Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vacancy defect configurations in the metal–organic framework UiO-66: energetics and electronic structure†
Katrine L.
Svane‡
 *a,
Jessica K.
Bristow
a,
Julian D.
Gale
b and
Aron
Walsh
*a,
Jessica K.
Bristow
a,
Julian D.
Gale
b and
Aron
Walsh
 cd
cd
aDepartment of Chemistry, University of Bath, Bath, UK. E-mail: kasv@dtu.dk
bCurtin Institute for Computation, Department of Chemistry, Curtin University, PO Box U1987, Perth, WA 6845, Australia
cDepartment of Materials, Imperial College London, London, UK
dDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, South Korea
First published on 24th April 2018
Abstract
Vacancy lattice sites in the metal–organic framework UiO-66 are known to have a profound effect on the material properties. Here we use density functional theory to compare the energies of defect arrangements containing missing linkers and missing metal clusters for different choices of charge compensation. Our results show that the preference for missing metal clusters or missing linker defects depends on the charge compensation as well as the overall concentration of defects in the crystal. Both regimes can be experimentally accessible depending on the synthesis conditions. We investigate the electronic structure of the different types of defects, showing that, despite some changes in the localisation of the frontier orbitals, the electronic energy levels are only weakly affected by the presence of point defects.
1 Introduction
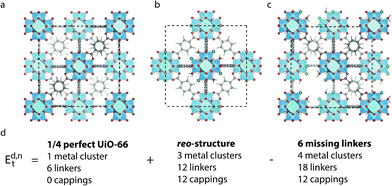
Metal–organic frameworks (MOFs) are porous, crystalline materials made from metal clusters connected by organic linker molecules.1,2 The many possible combinations of metal and organic component leads to a wealth of diverse structures which have been investigated for potential applications ranging from gas-adsorption and catalysis,3,4 exploiting the porous nature of these materials, to sensing and photocatalysis which relies on their electronic properties.5,6Recently, increasing attention has been focused on defects in MOFs, in recognition of their significant influence on the material properties as well as their surprisingly high concentration in some MOFs.7–9 A striking example is UiO-66 (ref. 10) (Fig. 1a), a popular MOF due to its chemical, mechanical and thermal stability.11–14 It consists of zirconium oxide clusters which, in the perfect crystalline structure, are connected to 12 benzene-1,4-dicarboxylate (BDC) linkers, however experiments suggest that between 1 and 4 of these linkers are missing from every cluster. The exact fraction of missing linkers varies depending on the synthesis method;15–19 in particular the addition of an acid modulator has been shown to increase the number of defects.20 This, in turn, leads to changes in the gas adsorption capacity,18,21–23 proton conductivity,24 catalytic activity,25–29 mechanical properties19,30–32 and chemical stability.33
Based on experiment, several different schemes for charge compensation of the metal cluster where a linker is missing have been suggested, including water and hydroxide,12,34–36 Cl− (ref. 15 and 37) and small acids such as formate, benzoic acid and (trifluoro) acetic acid.17,18,25,31,38 These different charge compensation schemes are not necessarily inconsistent with each other, as the charge capping depends on the species available during synthesis. Furthermore, defects incorporated during synthesis can be removed by post-synthetic treatment. It is well known that the as-synthesised hydroxylated metal cluster (Zr6O4(OH)4)12,35 in a defect-free environment is dehydrogenated upon heating around 300 °C, resulting in two water molecules leaving to form Zr6O6.12,29,37 In a similar fashion the charge capping of a defect can be removed with one hydroxyl hydrogen,25,29,37 which in the case of four defects on the same cluster transforms the hydroxylated metal cluster into a dehydroxylated Zr6O8 cluster.19
Theoretical studies have investigated the structure and energetics of several of the defect configurations mentioned above.26,29,32,39,40 In all cases the formation of defects was found to be energetically unfavourable,29,32,39 with acid coordination having the lowest free energy of formation, followed by Cl− (ref. 29) or Cl−/H2O.39 The probability of defect formation can be enhanced by increasing the capping![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC ratio during synthesis.
