MOF-derived α-NiS nanorods on graphene as an electrode for high-energy-density supercapacitors†
Abstract
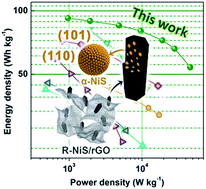
Hierarchically porous electrodes made of electrochemically active materials and conductive additives may display synergistic effects originating from the interactions between the constituent phases, and this approach has been adopted for optimizing the performances of many electrode materials. Here we report our findings in design, fabrication, and characterization of a hierarchically porous hybrid electrode composed of α-NiS nanorods decorated on reduced graphene oxide (rGO) (denoted as R-NiS/rGO), derived from water-refluxed metal–organic frameworks/rGO (Ni-MOF-74/rGO) templates. Microanalyses reveal that the as-synthesized α-NiS nanorods have abundant (101) and (110) surfaces on the edges, which exhibit a strong affinity for OH− in KOH electrolyte, as confirmed by density functional theory-based calculations. The results suggest that the MOF-derived α-NiS nanorods with highly exposed active surfaces are favorable for fast redox reactions in a basic electrolyte. Besides, the presence of rGO in the hybrid electrode greatly enhances the electronic conductivity, providing efficient current collection for fast energy storage. Indeed, when tested in a supercapacitor with a three-electrode configuration in 2 M KOH electrolyte, the R-NiS/rGO hybrid electrode exhibits a capacity of 744 C g−1 at 1 A g−1 and 600 C g−1 at 50 A g−1, indicating remarkable rate performance, while maintaining more than 89% of the initial capacity after 20 000 cycles. Moreover, when coupled with a nitrogen-doped graphene aerogel (C/NG-A) negative electrode, the hybrid supercapacitor (R-NiS/rGO/electrolyte/C/NG-A) achieved an ultra-high energy density of 93 W h kg−1 at a power density of 962 W kg−1, while still retaining an energy density of 54 W h kg−1 at an elevated working power of 46 034 W kg−1.
- This article is part of the themed collection: Materials and Nano Research in Atlanta


 Please wait while we load your content...
Please wait while we load your content...