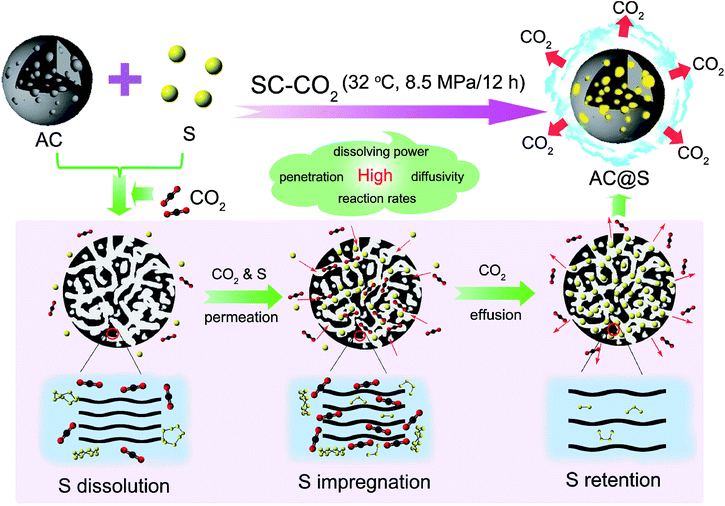Supercritical CO2 mediated incorporation of sulfur into carbon matrix as cathode materials towards high-performance lithium–sulfur batteries†
Ruyi
Fang‡
a,
Chu
Liang‡
a,
Yang
Xia
*a,
Zhen
Xiao
b,
Hui
Huang
a,
Yongping
Gan
a,
Jun
Zhang
a,
Xinyong
Tao
 a and
Wenkui
Zhang
a and
Wenkui
Zhang
 *a
*a
aCollege of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou, 310014, China. E-mail: nanoshine@zjut.edu.cn; msechem@zjut.edu.cn
bCollege of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China
First published on 24th November 2017
Abstract
Lithium–sulfur (Li–S) batteries are considered among the most promising candidates for the next-generation electrochemical power sources. The incorporation of sulfur and carbon matrices is the most appropriate strategy to contain sulfur and suppress the soluble polysulfide shuttle. However, in the conventional methods including mechanical mixing, heat treatment and wet-chemistry synthesis, it is very difficult to guarantee the precise sulfur content, uniform sulfur distribution, and strong interaction between sulfur and carbon. Hence, a novel synthetic strategy that utilizes supercritical CO2 (SC-CO2) to fabricate C@S composites for Li–S batteries has been successfully developed. Taking the advantages of high infiltrability, excellent diffusivity and superior solvability, SC-CO2 not only serves as an intercalator that penetrates into the pores and interlayers of carbon matrices to expand/exfoliate the porous structure and tightly-stacked layered graphite structure, but also plays the role of a marvellous hydrophobic solvent to dissolve sulfur and transfer it into the inner pores and interlayers of carbon matrices. Taking AC@S as an example, it exhibits the highly reversible capacity of 817 mA h g−1 after 100 cycles at 0.1 A g−1, and excellent cycling stability with a satisfactory capacity retention of 90.5%. We believe that this novel strategy will open up the prospects for synthesizing more efficient C/S composites to suppress the diffusion of polysulfides and enhance the structural stability and reaction kinetics of the sulfur cathode.
1. Introduction
Rechargeable batteries are crucial for the fast-growing demands of various applications including consumer electronics, electric vehicles and grid-scale stationary storage systems.1 However, the high cost and low energy density of commercial lithium-ion batteries (LIBs) consisting of a transition-metal oxide cathode and graphite anode are major obstacles to fulfilling the large-scale applications of electric vehicles and smart grids,2 and tremendous efforts have been made to explore new battery systems in recent years. In this respect, the lithium–sulfur (Li–S) battery with high theoretical energy density (2600 W h kg−1), low cost and good environmental benignity is considered as one of the most promising candidates for the next-generation of electrochemical power sources.1,3 Nonetheless, investigations on Li–S batteries are still at the lab stage, compared to LIBs, and several fundamental issues need to be overcome before their commercialization, particularly for the sulfur cathode. (i) Unlike the transition-metal oxide cathode in conventional LIBs, the sulfur cathode involves a multi-electron-transfer electrochemical process that is based on the conversion reaction of S8 + 16Li+ + 16e− → 8Li2S.4,5 Although sulfur can electrochemically react with lithium and provide a high theoretical specific capacity of 1675 mA h g−1, sulfur and its final products of Li2S/Li2S2 are both insulators, leading to poor electrochemical activity and inferior specific capacity. (ii) In the discharging process, a series of intermediate products of long-chain polysulfides (Li2Sn, 4 ≤ n ≤ 8) are dissolved into the liquid electrolyte,6,7 and these soluble polysulfides shuttle between the sulfur cathode and lithium anode, resulting the continuous loss of active material, serious surface passivation of lithium anode and poor coulombic efficiency.8–10To address the aforementioned issues in Li–S batteries, extensive efforts have been devoted to incorporating sulfur into various carbon matrices (e.g. meso/microporous carbon,11–13 hollow carbon spheres,14–17 graphene/graphene oxide18–22 and carbon nanotubes23–29) to construct sulfur/carbon (S/C) composite cathodes. These carbon matrices can simultaneously act as an elaborate conducting framework to contain sulfur and facilitate electron transport, and as a strong adsorbing agent to suppress the soluble polysulfide shuttle, resulting in the significantly enhanced electrochemical performance of Li–S batteries.2,11 Although the incorporation of sulfur and carbon matrices can ameliorate the intrinsic inferior characteristics of the sulfur cathode, a suitable sulfur encapsulation technique is more important for realizing the high sulfur utilization, high cycling stability and rate capability in Li–S batteries. To date, various synthetic strategies have been developed for the incorporation of secondary materials into the carbon matrix, which can be classified as direct incorporation by the assembly process,30 or post-incorporation.31 The advantage of direct incorporation is that the secondary materials can be uniformly embedded into the carbon matrix; however, compared with direct incorporation, post-incorporation is more convenient to operate and the range of application is wider. The synthetic methods for C/S materials mainly involve the latter, which can be generally divided into three major categories including mechanical mixing,32 heat treatment33–36 and wet-chemistry synthesis.37–39 Conventional methods often involve complex manufacturing processes, high toxic solvents and high energy consumption, and it is very difficult to guarantee the precise sulfur content, uniform sulfur distribution and good affinity between sulfur and carbon. Specifically, sulfur can hardly reach the inner pores and voids of carbon matrices via the aforementioned methods, leading the poor dispersion of sulfur and low effective utilization of carbon matrices. Therefore, the development of a more facile, efficient and green strategy is urgently needed for the synthesis of high-performance sulfur cathode in Li–S batteries.
Supercritical fluids (SCFs) are unique solvents with both “gas-like” and “liquid-like” physicochemical properties that offer huge opportunities to manipulate the reaction environment in terms of density, diffusivity, viscosity and surface tension via controlling pressure and temperature.40,41 Particularly, due to the relatively accessible critical parameters (Tc = 31.1 °C, Pc = 7.38 MPa), non-toxicity and non-inflammability, supercritical carbon dioxide (SC-CO2) is the most widely used in many fields such as materials processing, materials drying and separation.42–44 However, to date, the application of SC-CO2 to synthesize S/C composites has rarely been reported.