BDC ratio during synthesis.
The arrangement of defects on a larger scale has also been investigated. Reflections in an X-ray diffraction (XRD) pattern for UiO-66(Hf) synthesised with an acid modulator have been attributed to ordered structures of missing metal clusters with reo-topology (Fig. 1b), named the reo-structure in the following, which leaves the remaining metal clusters coordinated by only 8 linkers.38 A systematic study by Shearer et al. showed an increased intensity of the diffuse reflection with increasing amounts of acid modulator, and with decreasing pKa values, which effectively corresponds to an increasing concentration of deprotonated modulator.16 The presence of diffuse reflections was further shown to correlate with a lower thermal stability of some samples,12,16 however it is also possible to synthesise a crystal where no such reflections are observed.15 This could suggest a lack of ordering of the missing clusters at low defect concentrations (i.e. small domain size), or simply that a different arrangement of defects is preferred in this situation. The energetics of different arrangements of missing linkers around dehydroxylated metal clusters has been investigated computationally,26,32 however to our knowledge no studies comparing the energetics of missing linker and missing metal cluster defects have been performed.
Here we use density functional theory (DFT) to investigate the energetics of different defect arrangements in UiO-66. We present a scheme for calculating the energetic preference for missing metal clusters relative to missing linkers only. Furthermore, we investigate the effect of the different types of defects on the band gap and frontier orbital localisations. Previous investigations have shown that the length of the linker in the UiO-family of materials and the addition of functional groups to the linker can change the electronic properties of the material,41–43 and it is therefore interesting to see if defects can have similar effects. We consider defects capped by trifluoroacetic acid (tfaa, CF3COO−), acetic acid (aa, CH3COO−), formic acid (fa, HCOO−), Cl−/H2O (cl) as well as defects compensated by dehydroxylation of the metal cluster (e). The first four cappings are chosen because they are likely to be present in solution during synthesis, and remain in the structure after drying under mild conditions. tfaa, aa and fa are commonly used as modulators to enhance the formation of defects during synthesis, while Cl− is present when ZrCl4 is used as the Zr source and when HCl is added to the synthesis solution. The e defect only arises upon heating of the structure after synthesis, or as a short-lived state in solution.
2 Methods
2.1 Density functional theory calculations
The Vienna Ab initio Simulation Package (VASP) code44 was used to perform calculations under the Kohn–Sham density functional theory (DFT) framework. The PBEsol exchange–correlation functional45 with D3 dispersion correction46 was used for structure optimisation, using the projector augmented wave method for interaction between core and valence electrons of all atoms in the system. Forces were converged to 0.01 eV Å−1 and SCF convergence was set to 10−8 eV, with a plane wave basis set cutoff of 500 eV. Owing to the dimensions of the unit cell of UiO-66, Γ-point sampling of the Brillouin zone was found to be sufficient. This is also justified by the almost flat bandstructure of the material (c.f. ESI†).For the parent UiO-66 structure the phonon spectrum was calculated to verify that the structure is a local minimum (i.e. has no imaginary frequencies), but calculation of phonons for the defective structures where symmetry is (partially) broken would be computationally unfeasible. We thus compare the enthalpies rather than the free energies in the following, however in previous investigations the influence of entropy on different arrangements with the same number of defects was found to be modest.32
The unit cell volume was optimised for all structures, since the presence of defects can lead to strain in the material. We note that comparison of energies across cells that are not the same size can lead to small errors, which should vanish in the limit of a large energy cutoff. We compared the energy difference between two different defect arrangements (Ed,nt, see Section 3) for the aa capping calculated with a 500 eV and a 600 eV cutoff and found the difference to be less than 10 meV, thus this effect is expected to be small.
