direct evidence of the good predicting power of our calculation methodology. Our results will be useful to guide the experimental synthesis of new MOF membranes to achieve high performance H2/CH4 separations and the computational methodology employed in this work will be used to identify the promising MOF membranes for various industrially important gas separations.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
S. K. acknowledges ERC-2017-Starting Grant. This study has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC-2017-Starting Grant, grant agreement No. 756489-COSMOS).References
- M. Eddaoudi, H. Li and O. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391–1397 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- N. Stock and S. Biswas, Chem. Rev., 2011, 112, 933–969 CrossRef PubMed.
- V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141–6172 RSC.
- Y. Basdogan and S. Keskin, CrystEngComm, 2015, 17, 261–275 RSC.
- S. Keskin, T. M. Van Heest and D. S. Sholl, ChemSusChem, 2010, 3, 879–891 CrossRef CAS PubMed.
- L. B. Li, R. B. Lin, R. Krishna, X. Q. Wang, B. Li, H. Wu, J. P. Li, W. Zhou and B. L. Chen, J. Mater. Chem. A, 2017, 5, 18984–18988 CAS.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef CAS PubMed.
- R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444–1230456 CrossRef PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2011, 112, 869–932 CrossRef PubMed.
- M. Shah, M. C. McCarthy, S. Sachdeva, A. K. Lee and H. K. Jeong, Ind. Eng. Chem. Res., 2012, 51, 2179–2199 CrossRef CAS.
- E. Adatoz, A. K. Avci and S. Keskin, Sep. Purif. Technol., 2015, 152, 207–237 CrossRef CAS.
- S. L. Qiu, M. Xue and G. S. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC.
- A. Kasik and Y. S. Lin, Sep. Purif. Technol., 2014, 121, 38–45 CrossRef CAS.
- Z. X. Zhao, X. L. Ma, A. Kasik, Z. Li and Y. S. Lin, Ind. Eng. Chem. Res., 2013, 52, 1102–1108 CrossRef CAS.
- Z. X. Zhao, X. L. Ma, Z. Li and Y. S. Lin, J. Membr. Sci., 2011, 382, 82–90 CrossRef CAS.
- Y. Yoo, Z. P. Lai and H. K. Jeong, Microporous Mesoporous Mater., 2009, 123, 100–106 CrossRef CAS.
- Y. Y. Liu, Z. F. Ng, E. A. Khan, H. K. Jeong, C. B. Ching and Z. P. Lai, Microporous Mesoporous Mater., 2009, 118, 296–301 CrossRef CAS.
- Y. Y. Mao, W. Cao, J. W. Li, Y. Liu, Y. L. Ying, L. W. Sun and X. S. Peng, J. Mater. Chem. A, 2013, 1, 11711–11716 CAS.
- J. P. Nan, X. L. Dong, W. J. Wang, W. Q. Jin and N. P. Xu, Langmuir, 2011, 27, 4309–4312 CrossRef CAS PubMed.
- V. V. Guerrero, Y. Yoo, M. C. McCarthy and H. K. Jeong, J. Mater. Chem., 2010, 20, 3938–3943 RSC.
- D. F. Liu, X. L. Ma, H. X. Xi and Y. S. Lin, J. Membr. Sci., 2014, 451, 85–93 CrossRef CAS.
- M. Shah, H. T. Kwon, V. Tran, S. Sachdeva and H. K. Jeong, Microporous Mesoporous Mater., 2013, 165, 63–69 CrossRef CAS.
- Y. C. Pan, B. Wang and Z. P. Lai, J. Membr. Sci., 2012, 421, 292–298 CrossRef.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef.
- R. Krishna and J. M. van Baten, Phys. Chem. Chem. Phys., 2011, 13, 10593–10616 RSC.
- Z. W. Qiao, K. Zhang and J. W. Jiang, J. Mater. Chem. A, 2016, 4, 2105–2114 CAS.
- Z. W. Qiao, C. W. Peng, J. Zhou and J. W. Jiang, J. Mater. Chem. A, 2016, 4, 15904–15912 CAS.
- T. Watanabe and D. S. Sholl, Langmuir, 2012, 28, 14114–14128 CrossRef CAS PubMed.
- M. Hong, S. Li, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2008, 307, 277–283 CrossRef CAS.
- S. Adhikari and S. Fernando, Ind. Eng. Chem. Res., 2006, 45, 875–881 CrossRef CAS.
