Crystal structure and compositional effects on the electrical and electrochemical properties of GdBaCo2−xMnxO5+δ (0 ≤ x ≤ 2) oxides for use as air electrodes in solid oxide fuel cells†
Abstract
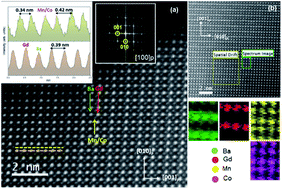
The effects of the substitution of Co with Mn in the crystal structure, oxygen content, thermal stability and expansion and electrical properties of GdBaCo2−xMnxO5+δ (0 ≤ x ≤ 2) oxides are reported. Composites of GdBaCo2−xMnxO5+δ–Ce0.9Gd0.1O2−δ (70 : 30 wt%) are used as cathode materials and their electrochemical behaviour is presented. Layered-type ordering of Ba and Gd cations in the perovskite structure occurs in the whole system when the materials are prepared in argon but only for compositions in the range corresponding to x < 1.4 when the materials are prepared in air. The oxygen content increases with increasing the Mn content, causing thermal stability to improve and thermal expansion to decrease. However, lowering of the dc conductivity and an increase of electrode polarization resistances are observed by Mn substitution for Co. Cation ordering of the Gd and Ba atoms seems to affect the electrochemical properties of the materials.
- This article is part of the themed collection: Advances in Solid State Chemistry and its Applications


 Please wait while we load your content...
Please wait while we load your content...