Single-point HSE06 (ref. 47 and 48) hybrid DFT calculations were carried out on the optimised geometries of UiO-66 and its defective structures for calculating electronic densities of states (DOS), optical band gap values and frontier orbital compositions. Band alignment was performed following the method of Butler et al., using a plateau in the electrostatic potential in the centre of the MOF pores to align the vacuum potentials of each system.49,50 The average of the electrostatic potential (Φav(r)) is calculated within a defined cube centered at r following the equation:
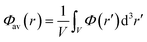 | (1) |
The variance of the electrostatic potential in the sampled volume in the centre of the MOF pores are given in the ESI,† demonstrating that the sampled points correspond to a plateau in the electrostatic potential of the systems. The side length of the cube used for calculating Φav(r) was set to 0.75 Å.
3 Results
3.1 Defect energetics
All calculations are performed in the cubic unit cell which contains 4 metal clusters and 24 linkers for the defect-free structure (Fig. 1a), and the metal clusters are fully hydroxylated except when e defects are present, in which case a hydrogen is removed from the metal cluster for each missing linker.We now want to investigate if a structure with only missing linkers is energetically favoured over the reo-structure. Starting with a given defect concentration corresponding to n missing linkers of type d in the cubic unit cell, it is possible to convert this structure into the reo-structure, which contains missing metal cluster defects, and an amount of defect-free UiO-66. The energy required for this transformation, Ed,nt can be calculated following the equation:
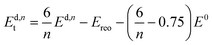 | (2) |
We have calculated Ed,1t and Ed,6t for the five different types of cappings, corresponding to a low defect concentration of 4% and a more realistic concentration of 25%, respectively. The calculation with one defect is straightforward, since there is only one symmetry-inequivalent configuration of one defect in the cubic unit cell, however to get an accurate value of the transformation energy with 6 defects we must in principle first identify the lowest energy configuration of 6 missing linkers. Due to the many possible combinations in this case, it is beyond our computational resources to calculate all the possibilities. Instead we consider two different structures for each capping; one in which each metal cluster has three defects next to each other and the defects are clustered to form a missing tetrahedral cage, and one where the defects are dispersed and each metal cluster has three defects as far from each other as possible (c.f. ESI† for schematics of these structures). Since the two structures represent the limiting cases of clustered and dispersed defects, we would expect an energy difference if there are attractive or repulsive interactions between neighbouring defects. Following the naming convention in ref. 26 the structures would be named (9h,9h,9h,9h)111111111111222 and (9c,9c,9c,9c)333333333333222, respectively. We denote them by 9h (missing tetrahedral cage) and 9c (dispersed defects) in the following.
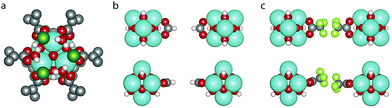
The difference in energy between the two defect arrangements, ΔE6def = E9h − E9c, is given in Table 1, a negative value indicating a preference for the clustered 9h arrangement. It can be seen that the cl capping shows a strong preference for the clustered defects, which arises because it allows the water molecules to make hydrogen bonds with two neighbouring Cl atoms (c.f.Fig. 2a). For the other cappings the numerical value of ΔE6def is much smaller, suggesting that the relative positions of defects have a limited impact on the energy, as has also been found in previous studies comparing different defect arrangements.26,32,39 We have used the lower energy value for our calculations of Ed,nt.