In this work, we attempt to develop a novel SC-CO2 method for the synthesis of a S/C composite with high sulfur content, uniform sulfur distribution and strong sulfur affinity, as shown in Fig. 1. Compared with routine methods, the exploitation of SC-CO2 has several unparalleled merits. First of all, SC-CO2 is a promising hydrophobic solvent that has the comparable dissolving ability of nonpolar sulfur to that of the highly toxic carbon disulfide (CS2), guaranteeing that sulfur can be effectively dissolved at the molecular level. The surface tension of the interface between carbon matrices and SC-CO2 is much smaller than that of a carbon–liquid (e.g. CS2) interface or a carbon–solid (e.g. sulfur powder) interface, since SC-CO2 has low interfacial tension and high diffusivity. The excellent wetting interfaces allow better penetration of sulfur into the pores of carbon matrices, resulting in homogeneous sulfur distribution and high sulfur content. More interestingly, SC-CO2 as an intercalator can expand and exfoliate tightly-stacked, layered carbon materials during the abrupt pressure release process.45 This ability of SC-CO2 to synchronously tune the layer structure or porous structure of carbon matrices will offer plenty of space to store small sulfur allotropes (S2–4) in carbon matrices. Active carbon (AC) (amorphous carbon), MCMB (graphitic carbon) and MWCNT (1D carbon) are three typical carbon materials, which are selected in this work as examples to study the universality of the SC-CO2 method for synthesizing C/S cathodes in Li–S batteries. It is highly expected that SC-CO2 technology may synchronously realize the highly efficient sulfur transfer and precise microstructure regulation of S/C composites.
2. Experimental
2.1 Materials preparation
All reagents were used as purchased without any further purification. In a typical procedure, 0.5 g carbon (active carbon (AC), mesocarbon microbeads (MCMB), multi-walled carbon nanotubes (MWCNTs)) and 0.6 g sulfur were transferred into a 100 mL stainless-steel milling jar. Subsequently, CO2 (99.9%) was pumped into the milling jar until the pressure reached 8.5 MPa. A planetary ball mill (Nanjing, QM-1SP2) at 350 rpm was used in the milling process, and the ambient temperature was strictly kept at 32 °C for 12 h. After milling, CO2 gas was immediately released, and the as-prepared C@S composites were denoted as AC@S, MCMB@S and MWCNTs@S, respectively. Meanwhile, C–CO2 samples were synthesized under the same conditions without adding sulfur, and were denoted as AC-CO2, MCMB-CO2 and MWCNTs-CO2. In order to make a comparison, the control samples were prepared by the routine melt-diffusion method as well. In detail, sulfur and AC were mixed in a mass ratio of 1.2 to 1, and then the mixture was heated in a vacuum oven at 155 °C for 12 h to obtain the final products, denoted as AC/S-155.2.2 Materials characterization
X-ray diffraction (XRD) patterns were obtained using a Rigaku Ultima IV X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm). The Raman spectra were recorded using a DXR Raman microscope (Thermo Fisher Scientific) with He–Ne 532 nm laser excitation in the range of 200–2000 cm−1. The surface area was determined by the Brunauer–Emmett–Teller (BET) method based on nitrogen adsorption–desorption tests using an ASAP 2020 (Micromeritics Instruments). The pore size distributions were calculated by the Barrett–Joyner–Halenda method. Thermogravimetric analysis was performed on a SDT Q600 (TA Instruments) under Ar flowing atmosphere with a heating rate of 10 °C min−1 from room temperature to 500 °C. The morphology and microstructure were observed using scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, FEI, Tecnai G2 F30) with an energy dispersive spectroscopy (EDS) detector. X-ray photoelectron spectroscopy (XPS) was performed on a Kratos Analytical spectrometer with an Al Kα monochromatic X-ray source. The peak positions were calibrated based on the C 1s peak at 284.8 eV.2.3 Electrochemical measurements
The cathode slurry was comprised of 10 wt% Super-P, 10 wt% polyvinylidene fluoride (PVDF) binder and 80 wt% active materials. The resultant viscous slurry was coated onto Al foil via a glass rod. The working electrodes were dried in a vacuum oven at 60 °C overnight. The areal mass loading of sulfur on each electrode was approximately 2.1 mg cm−2. Lithium foil and Celgard 2300 membrane were used as the anode and separator, respectively. The electrolyte was 1 M LiCF3SO3 and 0.2 M LiNO3 in the co-solvent mixture of 1,2-dimethoxyethane and 1,3-dioxolane (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The volume of electrolyte in the coin cells was approximately 80 μL (electrolyte/sulfur ratio = 15 μL mg−1). Cyclic voltammogram (CV) measurements were recorded on a CHI650B electrochemical workstation (Chenhua, Shanghai, China) at a scanning rate of 0.1 mV s−1 in the voltage range of 1.8–2.6 V. The galvanostatic charge–discharge profiles were recorded on a battery testing system (Shenzhen Neware Technology Co. Ltd.) at various current densities in the voltage window of 1.8–2.6 V. All electrochemical tests were performed based on CR2025 button cells in ambient environment.
1). The volume of electrolyte in the coin cells was approximately 80 μL (electrolyte/sulfur ratio = 15 μL mg−1). Cyclic voltammogram (CV) measurements were recorded on a CHI650B electrochemical workstation (Chenhua, Shanghai, China) at a scanning rate of 0.1 mV s−1 in the voltage range of 1.8–2.6 V. The galvanostatic charge–discharge profiles were recorded on a battery testing system (Shenzhen Neware Technology Co. Ltd.) at various current densities in the voltage window of 1.8–2.6 V. All electrochemical tests were performed based on CR2025 button cells in ambient environment.
