Carbon doping of hexagonal boron nitride porous materials toward CO2 capture†
Abstract
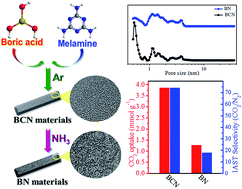
CO2 capture via solid adsorption technology relies on the development of low-cost and high-performance new solid adsorbents. The robust and large-scale produced hexagonal boron nitride (h-BN) porous materials are an ideal candidate but present low CO2 adsorption properties. Here, we report that in situ carbon doping of BN materials (BCN) can be facilely controlled by thermal annealing of boric acid and melamine mixtures in various atmospheres. CO2 uptake of the BCN materials is in the range of 3.74–3.91 mmol CO2 g−1 (165–172 mg CO2 g−1) at 298 K and ambient pressure, whereas it is only 1.16–1.66 mmol CO2 g−1 (53.0–73.1 mg CO2 g−1) over the pure BN materials. The separation coefficient between CO2 and N2 is up to 74 and no decrease in the adsorption performance was observed after nine adsorption/desorption cycles. The BCN material presents the best performance among known BN-based sorbents, which is comparable to many excellent carbon materials. Detailed characterization reveals that the incorporation of carbon into the BN matrix increases the density of ultramicropores (<0.7 nm) and chemical defects, consequently enhancing the CO2 adsorption capacity and the selectivity over N2 molecules.


 Please wait while we load your content...
Please wait while we load your content...