| Capping | e | cl | fa | aa | tfaa |
|---|---|---|---|---|---|
| ΔE6def [eV] | 0.17 | −1.26 | −0.03 | 0.10 | 0.73 |
| E d,1t [eV] | −0.90 | 2.17 | 1.89 | 1.81 | −0.71 |
| E d,6t [eV] | −1.72 | 1.31 | 0.24 | 0.05 | −0.82 |
| pKa | — | — | 3.77 | 4.76 | 0.23 |
The calculated values of the transformation energy are shown in Table 1, for the different choices of charge cappings. Negative values indicate that formation of the reo-structure is preferred, while positive values indicate that a structure with only missing linkers is preferred. The results show that the preferred defect structure depends on which capping agents are available from the synthesis solution, as well as the total number of defects. The cl, fa and aa cappings shows a clear preference for missing linkers at low defect concentrations. For fa and aaEd,6t is small, indicating that both types of defects could be present at higher defect concentrations. For the tfaa and e defects formation of the reo-structure is preferred, however we note that e defects are formed from a structure with capped defects and the defect arrangement is thus expected to be inherited from this parent structure. In ref. 16 the total amount of missing linker defects obtained from thermogravimetric analysis (TGA) was compared to the intensity of the XRD-reflection that characterises the reo-structure. While the data is too sparse to be conclusive it seems plausible that there are differences in the relative amount of reo-structure for different types of modulator, and in particular tfaa appears to give rise to the largest relative amount of reo-structure.
To understand the different preferences for the different cappings we look at the optimised defect structures. Fig. 2b and c shows a missing linker defect with the fa and tfaa cappings. In the fa defect (Fig. 2b) the two cappings point directly towards each other and the unit cell dimensions in the plane of the defect are only slightly changed from 20.74 Å in defect-free UiO-66 to 20.76 Å (see ESI† for unit cell parameters of all optimised structures and comparison with experimental values). For tfaa we investigated two different structures of the single defect, one in which the tfaa cappings point directly towards each other, and one where they are misaligned as seen in Fig. 2c. In both cases the defect leads to an expansion of the unit cell in the plane of the defect to an average of 20.85/20.86 Å for the misaligned/aligned defect. This suggests a steric repulsion between the two cappings as can also be imagined by considering the proximity of atoms in Fig. 2c. Such steric repulsions are only present for a missing linker defect and not in the reo-structure where the distance between opposing cappings is larger, and indeed the reo-structure is found to have lattice parameters of 20.71 Å, close to the value for the defect-free UiO-66. We note that the misaligned tfaa single defect is only 36 meV more stable than the aligned defect, suggesting a soft energy landscape due to the balance between steric repulsion between the tfaa cappings and angular strain when bending the cappings away from the planar configuration. We therefore used several different starting structures including aligned and misaligned configurations for the tfaa structures containing missing ligand(s), however a complete investigation of all possible relative defect misalignments when multiple defects are involved was judged to be computationally unfeasible.
The aa capping is similar in size to the tfaa capping and we have treated it in a similar manner, considering both aligned and misaligned capping arrangements. Again, a small energetic preference for the misaligned configuration is found, however for this capping the missing linker and missing metal cluster structures are almost equal in energy, suggesting a smaller steric effect, as would be expected since the hydrogen atoms have a lower electron density than the fluorine atoms. The fa and cl cappings are both so small that no steric effects are expected, and both show preference for the structure with missing linkers only. For Ed,1t the preference is 0.28 eV larger for cl than for fa, while for Ed,6t it is 1.07 eV larger for cl, which we attribute to the stabilising hydrogen bonding pattern in the 9h missing linker structure (Fig. 2a) which is not formed in the reo-structure. Finally, the e defect shows a clear preference for the reo-structure. This could be a result of strain in the partially dehydrogenated metal clusters of the missing linker structures. It has previously been shown that the metal cluster condenses and becomes asymmetric when the cappings are removed from the reo-structure in UiO-66(Hf) by dehydroxylation (to form e-type defects).19 The partially dehydroxylated metal clusters in our missing linker structures are geometrically distorted from both the hydroxylated and the completely dehydroxylated metal cluster (c.f. ESI for details†).
From the results in Table 1 it appears that formation of the reo-structure becomes more favourable with increasing defect concentration, since Ed,1t is generally higher than Ed,6t, however, since we have not calculated all possible structures with 6 missing linkers the values of Ed,6t in Table 1 could in principle be higher, if a more stable structure with 6 missing linkers existed. To investigate the concentration-dependence further we calculated the transformation energy for aa, cl and e, using the lowest energy defect structures with two and three missing linkers determined in our previous force field calculations39 (aa, cl) and in ref. 26 (e) (see ESI† for schematics of these defect arrangements). The results are plotted in Fig. 3, and show that for aa the preference for missing linkers decreases with increasing defect concentration. A similar trend is observed for cl, however here the transformation energy plateaus for higher defect concentrations, perhaps because more hydrogen bonds can be formed. The curve for the e defect has a different shape, the origin of which we can only speculate about, but formation of the reo-structure is always favoured.
