Optically induced motion of liquid crystalline droplets†
Abstract
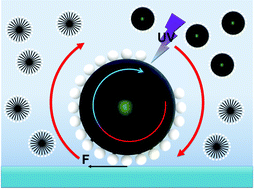
The controlled motion of a liquid crystalline active droplet was demonstrated in a surfactant solution and by irradiation with UV light. The droplet could be induced to roll on a glass substrate toward the UV light source. This was explained by the Marangoni flow induced by the UV-induced desorption of surfactants.


 Please wait while we load your content...
Please wait while we load your content...