3. Results and discussion
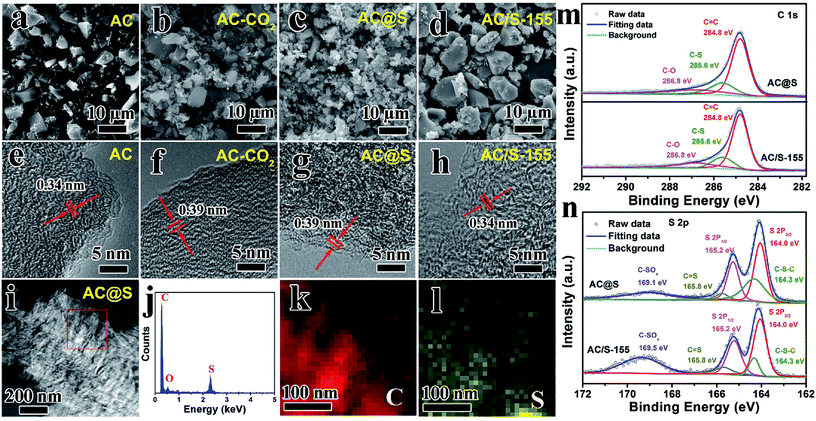
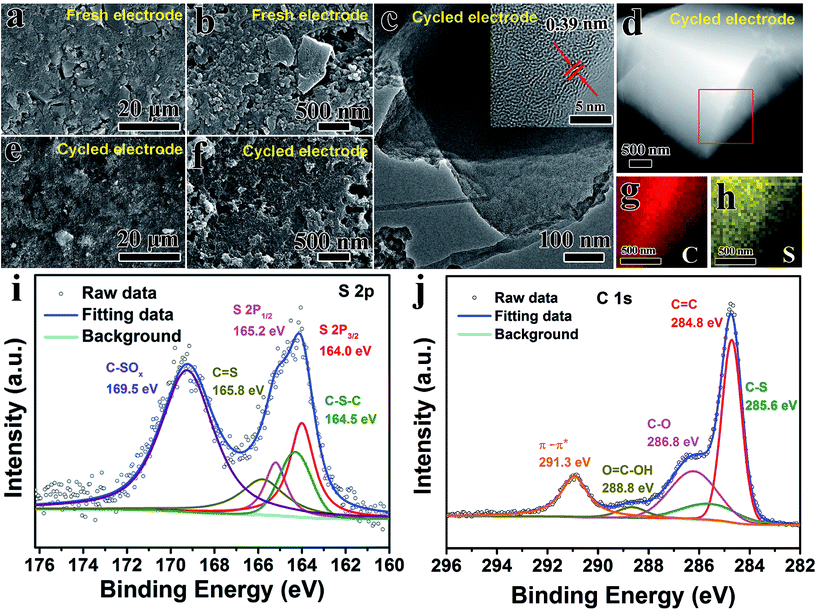
Fig. 2a and b vividly illustrates the morphology and structure of AC before/after the SC-CO2 process. The pristine AC sample is composed of irregular particles with size of ca. 5 μm (Fig. 2a). After SC-CO2 treatment, the particle size of AC-CO2 sample gradually decreased; some small cracked particles are observed in Fig. 2b. Additionally, when sulfur was incorporated into AC matrices via the SC-CO2 process, lots of fine particles were obtained in the AC@S sample, and the particle size was smaller than that of AC-CO2. The reason for this phenomenon could be explained as follows. During the SC-CO2 process, sulfur penetrates into the pores of the AC matrices with the assistance of the SC-CO2 fluid. When exposed, there was an abrupt decrease in pressure, SC-CO2 fluid was immediately transformed into CO2 gas, resulting in a large pressure difference between the inner pores and the ambient environment. Synchronously, sulfur remained in the pores and in intervals of AC matrices, causing huge mechanical stress. As a result, the AC@S sample was crushed into small pieces, compared to the AC-CO2 sample. However, the AC/S-155 sample prepared by the routine melt-diffusion method had no distinct change in morphology or particle size (Fig. 2d). Furthermore, the effects of the SC-CO2 process on MCMB and MWCNTs carbon matrices were also investigated. As shown in Fig. S1a,† MCMB the sample has a typically spherical morphology. After SC-CO2 treatment, MCMB-CO2 and MCMB@S samples were both exfoliated into small pieces of sheet-like particles (Fig. S1b and c†). Interestingly, there was no obvious morphological change in the MWCNTs sample (Fig. S2a–c†). MWCNTs-CO2 and MWCNTs@S samples still maintained the 1D tube-like structure. Since the microstructures of the three carbon matrices are quite different, this might be responsible for the significant morphological changes. The MCMB sample is composed of planar and curved graphitic layers via weak van der Waals forces, therefore, SC-CO2 can directly intercalate into the intervals of layers in MCMB and exfoliate it into graphite sheets. The MWCNTs sample is dominated by strong C–C bonds that are not easily exfoliated or broken during the SC-CO2 process.The inner microstructure and chemical composition of AC, AC-CO2, AC@S and AC/S-155 samples were observed by high-resolution TEM (HRTEM), scanning TEM (STEM) and EDS tests. As shown in Fig. 2e–h, the interplanar distances of AC-CO2 and AC@S samples are both 0.39 nm, which is larger than that of AC (0.34 nm), indicating that well-defined expansion exists in the graphitic interlayers of AC. This phenomenon can also be detected in MCMB and MWCNTs samples as shown in Fig. S3a, b and S4a–c.† After incorporating sulfur into MCMB via the SC-CO2 process, the interlayer spacing between two lattice fringes expands from 0.34 to 0.39 nm. Such enlarged interlayer spacing in carbon matrices is beneficial for increasing sulfur content, suppressing the polysulfide shuttle, and facilitating electrolyte penetration. Furthermore, according to the STEM micrograph and EDS results (Fig. 2i–l and S3e–h†), the main elements in AC@S and MCMB@S samples are sulfur and carbon, matching well with the chemical composition of AC@S and MCMB@S. The EDS mapping results vividly display that the sulfur mapping and carbon mapping are well overlapped, indicating that sulfur is homogeneously embedded into carbon matrices.
The high-resolution C 1s and S 2p spectra of AC@S and AC/S-155 composites are exhibited in Fig. 2m and n. As depicted in Fig. 2m, the high-resolution C 1s spectra of AC@S and AC/S-155 samples can be assigned to three peaks with binding energies of 284.8 eV (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 286.8 eV (C–O), and 285.6 eV (C
C), 286.8 eV (C–O), and 285.6 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S).26,46 Additionally, according to the high-resolution S 2p spectra, the peaks located at 164.3 eV and 165.8 eV are attributed to C–S–C and C
S).26,46 Additionally, according to the high-resolution S 2p spectra, the peaks located at 164.3 eV and 165.8 eV are attributed to C–S–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, respectively, whereas the peak at 169.1 eV is related to C–SOx species.46,47 It is worth noting that the percentage of C–S–S/C
S, respectively, whereas the peak at 169.1 eV is related to C–SOx species.46,47 It is worth noting that the percentage of C–S–S/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S in AC@S is higher than that of AC/S-155 (Table S1†), suggesting the better interaction between C and S in the AC@S sample. Moreover, the intensity of C–SOx species in AC/S-155 is much higher than AC@S, indicating that sulfur is easily oxidized during the melt-diffusion process.
S in AC@S is higher than that of AC/S-155 (Table S1†), suggesting the better interaction between C and S in the AC@S sample. Moreover, the intensity of C–SOx species in AC/S-155 is much higher than AC@S, indicating that sulfur is easily oxidized during the melt-diffusion process.
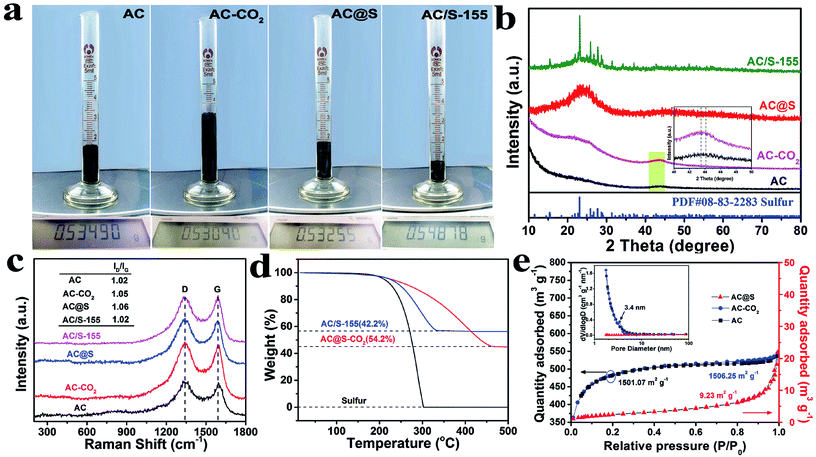
In order to visually display the volume changes of various carbon matrices before/after SC-CO2 treatment, the digital photographs of AC, MCMB and MWCNTs are presented in Fig. 3a, S1d and S2d,† respectively. Although all the samples have detectable volume expansion compared to their raw materials, the volumes of AC-CO2 and MCMB-CO2 samples are drastically expanded two times and three times, respectively. As shown in Fig. S5,† the apparent densities of AC (0.3 g cm−3), MCMB (0.58 g cm−3) and MWCNTs (0.1 g cm−3) decreased sharply to 0.15 g cm−3 (AC-CO2), 0.11 g cm−3 (MCMB-CO2) and 0.07 g cm−3 (MWCNT-CO2), respectively. Meanwhile, the apparent densities of AC@S, MCMB@S and MWCNTs@S increased to 0.28 g cm−3, 0.19 g cm−3 and 0.1 g cm−3, respectively, implying that sulfur was successfully incorporated into the carbon matrices. Combined with the aforementioned TEM results, the corresponding expansion mechanism can be described as follows. SC-CO2 as a nonpolar solvent has a powerful dissolving capability, and can easily dissolve nonpolar sulfur powder at the molecular level.48 Therefore, CO2 molecules and sulfur molecules carried by SC-CO2 can penetrate into graphitic layers and pores of carbon matrices and remain as stable species during the initial stage of the SC-CO2 process. When exposed to an abrupt decrease in pressure, the intercalated CO2 molecules immediately transform into CO2 gas, which creates a large pressure difference between graphitic layers/inner pores and the ambient environment. This large pressure difference can generate enough force to peel off graphitic sheets along the c-axis direction or greatly weaken the van der Waals interplanar interactions of carbon matrices. Meanwhile, the dissolved sulfur remains at the interlayers or pores of carbon matrices when SC-CO2 fluid transforms to CO2 gas. As a result, the SC-CO2 process will not only enlarge the interlayer spacings or pores of carbon materials, but also may exfoliate graphite into small graphitic sheets to further increase the specific surface area and volume of carbon materials. More importantly, these expanded interlayer spacings, pores and increased specific surface area will be favorable for impregnating sulfur and trapping polysulfides, which may greatly enhance the electrochemical performance in Li–S batteries.