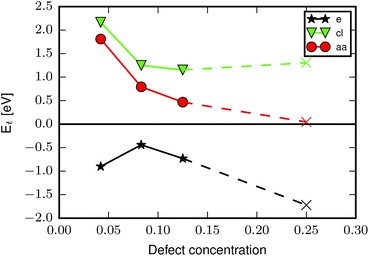 | ||
| Fig. 3 Transformation energy as a function of defect concentration for the aa (red circles), cl (green triangles) and e (black stars) cappings. Circles/triangles/stars mark the concentrations where the best defect configuration has previously been identified by calculating all possible structures,26,39 while × indicates that only selected configurations of missing linkers were optimised at that defect concentration. | ||
The formation of defects has previously been calculated to be energetically unfavourable,29,32,39 however these calculations did not include configurational entropy contributions to the free energy which will lead to an equilibrium population of defects. Furthermore, the concentrations in real samples will be influenced by the kinetics of nucleation and growth. The kinetics can be changed by varying synthesis parameters such as temperature and reaction time, and more intuitively also the ratio of modulator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC in the synthesis. The pKa value determines the fraction of tfaa/aa/fa modulator molecules that are deprotonated in solution at any given time, and thereby the effective modulator
BDC in the synthesis. The pKa value determines the fraction of tfaa/aa/fa modulator molecules that are deprotonated in solution at any given time, and thereby the effective modulator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC ratio. In our calculations we consider the different modulators for a given concentration of defects, which would arise from different molar concentrations of modulator during synthesis. The results suggest that in changing the amount of defects (i.e. the concentration of the modulator), the ratio between missing linker and missing metal cluster defects is also changed.
BDC ratio. In our calculations we consider the different modulators for a given concentration of defects, which would arise from different molar concentrations of modulator during synthesis. The results suggest that in changing the amount of defects (i.e. the concentration of the modulator), the ratio between missing linker and missing metal cluster defects is also changed.
3.2 Electronic structure
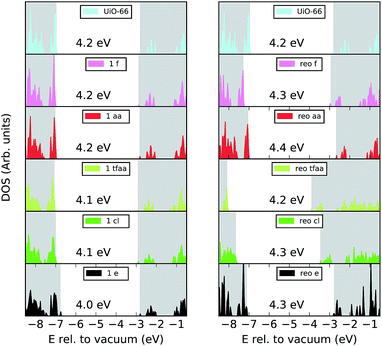
In inorganic semi-conductors a common approach for modulating the optical band gap and electronic properties of a material is to introduce defects.51 The question as to if the missing linker phenomenon also modulates the electronic properties of UiO-66 was recently addressed in ref. 26 in the case of missing linkers only, and considering the e type defect. It was shown that the introduction of defects leads to a small decrease in the band gap and a decrease in the metal to ligand charge transfer energy, which is favourable for photocatalysis. While the absence of cappings gives easier access to the active sites on the metal cluster, such uncoordinated sites are unlikely to be abundant in solution and we therefore investigate the effect of different cappings on the electronic structure of the material.We calculate the electronic density of states (DOS), band alignment and valence orbital localisation for a single missing linker defect and for the reo-structure for the different capping types. The normalised DOS of UiO-66 and its defective structures are shown in Fig. 4. Since the position of the bands relative to the vacuum level is of importance for charge transfer processes (e.g. from the framework to a guest molecule or reactant) the plots are aligned relative to vacuum and the position of the band gap is marked by a white background. Our calculations give a band gap of 4.2 eV for defect-free UiO-66 in good agreement with previous calculations at this level of theory (3.9–4.6 eV) and experimental values which range from 3.8–4.1 eV.12,42,43,52,53Fig. 4 shows that the band gap variation between defect-free UiO-66 and the defective structures are up to 0.2 eV, with the largest changes observed for the single e defect and the aa-capped reo-structure. It is also clear from the plot that the aa and fa cappings preserve the shape and energetic position of the DOS, both for the single defect and for the reo-structure. This is perhaps not surprising given the similarity between these cappings and the BDC linker. For the cl, tfaa and e cappings some broadening of the highest occupied states are observed, and the cl and tfaa capped reo-structures are down-shifted in energy with respect to the defect-free structure.