- Q. P. Yang, L. J. Li, W. Q. Tan, Y. J. Sun, H. L. Wang, J. P. Ma and X. B. Zhao, Chem. Commun., 2017, 53, 9797–9800 RSC.
- S. Keskin and D. S. Sholl, Langmuir, 2009, 25, 11786–11795 CrossRef CAS PubMed.
- S. Keskin, Ind. Eng. Chem. Res., 2010, 49, 11689–11696 CrossRef CAS.
- S. Keskin, J. Phys. Chem. C, 2012, 116, 1772–1779 CAS.
- G. Yilmaz, A. Ozcan and S. Keskin, Mol. Simul., 2015, 41, 713–726 CrossRef CAS.
- T. N. Ozturk and S. Keskin, J. Phys. Chem. C, 2014, 118, 13988–13997 CAS.
- E. Haldoupis, S. Nair and D. S. Sholl, J. Am. Chem. Soc., 2010, 132, 7528–7539 CrossRef CAS PubMed.
- I. Erucar and S. Keskin, J. Membr. Sci., 2016, 514, 313–321 CrossRef CAS.
- P. Z. Moghadam, A. Li, S. B. Wiggin, A. Tao, A. G. P. Maloney, P. A. Wood, S. C. Ward and D. Fairen-Jimenez, Chem. Mater., 2017, 29, 2618–2625 CrossRef CAS.
- C. Altintas, I. Erucar and S. Keskin, ACS Appl. Mater. Interfaces, 2018, 10, 3668–3679 CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS.
- M. Pinheiro, R. L. Martin, C. H. Rycroft, A. Jones, E. Iglesia and M. Haranczyk, J. Mol. Graphics Modell., 2013, 44, 208–219 CrossRef CAS PubMed.
- R. L. Martin, B. Smit and M. Haranczyk, J. Chem. Inf. Model., 2012, 52, 308–318 CrossRef CAS PubMed.
- D. Frenkel and B. Smit, Understanding Molecular Simulation: From Algorithms to Applications, Academic Press, San Diego, 2nd edn, 2002 Search PubMed.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, Mol. Simul., 2016, 42, 81–101 CrossRef CAS.
- V. Buch, J. Chem. Phys., 1994, 100, 7610–7629 CrossRef CAS.
- M. G. Martin and J. I. Siepmann, J. Phys. Chem. B, 1998, 102, 2569–2577 CrossRef CAS.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- T. N. Ozturk and S. Keskin, Ind. Eng. Chem. Res., 2013, 52, 17627–17639 CrossRef CAS.
- K. B. Sezginel, A. Uzun and S. Keskin, Chem. Eng. Sci., 2015, 124, 125–134 CrossRef CAS.
- C. Altintas and S. Keskin, RSC Adv., 2017, 7, 52283–52295 RSC.
- Y. Basdogan, K. B. Sezginel and S. Keskin, Ind. Eng. Chem. Res., 2015, 54, 8479–8491 CrossRef CAS.
- E. Adatoz and S. Keskin, J. Nanomater., 2015, 2015, 1–9 CrossRef.
- D. Dubbeldam, A. Torres-Knoop and K. S. Walton, Mol. Simul., 2013, 39, 1253–1292 CrossRef CAS.
- R. Krishna and J. M. van Baten, J. Membr. Sci., 2010, 360, 323–333 CrossRef CAS.
- H. Chen and D. S. Sholl, J. Membr. Sci., 2006, 269, 152–160 CrossRef CAS.
- J. C. Liu, S. Keskin, D. S. Sholl and J. K. Johnson, J. Phys. Chem. C, 2011, 115, 12560–12566 CAS.
Footnotes |
† Electronic supplementary information (ESI) available: Supporting data associated with this article can be found in the online version: comparison of our results and experiments for single-component and mixture gas permeances through various MOF membranes; relations between Henry's constants, diffusivities, permeabilities, adsorption selectivities, diffusion selectivities, membrane selectivities and PLD, LCD, SA, ϕ, ρ of MOFs; similarity indexes and unit cell representations of the top ten promising MOFs; comparison of 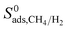 with with 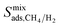 ; comparison of the single-component and mixture calculations for the permeability and selectivities of the top promising MOF membranes. See DOI: 10.1039/c8ta01547c ; comparison of the single-component and mixture calculations for the permeability and selectivities of the top promising MOF membranes. See DOI: 10.1039/c8ta01547c |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2018 |