The phase structures of AC, AC-CO2, AC@S and AC/S-155 samples are further analyzed by XRD. As shown in Fig. 3b, AC and AC-CO2 samples have two broad diffraction peaks at around 23° and 45°, indicating that the SC-CO2 process does not alter the amorphous structure of the AC sample. After impregnating sulfur into the AC matrices with the assistance of SC-CO2 fluid, the XRD pattern of AC@S was similar to AC and AC-CO2 samples, and no obvious sulfur peaks were detected in the AC@S sample. It is worth noting that after SC-CO2 treatment the phase structure of the pristine sulfur does not change, and it retains good crystallinity as shown in Fig. S6.† The above results demonstrate that sulfur is completely encapsulated within the interlayers and pores of AC matrices with a uniform distribution. In contrast, the well-defined diffraction peaks of crystalline S8 exist in the AC/S-155 sample, revealing that there are large numbers of sulfur particles still on the surface of the AC matrices. This result also confirms that the SC-CO2 method is more effective than the thermal diffusion method for achieving the highly homogeneous distribution of sulfur in carbon matrices. Additionally, due to the graphitic structure of MCMB, which is quite different from AC, the MCMB and MCMB@S samples were employed to verify the differences in using amorphous carbon and crystalline carbon as sulfur hosts during the SC-CO2 process. As shown in Fig. S7a,† the MCMB sample shows one sharp peak at around 26.5°, which belongs to the typical diffraction peak of graphite. Interestingly, this peak shifts to a lower angle after sulfur incorporation via the SC-CO2 treatment. This result vividly proves that the interlayer spacings of MCMB are greatly enlarged during SC-CO2 treatment, matching TEM results (Fig. S3a–d†). Moreover, the diffraction peaks arising from the sulfur phase can be clearly detected in MCMB@S (Fig. S7a†) and MWCNTs@S (Fig. S4d†) samples, implying that some sulfur particles may still attach to the surface of MCMB@S and MWCNTs@S samples. Thus, it can be deduced that amorphous AC as sulfur hosts may be better than MCMB and MWCNTs for fabricating C@S composites via SC-CO2 method.
The degrees of graphitization of AC, AC-CO2, AC@S and AC/S-155 samples were identified by Raman spectroscopy analysis. As shown in Fig. 3c, all the samples exhibited two broad peaks located at 1350 and 1595 cm−1, confirming the coexistence of disordered graphite (D band) and crystalline graphite (G band).16 Generally, the intensity ratio of the D band to the G band (ID/IG) is an important index for evaluating the graphitization degree of carbon materials. A small ID/IG value means a high degree of graphitic crystallinity. After careful comparison, ID/IG ratios of AC-CO2 and AC@S samples were 1.05 and 1.06, respectively, which are slightly larger than that of AC and AC/S-155 samples, suggesting that some defects emerge in AC after the SC-CO2 process. The same tendency also can be found in MCMB based samples (Fig. S7b†). The small value of the ID/IG ratio in the AC@S sample implies good electrical conductivity, which is favorable for boosting the electron transfer of electrochemical reactions. Moreover, there was no sulfur related peak in the, AC@S sample, whereas two sharp peaks of sulfur located at 220 and 470 cm−1, were detected in the MCMB@S sample (Fig. S7b†). This result is consistent with XRD results, which demonstrates that many sulfur particles remain on the surface of carbon matrices, and are not completely embedded into pores, interlayers and voids of carbon matrices.
To quantify the sulfur content in AC@S and AC/S-155 samples, TGA measurements are carried out. As shown in Fig. 3d, large weight losses exist in AC@S and AC/S-155 samples, suggesting that the actual sulfur contents in AC@S and AC/S-155 are 54.2 wt% and 42.2 wt%, respectively. Apparently, an inevitable sulfur loss occurs during the sulfur impregnation via the routine melt-diffusion method. Meanwhile, the weight loss of the AC@S sample finally ends at 450 °C, which is much higher than that of AC/S-155 (350 °C) and pristine sulfur (300 °C). These results reveal that SC-CO2 technology is not only a facile method for realizing the highly efficient impregnation of sulfur into carbon matrices, but can also achieve the enhanced thermal stability of sulfur and the strong affinity between sulfur and AC matrices.
Fig. 3e shows N2 adsorption–desorption isotherm curves and pore size distributions of AC, AC-CO2 and AC@S samples. A joint curve of the type I N2 adsorption–desorption isotherm confirms the existence of micropores in the AC sample. The specific surface area and pore volume of the AC sample were 1501.07 m2 g−1 and 0.694 cm3 g−1, respectively. After SC-CO2 treatment, the specific surface area and pore volume of the AC-CO2 sample slightly increased to 1506.25 m2 g−1 and 0.696 cm3 g−1, respectively. It is worth noting that the specific surface area of the MCMB sample (<2 m2 g−1) was drastically increased to 23.0 m2 g−1 (MCMB-CO2 sample), as depicted in Fig. S7c,† which demonstrates that the SC-CO2 process can expand the interplanar spacings and exfoliate graphite into graphitic sheets. However, after sulfur impregnation, it was found that the micropores and mesopores in AC matrices disappeared, and the specific surface area of the AC sample also sharply decreased from 1501.07 m2 g−1 to 9.23 m2 g−1, indicating that sulfur was successfully impregnated into the AC matrices. Meanwhile, the specific surface area of MCMB@S (11.9 m2 g−1) was larger than that of pristine MCMB (<2 m2 g−1), but smaller than that of MCMB-CO2 (23.0 m2 g−1). The distinct surface area changes further confirmed that SC-CO2 can tune the microstructure of carbon matrices to create more space for sulfur storage, and synchronously guarantee the highly efficient sulfur transfer in carbon matrices.