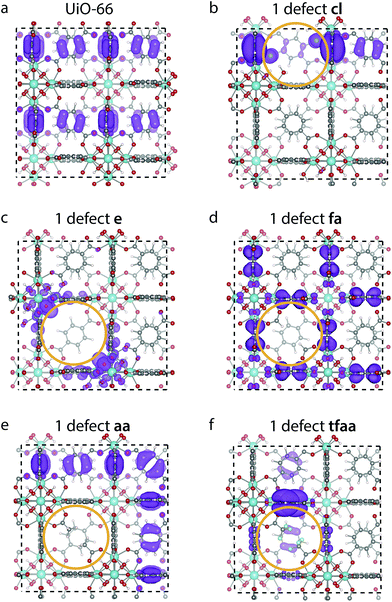
The changes in the DOS can be correlated with the changes in the shape of the frontier orbitals. The highest occupied crystalline orbital (HOCO) for perfect UiO-66 and a single missing linker defect with different cappings is shown in Fig. 5. The HOCO of perfect UiO-66 is delocalised over most of the organic linkers (Fig. 5a), but when a cl capped defect is introduced it becomes localised, with some weight on the Cl atom (Fig. 5b). The HOCO of the e defect likewise becomes localised around the defect, in particular with a high density on the oxygen atoms of the metal cluster (Fig. 5c). In contrast to this the HOCOs of the structures with the aa and fa cappings are still delocalised over many linkers, but with no weight on the cappings themselves (Fig. 5d and e). Finally, for the tfaa capping there is some change in the HOCO, but the density is not located on the defect itself (Fig. 5f). For all cappings the lowest unoccupied molecular orbital (LUCO) is largely unchanged (c.f. ESI†).
A similar investigation of the HOCO and LUCO of the reo-structure (c.f. ESI†) shows that the LUCO is still largely unchanged for all structures. Interestingly, the HOCO of the cl structure no longer has electron density on the Cl atoms, but the orbital is not fully delocalised over all linkers. A similar case is found for tfaa, while the HOCO of the aa and fa cappings again look similar to that of the defect-free structure. Only for the evacuated structure is the density still localised around the defect.
4 Conclusion
We have compared defect structures containing missing linker defects and missing metal clusters for different charge compensation schemes. Our results show that the preferred type of defect depends on the choice of capping, and is influenced by factors such as steric interactions and hydrogen bonding patterns. The preferred defect configuration is furthermore found to depend on the overall concentration of defects. This implies that the type of defects present in the material can be varied by the identity and concentration of the modulator during synthesis.For both missing ligand and missing metal type defects with the cl, fa, aa and tfaa cappings the electronic properties of the material are largely unchanged, and the main effect of the defects is to increase the porosity of the material. Upon removal of the capping to form an e defect, the HOCO becomes partly localised on the defect, indicating that this could be an important site of interaction for guest species. Furthermore, it implies that the excitation from the HOCO to the LUCO changes from a linker-to-linker transition to a metal-to-linker transition, which could affect the lifetime of the excited state.54 This type of defect is therefore particularly interesting in terms of changing the electronic properties of the material, however further experimental and theoretical efforts are needed to determine the availability of such open sites under relevant conditions. Finally, we note that first-principles simulations are limited to relatively small cell representations, so that a realistic distribution of defects will require the development of multi-scale simulation approaches for metal–organic frameworks.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. L. S. is funded by ERC programme grant no. 277757 and J. K. B. is funded by the EPSRC (grant no. EP/G03768X/1). J. D. G. thanks the Australian Research Council for funding through the Discovery Programme. The authors acknowledge computing support from the UK national supercomputing service (Archer), via membership of the UK Materials Chemistry Consortium which is funded by EPSRC (EP/L000202), and from the University of Bath computing services (Balena).References
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Ma and H.-C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- M. A. Nasalevich, M. van der Veen, F. Kapteijn and J. Gascon, CrystEngComm, 2014, 16, 4919–4926 RSC.
- E. A. Dolgopolova and N. B. Shustova, MRS Bull., 2016, 41, 890–896 CrossRef CAS.
- D. S. Sholl and R. P. Lively, J. Phys. Chem. Lett., 2015, 6, 3437–3444 CrossRef CAS PubMed.
- Z. Fang, B. Bueken, D. E. De Vos and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 7234–7254 CrossRef CAS PubMed.
- A. K. Cheetham, T. D. Bennett, F.-X. Coudert and A. L. Goodwin, Dalton Trans., 2016, 45, 4113–4126 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- P. G. Yot, K. Yang, F. Ragon, V. Dmitriev, T. Devic, P. Horcajada, C. Serre and G. Maurin, Dalton Trans., 2016, 45, 4283–4288 RSC.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-g. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 CAS.
- H. Wu, T. Yildirim and W. Zhou, J. Phys. Chem. Lett., 2013, 4, 925–930 CrossRef CAS PubMed.
- G. C. Shearer, S. Chavan, J. Ethiraj, J. G. Vitillo, S. Svelle, U. Olsbye, C. Lamberti, S. Bordiga and K. P. Lillerud, Chem. Mater., 2014, 26, 4068–4071 CrossRef CAS.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS.
- O. V. Gutov, M. G. Hevia, E. C. Escudero-Adán and A. Shafir, Inorg. Chem., 2015, 54, 8396–8400 CrossRef CAS PubMed.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- M. J. Cliffe, J. A. Hill, C. A. Murray, F.-X. Coudert and A. L. Goodwin, Phys. Chem. Chem. Phys., 2015, 17, 11586–11592 RSC.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. - Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- A. W. Thornton, R. Babarao, A. Jain, F. Trousselet and F.-X. Coudert, Dalton Trans., 2016, 45, 4352–4359 RSC.
- P. Ghosh, Y. J. Colón and R. Q. Snurr, Chem. Commun., 2014, 50, 11329–11331 RSC.
- W. Liang, C. J. Coghlan, F. Ragon, M. Rubio-Martinez, D. M. D'Alessandro and R. Babarao, Dalton Trans., 2016, 45, 4496–4500 RSC.
- J. M. Taylor, S. Dekura, R. Ikeda and H. Kitagawa, Chem. Mater., 2015, 27, 2286–2289 CrossRef CAS.
- F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed.
- A. De Vos, K. Hendrickx, P. Van Der Voort, V. Van Speybroeck and K. Lejaeghere, Chem. Mater., 2017, 29, 3006–3019 CrossRef CAS PubMed.
- F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck and D. E. De Vos, Angew. Chem., Int. Ed., 2012, 51, 4887–4890 CrossRef CAS PubMed.
- J. Canivet, M. Vandichel and D. Farrusseng, Dalton Trans., 2016, 45, 4090–4099 RSC.
- M. Vandichel, J. Hajek, F. Vermoortele, M. Waroquier, D. E. De Vos and V. Van Speybroeck, CrystEngComm, 2015, 17, 395–406 RSC.
- S. M. J. Rogge, J. Wieme, L. Vanduyfhuys, S. Vandenbrande, G. Maurin, T. Verstraelen, M. Waroquier and V. Van Speybroeck, Chem. Mater., 2016, 28, 5721–5732 CrossRef CAS PubMed.