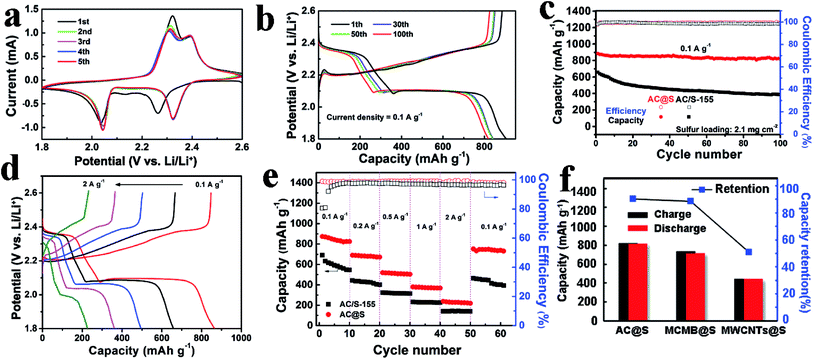
The lithium storage properties of AC@S sample were evaluated by CR2025 button cells. Fig. 4a displays the CV profiles of AC@S sample, which exhibit the typical features of multi-electron redox reactions in sulfur cathodes.49 Two cathodic peaks at 2.3 V and 2.0 V can be assigned to the stepwise reduction from S8 to soluble long-chain polysulfides (Li2Sn, 8 ≥ n ≥ 4) and further to insoluble Li2S2/Li2S.23 In the subsequent anodic scanning, two asymmetric oxidation peaks were observed at 2.3 and 2.4 V, which correspond to the reversible conversion of Li2S2/Li2S to Li2S8 and S.23 Notably, the succeeding CV profiles overlapped well with each other, suggesting a relatively good cycling stability. Moreover, the galvanostatic charge–discharge curves of the AC@S sample for the 1st, 30th, 50th and 100th cycles at 0.1 A g−1 are presented in Fig. 4b. Two discharge plateaus and two charge plateaus can be clearly detected, which are consistent with the CV results (Fig. 4a). During the 1st cycle, a high specific capacity of 905 mA h g−1 was obtained. After that, AC@S electrode still retained a stable specific capacity of 817 mA h g−1 after 100 cycles at 0.1 A g−1, along with a good capacity retention of 90.5% and a superior coulombic efficiency of approximately 100%.
In order to better clarify the merits of the AC@S sample, the long-term cycling performance comparison between AC@S and AC/S-155 was conducted. As shown in Fig. 4c, the AC@S electrode has a high reversible specific capacity and outstanding cyclic stability, compared to the AC/S-155 sample. After 100 cycles at 0.1 A g−1, the reversible discharge/charge capacities of AC@S and AC/S-155 were 819/817 mA h g−1 and 394/386 mA h g−1, respectively. Moreover, the capacity retention of AC@S was 90.5%, which is much higher that of AC/S-155 (58.7%). This remarkable cycling stability of the AC@S sample can be attributed to two factors. Firstly, with the assistance of SC-CO2, the AC@S sample has a more homogenous sulfur dispersion, guaranteeing good contact between sulfur and the conductive carbon framework. Secondly, SC-CO2 aids sulfur to deeply infiltrate the inner pores and interlayers of AC and form strong interactions between sulfur and AC, which could trap sulfur and polysulfides during cycling.
To verify the existence of sulfur in the interlayers of the AC matrices, we designed the following experiment to get deep insight into the Li storage mechanism of the AC@S sample. The AC@S sample was repeatedly washed by CS2 solution to remove sulfur in the pores or on the surface of the AC matrices. It should be mentioned that if sulfur is stably encapsulated in the interlayers, it will be hardly removed by CS2. As shown in Fig. S8a,† three reduction peaks appeared at 2.3 V, 2.0 V and 1.9 V. Two peaks located at 2.3 V and 2.0 V were assigned to the stepwise reduction from S8 to soluble long-chain polysulfides (Li2Sn, 8 ≥ n ≥ 4) and further to insoluble Li2S2/Li2S, corresponding to the characteristic electrochemical reactions of S8. This result indicates that sulfur (S8) can reach into the deep pores of AC matrices with the assistance of SC-CO2 fluid. Interestingly, a novel electrochemical behavior was observed, in which a reduction peak was detected at 1.9 V. According to the literature,56 small sulfur allotropes with chain-like structure, S2–4, have at least one dimension ≤0.39 nm. Thus, this peak is consistent with the fact that small S2–4 molecules instead of large cyclo-S8 exist in the composite.56 Furthermore, the HRTEM image (Fig. S8b†) shows that the lattice spacing of the AC@S sample washed by CS2 is 0.39 nm, which is suitable to accommodate small S2–4 molecules. EDS mapping results (Fig. S8c†) also vividly demonstrate that the sulfur signal is overlapped with carbon signal, implying the existence of sulfur and good distribution in AC matrices. It can therefore be concluded that a part of the small sulfur allotropes (S2–4) penetrated into the interlayers of carbon via the SC-CO2 method, which is favorable for alleviating the dissolution and shuttling problems of polysulfides.
The rate performances of AC@S and AC/S-155 electrodes are also compared in this work. Fig. 4d clearly illustrates the charge and discharge profiles of AC@S electrodes under various current densities from 0.1 to 2 A g−1. All the charge and discharge curves of the AC@S sample exhibit well-defined voltage plateaus and small polarization along with the gradually increased current density. This result vividly demonstrates that the AC@S sample has a superior electrochemical reversibility and fast electrochemical kinetics. Furthermore, as shown in Fig. 4e, the specific discharge capacities of the AC@S sample are 882, 697, 523, 381 and 242 mA h g−1 at 0.1, 0.2, 0.5, 1 and 2 A g−1, respectively, which are much higher than those of the AC/S-155 sample; the specific discharge capacity of the AC@S sample can be recovered well to 762 mA h g−1 at 0.1 A g−1 after multi-rate tests. In contrast, the specific capacity of the AC/S-155 sample fades very fast with increasing current density, and only retains 392 mA h g−1 after recycling at 0.1 A g−1. Obviously, both the specific capacity and coulombic efficiency of the AC@S samples are higher than that of AC/S-155 samples. This remarkable improvement in the rate performance of the AC@S sample can be greatly attributed to the strong interaction between the sulfur and carbon matrix with the aid of SC-CO2.
The universality of the SC-CO2 method was validated by the MCMB@S and MWCNTs@S samples. Fig. 4f and S9† present the charge/discharge capacity and capacity retention of three different C@S cathodes. After 100 cycles at 0.1 A g−1, the reversible discharge capacities of AC@S, MCMB@S and MWCNTs@S were 817, 715 and 441 mA h g−1, corresponding to capacity retentions of 90.5%, 88.6% and 51.2%, respectively. Meanwhile, as shown in Fig. S9 and Table S2,† the specific capacities, capacity retentions and coulombic efficiencies of C/S-155 samples prepared by melt-diffusion method were lower than that of C@S samples derived from the SC-CO2 method. These results confirm that the SC-CO2 method may be suitable for different carbon matrices to achieve various C@S cathodes with fascinating Li storage performance. Additionally, to better demonstrate the advantages of the SC-CO2 method, Table 1 provides a comparison of various synthesis methods for carbon–sulfur composites. Obviously, the SC-CO2 synthetic strategy significantly surpasses other conventional synthesis methods of carbon–sulfur composites. The merits of the SC-CO2 method can be summarized as follows. (1) The reaction media used in the SC-CO2 method is SC-CO2 fluid, which is a cheap, clean and non-toxic solvent. However, most of the routine methods (such as the dissolution method and melt-diffusion method) often use highly toxic CS2 as the solvent, which is not environmentally benign, and will even cause serious health issues for humans. (2) Compared to chemical techniques (chemical deposition and electrochemical deposition), the SC-CO2 method does not involve complex chemical reactions and expensive chemical reagents, and the final products need not be further purified. (3) The SC-CO2 method can guarantee the uniform sulfur distribution and high utilization of carbon matrices. In contrast, the pore-channel structure of the carbon matrix is easily blocked during the melt-diffusion process, meanwhile the sulfur precursor solution could not penetrate well into carbon matrices during the chemical synthesis process. Thus, it is difficult to take the advantages of carbon matrices with high specific surface area and abundant porous structures. (4) The reaction temperature of the SC-CO2 method is only 32 °C, which is much lower than that of the vapor-phase infiltration method (500 °C) and the melt-diffusion method (155 °C); therefore, the SC-CO2 method can save more energy and cut down the cost. (5) The electrochemical performances of the carbon–sulfur composites prepared via the SC-CO2 method surpasses those of the samples derived from other methods. Therefore, the reversible specific capacity, long-term cycling stability and coulombic efficiency have been greatly enhanced with the assistance of the SC-CO2 method.