- B. Van de Voorde, I. Stassen, B. Bueken, F. Vermoortele, D. De Vos, R. Ameloot, J.-C. Tan and T. D. Bennett, J. Mater. Chem. A, 2015, 3, 1737–1742 CAS.
- M. Vandichel, J. Hajek, A. Ghysels, A. De Vos, M. Waroquier and V. Van Speybroeck, CrystEngComm, 2016, 18, 7056–7069 RSC.
- Y. Jiao, Y. Liu, G. Zhu, J. T. Hungerford, S. Bhattacharyya, R. P. Lively, D. S. Sholl and K. S. Walton, J. Phys. Chem. C, 2017, 121, 23471–23479 CAS.
- C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162–11167 CrossRef CAS PubMed.
- S. Øien, D. Wragg, H. Reinsch, S. Svelle, S. Bordiga, C. Lamberti and K. P. Lillerud, Cryst. Growth Des., 2014, 14, 5370–5372 Search PubMed.
- M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC.
- G. C. Shearer, S. Forselv, S. Chavan, S. Bordiga, K. Mathisen, M. Bjørgen, S. Svelle and K. P. Lillerud, Top. Catal., 2013, 56, 770–782 CrossRef CAS.
- M. J. Cliffe, W. Wan, X. Zou, P. A. Chater, A. K. Kleppe, M. G. Tucker, H. Wilhelm, N. P. Funnell, F.-X. Coudert and A. L. Goodwin, Nat. Commun., 2014, 5, 4176 CAS.
- J. K. Bristow, K. L. Svane, D. Tiana, J. M. Skelton, J. D. Gale and A. Walsh, J. Phys. Chem. C, 2016, 120, 9276–9281 CAS.
- S. Ling and B. Slater, Chem. Sci., 2016, 7, 4706–4712 RSC.
- L.-M. Yang, E. Ganz, S. Svelle and M. Tilset, J. Mater. Chem. C, 2014, 2, 7111–7125 RSC.
- K. Hendrickx, D. E. P. Vanpoucke, K. Leus, K. Lejaeghere, A. Van Yperen-De Deyne, V. Van Speybroeck, P. Van Der Voort and K. Hemelsoet, Inorg. Chem., 2015, 54, 10701–10710 CrossRef CAS PubMed.
- E. Flage-Larsen, A. Røyset, J. H. Cavka and K. Thorshaug, J. Phys. Chem. C, 2013, 117, 20610–20616 CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou and K. Burke, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- A. V. Krukau, O. A. Vydrov, A. F. Izmaylov and G. E. Scuseria, J. Chem. Phys., 2006, 125, 224106 CrossRef PubMed.
- K. T. Butler, C. H. Hendon and A. Walsh, J. Am. Chem. Soc., 2014, 136, 2703–2706 CrossRef CAS PubMed.
- K. T. Butler, A. Walsh and J. M. Frost, MacroDensity, 2017 DOI:10.5281/zenodo.596631.
- A. Walsh and A. Zunger, Nat. Mater., 2017, 16, 964–967 CrossRef CAS PubMed.
- A. S. Yasin, J. Li, N. Wu and T. Musho, Phys. Chem. Chem. Phys., 2016, 18, 12748–12754 RSC.
- S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynska, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC.
- M. Nasalevich, C. Hendon, J. Garcia Santaclara, K. Svane, B. van der Linden, S. Veber, M. Fedin, M. van der Veen, F. Kapteijn, A. Walsh and J. Gascon, Sci. Rep., 2016, 6, 23676 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Further information on defect structures and band alignments, unit cell sizes and frontier orbital compositions. Optimised defect structures are available from DOI: 10.5281/zenodo.1064111. See DOI: 10.1039/c7ta11155j |
| ‡ Present address: Department of Energy Conversion and Storage, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. |
| This journal is © The Royal Society of Chemistry 2018 |