| Method | Sulfur source (solvent) | Temperature (°C) | Capacity (mA h g−1)/efficiency (%) | Sulfur distribution | Cost | Toxicity | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Physical techniques | Ball-milling | S(—) | RT | 643 | Bad | Low | Non-toxic | 32 |
| S(—) | RT | 615/96 | 50 | |||||
| Dissolution | S(CS2) | RT | 630/∼100 | Poor | Low | Highly toxic | 51 | |
| S(CS2) | RT | 541/∼98 | 22 | |||||
| S(CS2) | RT | 466/∼100 | 52 | |||||
| Vapor-phase infiltration | S(—) | 500 | ∼700/96 | Good | Moderate | Low toxic | 36 | |
| Melt-diffusion | S(CS2) | 155 | 792/92.8 | Good | Moderate | Highly toxic | 35 | |
| S(CS2) | 155 | 797.9/96 | 53 | |||||
| S(CS2) | 155 | 670/98.7 | 13 | |||||
| SC-CO2 | S(SC-CO2) | 32 | 817/∼100 | Good | Low | Non-toxic | This work | |
| Chemical techniques | Chemical deposition | Na2S + S(H2O) | 60 | 826/97 | Poor | High | Low toxic | 54 |
| C6H5NH2 + S(H2O) | 15 | 633.1/98 | 37 | |||||
| Electrochemical deposition | H2S/(Na2S + S + H2O) | RT | 756/∼90 | Poor | High | Highly toxic | 55 | |
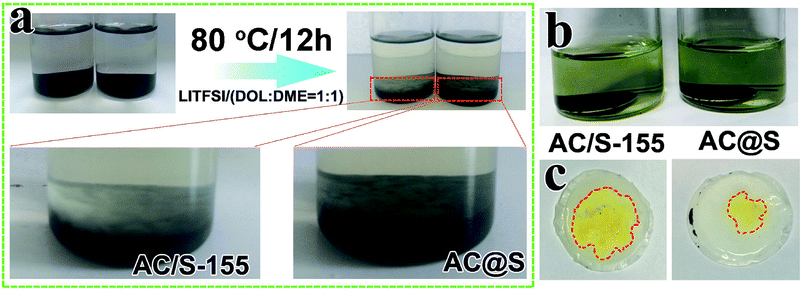
The structure stability and polysulfide adsorption capability of AC@S and AC/S-155 cathodes have been studied. As shown in Fig. 5a, AC@S and AC/S-155 samples were directly soaked in the electrolyte. After soaking for 12 h at 80 °C, the white flocculent precipitate was detected on the surfaces of both the AC@S and AC/S-155 samples. The color of the mixed solution also had a visible change, in which the colorless transparent solution became light yellow. However, compared to the AC@S sample, the AC/S-155 sample had more flocculent precipitate. The color of the AC@S sample was lighter, compared to the AC/S-155 sample. These results indicate that sulfur is tightly enveloped in the AC via the SC-CO2 method, compared to the routine melt-diffusion method. Moreover, the cycled cells of the AC@S and AC/S-155 samples were disassembled to verify their polysulfide trapping capabilities. Fig. 5b displays that the cycled cathodes with absorbed electrolyte were directly soaked into the DOL/DME (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solution. It is clear that the solution of AC@S cathode remained light yellow, suggesting that polysulfides are strictly confined within AC matrices. In contrast, a yellow solution of AC/S-155 sample was obtained after soaking, implying that the AC/S-155 sample (melt-diffusion method) could not effectively prevent polysulfides from continuously dissolving into the electrolyte during the long-term cycling. Additionally, as illustrated in Fig. 5c, only trace amounts of yellow polysulfides could be observed on the surface of the separator from the AC@S cell. In contrast, the yellow area and color depth on the separator of the AC/S-155 cell were much larger and deeper, compared to the AC@S cell. These observations clearly reveal that the AC@S cathode has superior structure stability during the long-term cycling.
1) mixed solution. It is clear that the solution of AC@S cathode remained light yellow, suggesting that polysulfides are strictly confined within AC matrices. In contrast, a yellow solution of AC/S-155 sample was obtained after soaking, implying that the AC/S-155 sample (melt-diffusion method) could not effectively prevent polysulfides from continuously dissolving into the electrolyte during the long-term cycling. Additionally, as illustrated in Fig. 5c, only trace amounts of yellow polysulfides could be observed on the surface of the separator from the AC@S cell. In contrast, the yellow area and color depth on the separator of the AC/S-155 cell were much larger and deeper, compared to the AC@S cell. These observations clearly reveal that the AC@S cathode has superior structure stability during the long-term cycling.
In order to gain deep insight into the chemical composition and microstructure evolution of the AC@S electrode, SEM, TEM, XPS and BET tests of the cycled AC@S electrodes were further carried out. The morphology of the fresh AC@S electrode is illustrated in Fig. 6a and b. It was found that AC@S and Super-P particles were uniformly dispersed on the surface of the fresh electrode. After 100 cycles, although the surface of the AC@S electrode became smooth (Fig. 6e and f), the structural integrity of the cycled AC@S electrode was maintained well, suggesting the superior cycling stability of the AC@S electrode. In addition, on the micro scale, TEM and HRTEM images (Fig. 6c) showed the same morphology and lattice spacing as the fresh electrode. EDS mapping results (Fig. 6d, g and h) confirmed that the bright and strong S signal overlapped well with the C signal after the long-term cycling, indicating that sulfur was still embedded uniformly in the cycled electrode. Moreover, the XPS results (Fig. 6i and j) further revealed that C–S–C (164.3 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (165.8 eV) and C–SOx species (169.1 eV) existed in the cycled AC@S electrode (Fig. 6i). However, compared with the fresh AC@S sample (Fig. 2n), the intensity of the C–SOx species in the cycled AC@S electrode was dramatically enhanced. Additionally, apart from C–C (284.8 eV), C–S (285.6 eV) and C–O (286.8 eV), a peak at 288.8 eV in the cycled AC@S electrode (Fig. 6j) was assigned to the carboxyl group (O
S (165.8 eV) and C–SOx species (169.1 eV) existed in the cycled AC@S electrode (Fig. 6i). However, compared with the fresh AC@S sample (Fig. 2n), the intensity of the C–SOx species in the cycled AC@S electrode was dramatically enhanced. Additionally, apart from C–C (284.8 eV), C–S (285.6 eV) and C–O (286.8 eV), a peak at 288.8 eV in the cycled AC@S electrode (Fig. 6j) was assigned to the carboxyl group (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH).26 The enhanced intensity of the C–SOx species and the new peak related to the carboxyl group may be due to the decomposition of electrolyte and the side reaction during long-term cycling. However, as shown in Fig. 6i and j, the interaction between C and S was still retained well after the long-term cycling, implying good affinity between sulfur and AC. Besides, the specific surface area of AC@S slightly increased to 25.46 m2 g−1 after 100 cycles (Fig. S10†). However, the specific surface area of AC@S was still much lower than that of pristine AC and AC-CO2. The increased specific surface area of the cycled AC@S sample may be attributed to the following. On the one hand, a small part of sulfur that is not well trapped by AC matrices will transform into polysulfides and further dissolve in the electrolyte, which may contribute to the specific surface area. On the other hand, Super-P with a large specific surface area was added to the electrode, and it cannot be completely removed from the cycled electrode when doing BET tests. The added Super-P will also increase the specific surface area of the cycled electrode, but this specific surface area change is very small in the cycled AC@S electrode. Therefore, it is easy to conclude that the SC-CO2 method is a facile and effective synthetic strategy for fabricating C@S cathodes with high cycling stability and strong polysulfide trapping capability.
C–OH).26 The enhanced intensity of the C–SOx species and the new peak related to the carboxyl group may be due to the decomposition of electrolyte and the side reaction during long-term cycling. However, as shown in Fig. 6i and j, the interaction between C and S was still retained well after the long-term cycling, implying good affinity between sulfur and AC. Besides, the specific surface area of AC@S slightly increased to 25.46 m2 g−1 after 100 cycles (Fig. S10†). However, the specific surface area of AC@S was still much lower than that of pristine AC and AC-CO2. The increased specific surface area of the cycled AC@S sample may be attributed to the following. On the one hand, a small part of sulfur that is not well trapped by AC matrices will transform into polysulfides and further dissolve in the electrolyte, which may contribute to the specific surface area. On the other hand, Super-P with a large specific surface area was added to the electrode, and it cannot be completely removed from the cycled electrode when doing BET tests. The added Super-P will also increase the specific surface area of the cycled electrode, but this specific surface area change is very small in the cycled AC@S electrode. Therefore, it is easy to conclude that the SC-CO2 method is a facile and effective synthetic strategy for fabricating C@S cathodes with high cycling stability and strong polysulfide trapping capability.
4. Conclusions
In conclusion, a facile, low cost and environment friendly SC-CO2 synthetic strategy has been successfully developed to fabricate C/S composite cathodes in Li–S batteries. Taking the advantages of high infiltrability, excellent diffusivity and superior solvability, SC-CO2 plays multiple roles in achieving the highly efficient sulfur transfer and highly homogeneous sulfur distribution in carbon matrices. On the one hand, SC-CO2 serves as intercalator, penetrating into pores, voids and interlayers of carbon matrices to expand and exfoliate the porous structure and tightly-stacked layered graphite structure. As a result, it can create extra space to store more sulfur in carbon matrices. On the other hand, SC-CO2 as a marvellous hydrophobic solvent has the powerful solubility of nonpolar sulfur. Hence, sulfur could be completely dissolved in SC-CO2 and be further transferred into the inner pores, voids and interlayer of carbon matrices to form a strong interaction between sulfur and carbon matrices. Benefiting from this novel SC-CO2 synthetic strategy, we use AC as an example to fabricate AC@S composites in Li–S batteries. The results clearly demonstrate that the obtained AC@S cathode exhibits high specific capacity (905 mA h g−1 at 0.1 A g−1), prolonged cycling life (817 mA h g−1 after 100 cycles) and remarkable coulombic efficiency (>99%). This novel strategy provides new insight into the rational design and controllable synthesis of C@S cathodes for Li–S batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial supports from National Natural Science Foundation of China (21403196, 51572240 and 51677170), Natural Science Foundation of Zhejiang Province (LY17E020010 and LY16E070004), Science and Technology Department of Zhejiang Province (2016C31012, 2016C33009 and 2017C01035) and Xinmiao Talents Program of Zhejiang Province (2016R403086).Notes and references
- S. Urbonaite, T. Poux and P. Novak, Adv. Energy Mater., 2015, 5, 20 CrossRef.
- A. Manthiram, S. H. Chung and C. Zu, Adv. Mater., 2015, 27, 1980–2006 CrossRef CAS PubMed.
- H. B. Yao, K. Yan, W. Y. Li, G. Y. Zheng, D. S. Kong, Z. W. Seh, V. K. Narasimhan, Z. Liang and Y. Cui, Energy Environ. Sci., 2014, 7, 3381–3390 CAS.
- X. Tao, J. Wang, Z. Ying, Q. Cai, G. Zheng, Y. Gan, H. Huang, Y. Xia, C. Liang, W. Zhang and Y. Cui, Nano Lett., 2014, 14, 5288–5294 CrossRef CAS PubMed.
- F. Z. Zeng, A. B. Wang, W. K. Wang, Z. Q. Jin and Y. S. Yang, J. Mater. Chem. A, 2017, 5, 12879–12888 CAS.
- J. Wang, Y. S. He and J. Yang, Adv. Mater., 2015, 27, 569–575 CrossRef CAS PubMed.
- Y. X. Yang, Z. H. Wang, G. D. Li, T. Z. Jiang, Y. J. Tong, X. Y. Yue, J. Zhang, Z. Mao, W. Sun and K. N. Sun, J. Mater. Chem. A, 2017, 5, 3140–3144 CAS.
- R. Fang, S. Zhao, P. Hou, M. Cheng, S. Wang, H.-M. Cheng, C. Liu and F. Li, Adv. Mater., 2016, 28, 3374–3382 CrossRef CAS PubMed.
- R. Cao, J. Chen, K. S. Han, W. Xu, D. Mei, P. Bhattacharya, M. H. Engelhard, K. T. Mueller, J. Liu and J.-G. Zhang, Adv. Funct. Mater., 2016, 26, 3059–3066 CrossRef CAS.
- H. Hu, H. Cheng, Z. Liu, G. Li, Q. Zhu and Y. Yu, Nano Lett., 2015, 15, 5116–5123 CrossRef CAS PubMed.
- X. Fang, W. Weng, J. Ren and H. Peng, Adv. Mater., 2016, 28, 491–496 CrossRef CAS PubMed.
- X. L. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- C. Oh, N. Yoon, J. Choi, Y. Choi, S. Ahn and J. K. Lee, J. Mater. Chem. A, 2017, 5, 5750–5760 CAS.
- Q. Sun, B. He, X. Q. Zhang and A. H. Lu, ACS Nano, 2015, 9, 8504–8513 CrossRef CAS PubMed.
- F. Wu, J. Li, Y. Su, J. Wang, W. Yang, N. Li, L. Chen, S. Chen, R. Chen and L. Bao, Nano Lett., 2016, 16, 5488–5494 CrossRef CAS PubMed.
- S. Rehman, S. Guo and Y. Hou, Adv. Mater., 2016, 28, 3167–3172 CrossRef CAS PubMed.
- C. B. Bucur, J. Muldoon and A. Lita, Energy Environ. Sci., 2016, 9, 992–998 CAS.
- C. Y. Fan, H. H. Li, L. L. Zhang, H. Z. Sun, X. L. Wu, H. M. Xie and J. P. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 23481–23488 RSC.
- C. Tang, B.-Q. Li, Q. Zhang, L. Zhu, H.-F. Wang, J.-L. Shi and F. Wei, Adv. Funct. Mater., 2016, 26, 577–585 CrossRef CAS.
- K. L. Zhang, Y. H. Xu, Y. Lu, Y. C. Zhu, Y. Y. Qian, D. F. Wang, J. B. Zhou, N. Lin and Y. T. Qian, J. Mater. Chem. A, 2016, 4, 6404–6410 CAS.
- R. Li, M. Zhang, Y. R. Li, J. Chen, B. W. Yao, M. P. Yu and G. Q. Shi, Phys. Chem. Chem. Phys., 2016, 18, 11104–11110 RSC.
- G. M. Zhou, L. C. Yin, D. W. Wang, L. Li, S. F. Pei, I. R. Gentle, F. Li and H. M. Cheng, ACS Nano, 2013, 7, 5367–5375 CrossRef CAS PubMed.
- X. M. Ye, J. Ma, Y. S. Hu, H. Y. Wei and F. F. Ye, J. Mater. Chem. A, 2016, 4, 775–780 CAS.
- F. Wu, Y. Ye, R. Chen, J. Qian, T. Zhao, L. Li and W. Li, Nano Lett., 2015, 15, 7431–7439 CrossRef CAS PubMed.
- K. Mi, Y. Jiang, J. Feng, Y. Qian and S. Xiong, Adv. Funct. Mater., 2016, 26, 1571–1579 CrossRef CAS.
- L. Sun, D. Wang, Y. Luo, K. Wang, W. Kong, Y. Wu, L. Zhang, K. Jiang, Q. Li, Y. Zhang, J. Wang and S. Fan, ACS Nano, 2016, 10, 1300–1308 CrossRef CAS PubMed.
- Y. C. Jeong, K. Lee, T. Kim, J. H. Kim, J. Park, Y. S. Cho, S. J. Yang and C. R. Park, J. Mater. Chem. A, 2016, 4, 819–826 CAS.
- Q. Fan, W. Liu, Z. Weng, Y. Sun and H. Wang, J. Am. Chem. Soc., 2015, 137, 12946–12953 CrossRef CAS PubMed.
- J. H. Kim, K. Fu, J. Choi, S. Sun, J. Kim, L. B. Hu and U. Paik, Chem. Commun., 2015, 51, 13682–13685 RSC.
- Y. Zhou, S. Ko, C. W. Lee, S. G. Pyo, S. K. Kim and S. Yoon, J. Power Sources, 2013, 244, 777–782 CrossRef CAS.
- Y. Zhou, Y. Kim, C. Jo, J. Lee, C. W. Lee and S. Yoon, Chem. Commun., 2011, 47, 4944–4946 RSC.
- S. Wei, H. Zhang, Y. Huang, W. Wang, Y. Xia and Z. Yu, Energy Environ. Sci., 2011, 4, 736 CAS.
- C. Luo, Y. Zhu, O. Borodin, T. Gao, X. Fan, Y. Xu, K. Xu and C. Wang, Adv. Funct. Mater., 2016, 26, 745–752 CrossRef CAS.
- H. Liao, H. Wang, H. Ding, X. Meng, H. Xu, B. Wang, X. Ai and C. Wang, J. Mater. Chem. A, 2016, 4, 7416–7421 CAS.
- W. Deng, X. F. Zhou, Q. Fang and Z. P. Liu, J. Mater. Chem. A, 2017, 5, 13674–13682 CAS.
- J. Guo, Y. Xu and C. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed.
- X. Li, M. Rao, H. Lin, D. Chen, Y. Liu, S. Liu, Y. Liao, L. Xing, M. Xu and W. Li, J. Mater. Chem. A, 2015, 3, 18098–18104 CAS.
- L. Ji, M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. Zhang, Energy Environ. Sci., 2011, 4, 5053 CAS.
- L. Y. Zhang, H. Huang, Y. Xia, C. Liang, W. K. Zhang, J. M. Luo, Y. P. Gan, J. Zhang, X. Y. Tao and W. K. Zhang, J. Mater. Chem. A, 2017, 5, 5905–5911 CAS.
- C. Aymonier, A. Loppinet-Serani, H. Reverón, Y. Garrabos and F. Cansell, J. Supercrit. Fluids, 2006, 38, 242–251 CrossRef CAS.
- H. M. Woods, M. M. C. G. Silva, C. c. Nouvel, K. M. Shakesheff and S. M. Howdle, J. Mater. Chem., 2004, 14, 1663 RSC.
- A. H. Romang and J. J. Watkins, Chem. Rev., 2010, 110, 459–478 CrossRef CAS PubMed.
- R. Sui and P. Charpentier, Chem. Rev., 2012, 112, 3057–3082 CrossRef CAS PubMed.
- F. Cansell, B. Chevalier, A. Demourgues, J. Etourneau, C. Even, Y. Garrabos, V. Pessey, S. Petit, A. Tressaud and F. Weill, J. Mater. Chem., 1999, 9, 67–75 RSC.
- H. Gao and G. Hu, RSC Adv., 2016, 6, 10132–10143 RSC.
- G. Zhou, E. Paek, G. S. Hwang and A. Manthiram, Nat. Commun., 2015, 6, 7760 CrossRef CAS PubMed.
- S. Z. Niu, W. Lv, G. M. Zhou, Y. B. He, B. H. Li, Q. H. Yang and F. Y. Kang, Chem. Commun., 2015, 51, 17720–17723 RSC.
- Y. Xia, R. Y. Fang, Z. Xiao, L. Y. Ruan, R. J. Yan, H. Huang, C. Liang, Y. P. Gan, J. Zhang, X. Y. Tao and W. K. Zhang, RSC Adv., 2016, 6, 69764–69772 RSC.
- Y. Xia, R. Y. Fang, Z. Xiao, H. Huang, Y. P. Gan, R. J. Yan, X. H. Lu, C. Liang, J. Zhang, X. Y. Tao and W. K. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 23782–23791 CAS.
- T. Lin, Y. Tang, Y. Wang, H. Bi, Z. Liu, F. Huang, X. Xie and M. Jiang, Energy Environ. Sci., 2013, 6, 1283 CAS.
- S. Zheng, F. Yi, Z. Li, Y. Zhu, Y. Xu, C. Luo, J. Yang and C. Wang, Adv. Funct. Mater., 2014, 24, 4156–4163 CrossRef CAS.
- J. Xu, K. Zhou, F. Chen, W. Chen, X. Wei, X.-W. Liu and J. Liu, ACS Sustainable Chem. Eng., 2016, 4, 666–670 CrossRef CAS.
- K. Zhang, K. Xie, K. Yuan, W. Lu, S. Hu, W. Wei, M. Bai and C. Shen, J. Mater. Chem. A, 2017, 5, 7309–7315 CAS.
- Y. Mi, W. Liu, Q. Wang, J. Jiang, G. W. Brudvig, H. Zhou and H. Wang, J. Mater. Chem. A, 2017, 5, 11788–11793 CAS.
- B. He, W. C. Li, C. Yang, S. Q. Wang and A. H. Lu, ACS Nano, 2016, 10, 1633–1639 CrossRef CAS PubMed.
- S. Xin, L. Gu, N. H. Zhao, Y. X. Yin, L. J. Zhou, Y. G. Guo and L. J. Wang, J. Am. Chem. Soc., 2012, 134, 18510–18513 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM images and volume changes of MCMB, MCMB-CO2, MCMB@S, MWCNTs, MWCNTs-CO2 and MWCNTs@S samples; TEM, HRTEM images and XRD patterns of MWCNTs, MWCNTs-CO2 and MWCNTs@S samples; XRD, Raman, N2 adsorption–desorption, TEM and HRTEM analysis of MCMB and MCMB@S; STEM, EDS spectrum and EDS mapping of MCMB@S; apparent density of raw C, C–CO2 and C@S samples; long-term cycling performance of AC@C, MCMB@S, MWCNTs@S, AC/S-155, MCMB/S-155 and MWCNTs/S-155 samples; N2 adsorption–desorption curve and pore size distribution of the cycled AC@S sample; CV curves of AC@S sample washed by CS2 at a scan rate of 0.1 mV s−1. See DOI: 10.1039/c7ta08768c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |