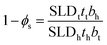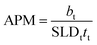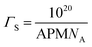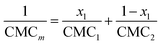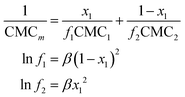Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembly and surface behaviour of pure and mixed zwitterionic amphiphiles in a deep eutectic solvent†
A.
Sanchez-Fernandez‡
 ab,
G. L.
Moody‡
a,
L. C.
Murfin‡
ab,
G. L.
Moody‡
a,
L. C.
Murfin‡
 a,
T.
Arnold
a,
T.
Arnold
 abc,
A. J.
Jackson
abc,
A. J.
Jackson
 *bd,
S. M.
King
*bd,
S. M.
King
 e,
S. E.
Lewis
e,
S. E.
Lewis
 a and
K. J.
Edler
a and
K. J.
Edler
 a
a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK
bEuropean Spallation Source, SE-221 00, Lund, Sweden. E-mail: andrew.jackson@esss.se
cDiamond Light Source, Harwell Campus, Didcot OX11 0DE, UK
dDepartment of Physical Chemistry, Lund University, SE-221 00, Lund, Sweden
eISIS Pulsed Neutron & Muon Source, Harwell Campus, Didcot OX11 0QX, UK
First published on 8th June 2018
Abstract
Recent investigations have shown that deep eutectic solvents provide a suitable environment for self-organisation of biomolecules, in particular phospholipids and proteins. However, the solvation of complex lyophilic moieties by deep eutectic solvents still remains unclear. Here we explore the behaviour of zwitterionic surfactants in choline chloride:glycerol eutectic mixture. Dodecyl-2-(trimethylammonio)ethylphosphate and N-alkyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (alkyl = dodecyl, tetradecyl) surfactants were investigated by means of surface tension, X-ray reflectivity and small-angle neutron scattering. These surfactants were found to remain surface active and form globular micelles in deep eutectic solvents. Still, the surface behaviour of these species was found to differ depending on the headgroup and tail structure. The morphology of the micelles also slightly varies between surfactants, demonstrating differences in the packing of individual monomers. The characteristics of mixtures of the dodecyl surfactants is also reported, showing a deviation from ideal mixing associated with attractive interactions between sulfobetaine and phosphocholine headgroups. Such non-ideality results in variation of the surface behaviour and self-assembly of these surfactant mixtures. The results presented here will potentially lead to the development of new alternatives for drug-delivery, protein solubilisation and biosensing through a better fundamental understanding of the behaviour of zwitterionic surfactants in deep eutectic solvents.
Introduction
Deep eutectic solvents (DES) have emerged as an alternative to traditional solvents in many applications.1,2 DES are green solvents obtained through the complexation of naturally occurring salts with compounds, such as sugar, alcohols, amines and carboxylic acids, among others.1,3–5 Furthermore through different combinations of precursors the properties of the solvent can be tailored,1 potentially providing sustainable solvents tailored for particular applications. Understanding the microscopic structure of the solvent and dynamics represents an essential step to predict and understand the macroscopic behaviour of the solvent, and this is the subject of considerable recent effort.6–9 Such investigations have shown that an extensive hydrogen bond interaction between the DES precursors is responsible for the formation and stability of the solvent. Thus, DES provides a H-bonding environment analogous to that in water.These solvents have been suggested as a non-aqueous environment where biomolecules may retain partial or total activity,2,10,11 presumably due to the existence of such hydrogen bond networks in DES. The investigation of biomolecules and bioprocesses involving DES has recently experienced a major upsurge, with relevant studies published on vesicle formation, phospholipid bilayers, DNA structuring and protein conformation and activity.12–20
Zwitterionic surfactants are molecules that contain both positively and negatively charged chemical groups within their headgroup structure. Such moieties are ubiquitous in biological systems, from proteins to phospholipid membranes. Naturally occurring di-chain phospholipids are the main components of cell membranes. As such, these are often used in simplified model systems such as phospholipid monolayers or bilayers, to investigate the properties of highly complex biological membranes.21,22 It is also possible to synthesise zwitterionic surfactants with single chain architectures and such molecules may open up new possibilities for applications in drug delivery, biosensors, protein stabilisation or those that require high degrees of biocompatibility.23–25
In this work we have investigated two classes of single chain zwitterionic surfactants; phosphocholine and sulfobetaine surfactants. The behaviour of these molecules is relatively well understood in water and as such they are good model systems to understand the effects of the DES solvent. Zwitterionic surfactants generally show high solubility in water, broad isoelectric ranges, and high resistance to changes in the pH and ionic strength of the media,26,27 and the surfactants studied here have been shown to form micelles in water.27–30
DES have been recently demonstrated to support surfactant self-assembly of cationic and anionic surfactants,31–33 as well as the formation of thermodynamically stable phospholipid vesicles.12,19 Such investigations provide new alternatives for applications in surfactant templating of nanostructured materials, formulations and drug delivery. However, the solvation of surfactants in DES is not yet fully understood. Our hope is that this study will add to this growing body of evidence to open new prospects in tailorable self-assembled systems. Here we examine three different zwitterionic surfactants in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
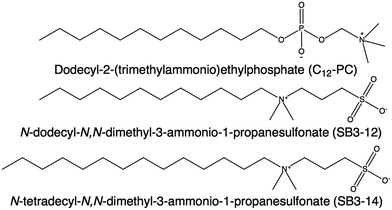
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol.34 Choline chloride:glycerol DES was selected as the solvent for this investigation as this has been previously reported as a suitable environment for both anionic and cationic moieties.33,35 These surfactants are dodecyl-2-(trimethylammonio)ethylphosphate (C12-PC), N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-12) and N-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-14). The structure of these surfactants is presented in Fig. 1. Note that these two headgroup types have the opposite orientation of charge separation relative to the alkyl tail. They have been studied by means of surface tension, X-ray reflectivity (XRR) and small-angle neutron scattering (SANS). These experiments have been performed at a temperature above room temperature (50 °C) to avoid the crystallisation of the C14 surfactant below its Krafft temperature. Furthermore, mixtures of C12-PC and SB3-12 surfactants were studied at different molar ratios in order to explore whether the relative interactions between the ionic headgroups synergistically influence aggregate behaviour.36
glycerol.34 Choline chloride:glycerol DES was selected as the solvent for this investigation as this has been previously reported as a suitable environment for both anionic and cationic moieties.33,35 These surfactants are dodecyl-2-(trimethylammonio)ethylphosphate (C12-PC), N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-12) and N-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-14). The structure of these surfactants is presented in Fig. 1. Note that these two headgroup types have the opposite orientation of charge separation relative to the alkyl tail. They have been studied by means of surface tension, X-ray reflectivity (XRR) and small-angle neutron scattering (SANS). These experiments have been performed at a temperature above room temperature (50 °C) to avoid the crystallisation of the C14 surfactant below its Krafft temperature. Furthermore, mixtures of C12-PC and SB3-12 surfactants were studied at different molar ratios in order to explore whether the relative interactions between the ionic headgroups synergistically influence aggregate behaviour.36
Experimental
Materials
Choline chloride (>98%, Sigma, h-ChCl) and glycerol (>99%, Sigma, h-Glyc) were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio at 80 °C on a hotplate until a transparent, homogeneous liquid was obtained. The deuterated version of the DES was prepared following the same procedure as above with d9-choline chloride (99%, 99.9%D, d-ChCl, Cambridge Isotope Laboratory) and d8-glycerol (98%, 99%D, d-Glyc, Cambridge Isotope Laboratory). Solvents were equilibrated at 40 °C for 24 hours. In an attempt to control and minimise the water content in the systems, solvents were freeze-dried prior to surfactant solution preparation, sealed and stored under a dry atmosphere. The purity of the solvents was checked through NMR and Karl–Fischer titration (Mettler Toledo DL32 Karl–Fischer Coulometer Aquiline electrolyte A (Fisher Scientific), Aqualine Catholyte CG A). Measurements on aliquots of pure solvent stored under the same conditions as the samples were characterized and showed that the water content of the system was maintained below ∼3000 ppm (0.3 wt%) during the experimental procedures presented here.
2 molar ratio at 80 °C on a hotplate until a transparent, homogeneous liquid was obtained. The deuterated version of the DES was prepared following the same procedure as above with d9-choline chloride (99%, 99.9%D, d-ChCl, Cambridge Isotope Laboratory) and d8-glycerol (98%, 99%D, d-Glyc, Cambridge Isotope Laboratory). Solvents were equilibrated at 40 °C for 24 hours. In an attempt to control and minimise the water content in the systems, solvents were freeze-dried prior to surfactant solution preparation, sealed and stored under a dry atmosphere. The purity of the solvents was checked through NMR and Karl–Fischer titration (Mettler Toledo DL32 Karl–Fischer Coulometer Aquiline electrolyte A (Fisher Scientific), Aqualine Catholyte CG A). Measurements on aliquots of pure solvent stored under the same conditions as the samples were characterized and showed that the water content of the system was maintained below ∼3000 ppm (0.3 wt%) during the experimental procedures presented here.
The sulfobetaine surfactants (SB3-12 and SB3-14) were synthesised following the procedure from Qu et al.28 After synthesis the surfactants were purified via recrystallization with hot methanol/acetone. The purity of the final products was assessed by 1H NMR, 13C NMR and High Resolution Mass Spectrometry. A detailed description of the synthesis procedure and the results from the characterisation of the final products are included in the ESI.† Dodecyl-2-(trimethylammonio)ethylphosphate (>99%) was supplied by Glycon Biochemicals GmbH and used without further purification.
Methods
Samples for surface tension measurements were prepared by dilution of high concentration stock solutions. These stock solutions were prepared by direct mixing each surfactant with DES and subsequently diluted using pure solvent in order to obtain lower concentrations, minimising the variability between samples. Surfactant mixtures were prepared at different surfactant molar ratios following the same procedure, C12-PC/SB3-12: 0.2/0.8, 0.35/0.65, 0.5/0.5, 0.65/0.35 and 0.8/0.2.
Samples for reflectivity measurements of pure surfactants and surfactant mixtures in DES were prepared at the critical micelle concentration (CMC) of each system. Pure hydrogenous surfactant powders or homogenous mixtures of surfactants were mixed with hydrogenous solvent and subsequently equilibrated for 24 hours at 50 °C before measurement.
XRR data were fitted using the Abelés formalism implemented in Motofit.38,39 This method, also known as the Dynamic Approximation, uses classical optics to simulate the reflectivity pattern from a given electron density profile. A two-layer plus subphase geometry was found as the simplest model that allowed fitting of the data from pure phosphocholine surfactant solutions, whereas one layer was enough to satisfactorily fit data from pure sulfobetaine solutions. Whilst the use of a one-layer model for the sulfobetaine systems in water has been previously validated,40 here, data have been fitted using the two-layer plus subphase model in order to make the results from all of the systems presented directly comparable. The model uses a layer to describe the “air-solvated” tail-region of the surfactant and a headgroup layer containing a certain amount of DES. An infinitely thick subphase is used to describe the solvent. The parameters used to describe the layers are: thickness (t), scattering length density of the layer (SLD) and roughness (σ), where the subscripts s, t and h stand for solvent, tails and headgroup layers respectively. The SLDs of the headgroup and tail of each surfactant were calculated by accounting for the scattering length of each group and the volume it occupies (see ESI† for further details). The SLDs used in the model therefore account for the amount of surfactant in each layer, allowing calculation of the volume fraction of each component in the layer: volume fraction of tails (ϕt) in the tail layer, and volume fraction of headgroups (ϕh) and solvent (ϕs) in the headgroup layer. The subphase is described by the SLD (SLDs) and the roughness of the solvent (σs). A background term is used to account for the residual background that remains after data subtraction.
To be physically realistic, the model is constrained to ensure that surface excess of both layers is the same, i.e. the two layers contain the same number of headgroups and tails.41 This has been done through the introduction of the following mathematical constraint.
where NA is Avogadro's number.
The data collected were reduced to absolute units (I(q), cm−1vs. q, Å−1) following the standard procedures on the instrument using the Mantid framework.43,44 Data were corrected for detector efficiency, background noise, sample transmission and the scattering from an empty cell, after which the intensity was place on an absolute scale by reference to the scattering from a partially-deuterated polystyrene blend of known molecular weight. The contribution from the solvent was then subtracted from each sample accounting for the incoherent contribution using SasView 4.1, using a procedure previously described.45
Samples of the pure surfactants for SANS were prepared at various surfactant concentrations above the CMC at three different contrasts: h-surfactant in d-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-glycerol, h-surfactant in h-choline chloride
d-glycerol, h-surfactant in h-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-glycerol and h-surfactant in h/d-choline chloride
d-glycerol and h-surfactant in h/d-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h/d-glycerol (mole ratios: 0.38 h-choline chloride/0.62 d-choline chloride; 0.56 h-glycerol/0.44 d-glycerol). Equivalent mole fractions of each system were prepared for the three surfactants (C12-PC, SB3-12 and SB3-14) by mixing each protonated surfactant with the solvent. Samples of surfactant mixtures were prepared at one contrast following the same procedure. A homogeneous powder mixture of the protonated surfactants was mixed with d-choline chloride:d-glycerol, at two different concentrations and at the aforementioned surfactant ratios.
h/d-glycerol (mole ratios: 0.38 h-choline chloride/0.62 d-choline chloride; 0.56 h-glycerol/0.44 d-glycerol). Equivalent mole fractions of each system were prepared for the three surfactants (C12-PC, SB3-12 and SB3-14) by mixing each protonated surfactant with the solvent. Samples of surfactant mixtures were prepared at one contrast following the same procedure. A homogeneous powder mixture of the protonated surfactants was mixed with d-choline chloride:d-glycerol, at two different concentrations and at the aforementioned surfactant ratios.
A systematic procedure was used to analyse the data presented here. Data from pure surfactant systems were simultaneously fitted to all three contrasts, whereas the single contrast surfactant mixture data was fitted individually. Three models were initially compared using the Chi Square goodness-of-fit parameter in order to evaluate the best option to fit the intramicellar contribution to the scattering data: monodisperse and polydisperse homogeneous spheres, and monodisperse homogeneous ellipsoids models.46 The results from all three models are compared for one of the systems (h-C12-PC in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 d-choline chloride
2 d-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-glycerol) in the ESI.† The ellipsoid model was shown to provide the best fits to the data and therefore used for the detailed analysis of the data. This model uses the following parameters: equatorial radius or radius of rotation (req), aspect ratio (AR = rpo/req, where rpo is the polar radius of the scatterer), volume fraction of scatterers (ϕP(q)) and SLD of the solvent and the surfactants.46 The radius of gyration of the ellipsoidal scatterers was calculated as follows:
d-glycerol) in the ESI.† The ellipsoid model was shown to provide the best fits to the data and therefore used for the detailed analysis of the data. This model uses the following parameters: equatorial radius or radius of rotation (req), aspect ratio (AR = rpo/req, where rpo is the polar radius of the scatterer), volume fraction of scatterers (ϕP(q)) and SLD of the solvent and the surfactants.46 The radius of gyration of the ellipsoidal scatterers was calculated as follows:
The SLD of the solvent was derived from the results obtained from reflectivity measurements. In terms of surfactant SLDs, the contribution of the headgroup region to the scattering will be considerably reduced due to solvation. Thus the limited contrast between the solvent and the headgroups did not permit a more detailed model of the micelle (e.g. core–shell structure) and so the SLD of the micelle was considered to be that of the surfactant tails. A full record of these values is included in the ESI.† No size polydispersity was included in the model-fitting. The reasons for this are discussed later.
Subsequently, an attempt to simultaneously fit the data using a core–shell ellipsoid model was performed in order to probe the structural heterogeneity of the micelles. However, the low signal-to-noise ratio limited the applicability of this model, more evident at low surfactant concentration where the statistical significance of the results was insufficient. One of the high concentrations was fitted to this model in order to show the core–shell density distribution of the micelle. This was performed following this systematic procedure: the aspect ratio of the aggregate was constrained to that obtained using a uniform ellipsoid model (AR), the thickness of the shell (teq,shell) was fitted using the size of the headgroup region from our reflectivity results as the initial guess, and the effective radius was recalculated for the new micelle morphology. A full record of these values is included in the ESI.†
Some of the main factors that determine the intermicellar interactions between charged aggregates are the ionic strength and the dielectric constant of the environment. However, the understanding of the ionic behaviour of DES is still not well understood and, although it is know to behave as a polar environment,47 those parameters remain rather unexplored. Thus, in order to account for any interaction between the micelles, a structure factor based on a rescaled Percus–Yevick approximation was used. The suitability of this model to account for the intermicellar scattering in DES has been previously discussed.32,33,48 This approach modifies the original Hard–Sphere structure factor in order to account for any weak electrostatic repulsion between anisotropic interacting particles.49,50 The structure factor model is built using two parameters: the volume fraction of interacting hard spheres (ϕS(q)) and the radius of interaction (Reff). Since the particles slightly deviate from sphericity, a correction for the radius of interaction was applied. The fitting procedure calculates the Reff as the second virial coefficient of the particle (Reff = (rporeq2)1/3) and fits ϕS(q).33,51,52
The SANS data was analysed using SasView 4.1.45 The model-fitting optimisation was performed using a Levenberg–Marquardt algorithm within a q-range between 0.007 and 0.5 Å−1. The models were smeared using a Gaussian distribution with a constant dq/q = 5% in order to account for the instrument resolution.
Results
Behaviour of pure surfactants
Zwitterionic surfactants were found to dissolve and remain surface active in choline chloride:glycerol. The surfactant behaviour at the interface is considered to be similar to that in water, where the surfactant diffuses to the surface due to the lyophobic effect. Once the equilibrium at the interface has been reached, the surface tension of the system is also equilibrated, as previously shown for alkyltrimethylammonium bromides in choline chloride:glycerol DES.33 In all the cases, the addition of surfactant initially produces a decrease of the surface tension of the system, indicative of the adsorption of surfactant at the interface. Above a certain concentration, the surface of the liquid is saturated with surfactant and further reduction of the surface tension is not observed. This point is the CMC and correlates with the formation of micelles in the continuous phase. The CMC of the pure surfactant systems (C12-PC, SB3-12 and SB3-14), the limiting surface tension at the CMC (γCMC) and surface pressure at the CMC (π = γ0 − γCMC) are presented in Table 1. The surface tension of pure DES (γ0 = 62.9 ± 0.4 mN m−1) and water (72.4 ± 0.2 mN m−1) at room temperature were measured as controls and found to be consistent with our previous measurements.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 choline chloride
2 choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol. The values for those surfactants in water are presented for comparison with the results in DES
glycerol. The values for those surfactants in water are presented for comparison with the results in DES
Both headgroup and tail structure were found to affect the CMC. The phosphocholine surfactant shows a lower CMC (7.9 ± 0.3 mM) than its homologous C12 sulfobetaine, SB3-12 (14 ± 2 mM). In the case of sulfobetaines, increasing the number of carbons in the surfactant tail from 12 to 14, decreases the CMC from 14 ± 2 mM to 2.1 ± 0.2 mM.
Interestingly, this behaviour is very similar to that observed in water for the same surfactants, where the CMC of C12-PC is lower than that of SB3-12.28,29 In fact, in both solvents the CMC of C12-PC is about 44% lower than the CMC of SB3-12. Nonetheless the absolute values of the CMC were found to be consistently lower in water, suggesting higher monomer solubility in DES. An increase in the CMC was previously observed with the addition of ethylene glycol to the aqueous solutions of sulfobetaines.53 Those results suggest that the solvophobicity of non-polar moieties is reduced by the presence of ethylene glycol. Similarly, depression of the solvophobic effect has also been previously suggested for cationic surfactants in the same DES.33
The limiting surface tension in DES also follows the same trend as in water, although the extent of variation is much less pronounced. The system reaches the lowest value of surface tension at the CMC for SB3-14 and the highest for C12-PC (see Table 1). However, the total variation for these surfactants in DES is only 2.3 mN m−1, as opposed to nearly 8 mN m−1 in water. Actually, this difference is most significant between the PC surfactant and the two SB surfactants. This suggests that the headgroups are affected by the solvent to a different extent.
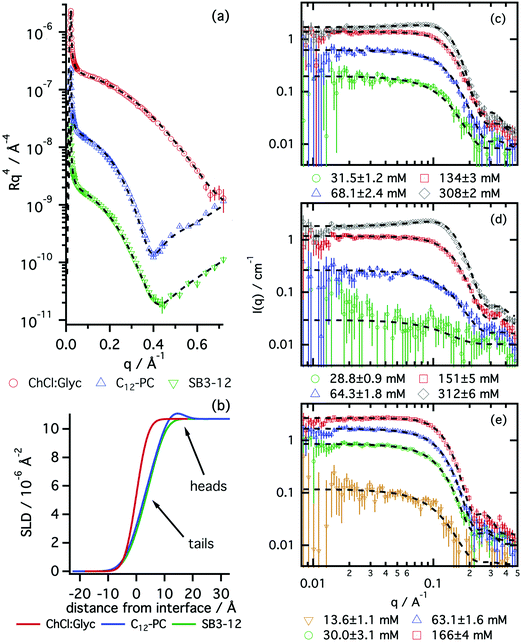
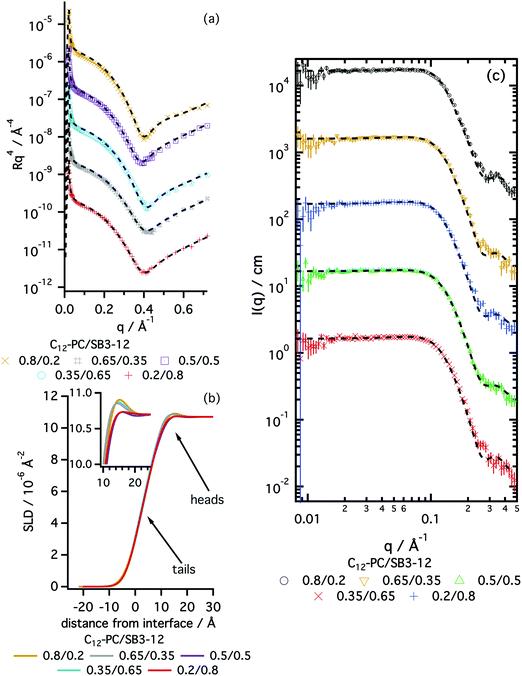
The structures of surface adsorbed layers of C12-PC and SB3-12 surfactants were measured at the CMC in order to identify any difference in the behaviour of these species at the interface. Fig. 2 shows the XRR data of a pure subphase, C12-PC at its CMC and SB3-12 at its CMC. The results from the best fits are included in Table 2. A complete record of the fits is included in the ESI.†
| System | t t/Å | ϕ t/10−2 | t h/Å | ϕ h/10−2 | Γ S,CMC/×10−6 mol m−2 | APM/Å2 |
|---|---|---|---|---|---|---|
| C12-PC | 8.3 ± 0.4 | 72 ± 4 | 6.7 ± 0.3 | 45 ± 4 | 2.8 ± 0.2 | 59 ± 4 |
| SB3-12 | 7.0 ± 0.5 | 68 ± 4 | 5.4 ± 0.4 | 48 ± 2 | 2.4 ± 0.2 | 70 ± 5 |
A bare choline chloride:glycerol surface was initially measured in order to determine its characteristics. The values obtained from the fits are: SLDs = 10.8 × 10−6 Å−2 and surface roughness, σs = 3.3 Å, in good agreement with the values previously reported.33 These values for the subphase were held fixed during subsequent data fitting from the surfactant systems.
The reflectivity results confirm the adsorption of the pure surfactants to the liquid surface, as both surfactants form a monolayer at the air–liquid interface. C12-PC was found to form a thicker monolayer (15.0 ± 0.3 Å) than SB3-12 (12.4 ± 0.4 Å), at a similar volume fraction of monomer within error. The thicknesses of the surfactant layers in DES were found to be thinner than those in water, where, at the CMC, a C12-PC monolayer fits to a total thickness of 20 ± 2 Å and a SB3-12 monolayer shows a total thickness of 14 ± 3 Å.29,54 Whereas the thickness of the headgroup layer in DES in both cases is comparable to that in water,40,55 thicker monolayers in water may indicate the presence of a stronger lyophobic interaction in water than in DES. The interfaces between different layers appear to be diffuse, potentially due to thermal-induced capillary waves. This effect is reflected in the smearing of the SLD profile on the reflectivity results (see Fig. 2b), as it has been previously reported for SDS on choline chloride:urea.31
The surface excess concentration and area per molecule at the interface of these surfactant solutions at the CMC were subsequently calculated from the reflectivity results. The calculations show that the surface excess concentration is higher for the phosphocholine surfactant than for the dodecyl sulfobetaine. Interestingly this is the opposite behaviour found for these amphiphiles in water. Neutron reflectivity results have shown that these surfactants on water, at the CMC, present a surface excess concentration of 3.35 × 10−6 mol m−2 and 3.70 × 10−6 mol m−2 for C12-PC and SB3-12 respectively.29,54 The higher values found in water are again indicative of the greater affinity of the DES for solvated surfactant free monomers, which thus leads to reduced surface excess concentrations. Finally, the calculated values indicate that the area per surfactant monomer at the air–DES interface is larger in the case of SB3-12 than C12-PC. This effect is, again, the opposite to the behaviour seen in water, where the area per molecule is slightly larger for C12-PC than for SB3-12.
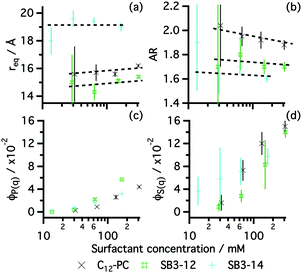
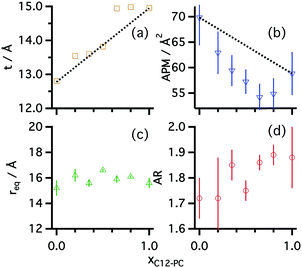
As anticipated by the results from surface tension, these surfactants were found to aggregate in solution. The morphology and behaviour of those micelles were investigated by means of SANS. Fig. 2 shows the scattering data at one contrast of the three zwitterionic surfactants in choline chloride:glycerol together with the best model fits. The results from those fits are presented in Fig. 3. A full record of the results from the fits is included in the ESI,† together with the plots for all the SANS contrasts.
All the surfactants presented here were found to form globular micelles above the CMC in DES, with a certain amount of intermicellar interaction. The uniform ellipsoid with a prolate distribution of mass (AR > 1) accurately represents the morphology of those micelles, reflected in the good agreement between the models and the experimental data (see Fig. 2c–e).
Previous investigations have used either polydisperse spheres and monodisperse ellipsoids to describe the structure of zwitterionic micelles in water.26,30 Our decision, based on both thermodynamic reasons and quality of the fits, was to use the monodisperse ellipsoidal model. As shown by Tanford, the formation of globular micelles with a certain deviation from sphericity constitutes a suitable scenario in terms of micelle morphology, as it would optimise the tail packing in the hydrocarbon core and the entropic contribution.56 Furthermore, it has been recently reported that surfactant aggregates, in thermodynamic equilibrium, show little polydispersity in DES.48 Therefore no polydispersity function was implemented in our model, as a realistic fit could be obtained without this extra parameter.
Our results show that the structure of the aggregates depends on the tail length and surfactant headgroup. As expected, SB3-14 shows bigger micelles than the SB3-12 driven by the presence of a larger hydrophobic moiety in the surfactant. SB3-14 micelles have a similar size to those in water (Rg = 19.1 ± 1 Å),57 whereas C12-PC micelles were found to be slightly smaller in DES.30 Both C12 surfactants show a similar equatorial radius and differences in the AR are small. Although the resolution of the experiment and the low signal-to-noise ratio did not allow the use of a more detailed model of the micelles, an underlying trend can be found in the AR of these surfactants. SB3-12 forms slightly shorter micelles, associated with a smaller AR, potentially driven by the interactions between headgroups at the micelle interface. Since the hydrophobic moiety of both C12 surfactants is identical, changes in the packing parameter (v/a0lc, where v is the volume of the lyophobic moiety, a0 is the area at the headgroup–tail interface and lc is the length of the fully extended tail) are strictly driven by differences in the area at the headgroup–tail interface.58 Assuming that geometrical effects will not change the trend in the area per monomer between the planar interface of the monolayer and the curved micelle interface, larger APM would imply larger a0. Thus, a larger AR would be expected for C12-PC than for SB3-12. These results therefore correlate with those obtained from reflectivity, where the area per monomer at the interface was found to be larger for SB3-12 than for C12-PC.
From the simultaneous fit of the three contrasts to the core–shell ellipsoid model, detailed structural information about the aggregate could be obtained (see ESI†). For the three surfactants, a model using a micelle core containing surfactant tails surrounded by solvated headgroups satisfactorily fitted the data. The core of C12-PC and SB3-12 were found to be the same within error (14 ± 1 Å), whereas SB3-14 showed a larger lyophobic core (19 ± 1 Å). Shell thickness was found to vary with the headgroup of the surfactant, in agreement with the reflectivity results, where the phosphocholine headgroup region is thicker than that of the sulfobetaine surfactants (7 ± 1 Å for C12-PC, 5 ± 1 Å for SB3-12 and SB3-14). Also, the volume fraction of the micelle headgroup (ϕhg) was found to be consistently smaller than that at the air–liquid interface, obtained through reflectivity (ϕh), which potentially causes the different arrangement of surfactant monomers (planar geometry at the interface, globular geometry at the bulk phase). However, the contrast resolution and limited signal-to-noise ratio did not allow further information about the characteristics of this region to be extracted.
Zwitterionic surfactants in pure water show an overall neutral charge, thus long-range electrostatic interactions between micelles are negligible.30 Meanwhile, intermicellar interactions in DES are not expected to appear until high surfactant concentration since the solvent has inherently high ionic strength.32,33 However, here we have found that hard-sphere interactions were insufficient to account for the intermicellar scattering, and therefore, intermicellar interactions must have a role in these systems. Fig. 3 indexes both the form and structure factor volume fractions as a function of the surfactant concentration. Our results show the clear decoupling between ϕS(q) and ϕP(q), where the contribution from micelle–micelle interactions consistently shows higher volume fractions than those from the intramicellar scattering. These differences may be related to the excess contribution arising from electrostatic interactions between the aggregates, commonly found in simple ionic surfactants but uncommon for zwitterionics in pure water.
We consistently find that the structure factor contribution from C12-PC micelles is greater than those for SB3-12. This may be indicative of differences in ion binding to the headgroup with varying charge distribution in the headgroup. This would therefore create a surface higher charge density by unbalancing the charge neutralisation within the headgroup and/or by affecting the solvent structure surrounding the micelle.59,60 Unfortunately, due to the limited physicochemical information of the solvent and instrument resolution, more information about this interaction cannot be extracted from the data presented here.
Surfactant mixtures in choline chloride:glycerol
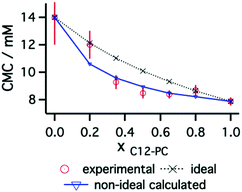
The behaviour of mixtures of the C12 phosphocholine and sulfobetaine surfactants was investigated at different molar ratios of surfactant: C12-PC/SB3-12: 0.2/0.8, 0.35/0.65, 0.5/0.5, 0.65/0.35 and 0.8/0.2. Surface tension measurements were used to find the CMC of the systems and elucidate the nature of mixing of the two surfactants (C12-PC and SB3-12). The surface tension plots of these systems are included in the ESI† and the CMC results are presented in Fig. 4 as a function of the mole fraction of C12-PC in the surfactant mixture.As seen for the pure surfactant systems, there is a decrease of surface tension with increasing surfactant concentration. The inflexion point indicates the limiting surface tension allowing the CMC to be identified for the various mixtures. When considering ideal mixing of surfactants, a theoretical determination of the CMCs of the mixed systems can be obtained using the pseudophase separation model:61
Interactions between the two surfactants when mixed will lead to deviations from the ideal case. Attractive interactions between the amphiphiles lead to a decrease in the experimental values of the CMC, whereas repulsive interactions show higher CMC values for the mixtures. The application of the regular solution theory approximation accounts for the non-ideality of the mixture through modifications in the activity coefficient of each surfactant in the mixture. Following the procedure introduced by Holland and Rubingh,62 the interaction between surfactants within a binary mixture can be described using a parameter β, for activity coefficients f1 and f2:
The interfacial behaviour of the mixtures of zwitterionic surfactants was further probed using X-ray reflectivity. Data and best fits are presented in Fig. 5, and results from those fits are summarised in Fig. 6. A complete record of the fitting results is included in the ESI.†
Mixed monolayers at the CMC show a similar structure to those of pure surfactants: a dry tail region and a solvated headgroup layer. The dimensions of the various layers change with the ratio of each surfactant in the mixture. At the CMC, the monolayer total thickness gradually varies from thinner values at low C12-PC mole fractions to thicker monolayers at high C12-PC mole fractions. These results fit the trend established for the pure surfactants at the interface, for which the C12-PC monolayer is thicker than that of pure SB3-12. The area per molecule also gradually varies, showing a decrease with increasing amount of C12-PC. Those values were found to negatively deviate from the ideal values, suggesting that mixed species allow tighter packing potentially due to attractive interactions between monomers.
The formation of micelles by these surfactant mixtures in DES was investigated by means of SANS. Two different concentrations of the mixtures were measured above the CMC in a single contrast and fitted using the homogeneous ellipsoid model. Fig. 5 shows the SANS data and best fits of the different h-surfactant mixtures in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 d-choline chloride
2 d-choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-glycerol. The results from the fits are summarised in Fig. 6 and a full record of the fitted parameters is included in the ESI.†
d-glycerol. The results from the fits are summarised in Fig. 6 and a full record of the fitted parameters is included in the ESI.†
As shown for the systems containing pure zwitterionic surfactants in choline chloride:glycerol, the agreement between the data and mathematical model demonstrates that prolate ellipsoid is a suitable model to represent zwitterionic mixed micelles in DES. The variation of surfactant mole fraction in the surfactant mixture leads to subtle changes in the morphology of the aggregates. Whereas the equatorial radius remains practically unchanged as the composition changes, the AR of the micelles gradually evolves with the molar ratio. These results indicate a variation in the area per surfactant monomer at the micelle interface and match the findings from XRR: increasing the amount of C12-PC in the mixture leads to a smaller area per surfactant monomer, and therefore promotes the formation of more elongated aggregates (see Fig. 6b and d).
The volume fractions of the mixed micelles extracted from the structure factor and the form factor were found to behave in a similar manner to those of the pure systems. Increasing the total volume fraction of micelles in solution leads to a considerable increase of the apparent S(q) volume fraction, the latter being considerably higher than the volume fraction of micelles (∼3 times higher at 185 ± 3 mM surfactant concentration). Due to systematic variability between samples it is however difficult to draw conclusions about the S(q) evolution as the composition of the mixture is varied.
Discussion
Zwitterionic surfactants were found to preserve their activity in choline chloride:glycerol DES. The solvation of zwitterionic moieties becomes highly complex when it occurs in DES. The presence of charged ions in the solvent and neutral compounds with H-bonding capability may promote the formation of solvating environments, where the solvent components are segregated.12,19,32,33 The presence of positively charged choline ions in close proximity to the sulfate or phosphate group may be favoured due to electrostatic interactions and H-bonding. Moreover, the choline group of the surfactant heads may favour the presence of glycerol in its local solvation environment.19,33 Such considerations could lead to the formation of solvation layers that would affect the behaviour of the amphiphiles and, ultimately, the morphology of the resultant aggregates. The effect of solvent layering has been explored and confirmed for the nanoparticles in DES, where electrostatics govern the formation of distinct choline-rich or hydrogen bond donor-rich layers surrounding the particles.60,63,64Surface tension results have shown differences in the CMC values between surfactants indicating differences in the solvation of the sulfobetaine and phosphocholine headgroups. These differences were corroborated by our XRR and SANS results. In both cases the micelles are globular and the surfactants show a relatively high resilience to the high ionic strength of the solvent, in that the morphologies are globular and therefore not profoundly altered compared to the analogous systems in water. This is in notable contrast to anionic surfactants in choline chloride:urea or cationic surfactants in choline chloride:malonic acid.13,32
As occurs in water, the presence of salts at the interface also promotes changes in the monomer–monomer electrostatic interactions, as widely seen for phospholipid monolayers and bilayers.22,40,55,65,66 Interestingly, such ion–ion interactions were reported to be more pronounced when counterions interact with the charged group adjacent to the tail than with the terminal group.40,65,66 Although the surfactants investigated here both have positive and negative charges, the relative position of those charges in the headgroup seem to alter the structure of the aggregates, presumably through modifications to the monomer packing or solvation shell. Unfortunately, limited resolution and SLD contrast (due to solvation) in the scattering data mean we cannot determine a more detailed structure of the headgroup and solvation shell of the micelles or monolayers.
Charge screening has been found to be a significant influence on the intermicellar interaction of anionic surfactants in DES. This was explained through the ion-pair formation between solvent cations and surfactant native counterions from the solvent and the anionic headgroups.32,35 However, cationic surfactants in the same solvent showed a stronger interaction between micelles. This was attributed to the weaker effect of chloride/bromide anions binding to the surfactant headgroups.33
Zwitterionic micelles also show intermicellar interactions that depend on the characteristics of the headgroup. The phosphocholine surfactant consistently showed higher structure factor volume fractions, an indicator of intermicellar repulsion, than its sulfobetaine analogue. These findings could be interpreted in two ways. Traditionally, long-range electrostatic interactions have been associated to the ionic character of the aggregates.67 In this case, DES may somehow behave as a relatively low ionic strength environment, where the electrostatic interactions are not totally screened. This correlates with results suggesting that ionic liquids behave as relatively dilute electrolytes and long-range electrostatic interactions are retained.68 The excess interactions observed here may be explained through partial adsorption of ions to the headgroups. In pure water, zwitterionic headgroups can be considered as neutral moieties,30 so the formation of ion-pairs between the solvent and one of the charges in the headgroup in DES could explain the differences between in behaviour in these solvents. In a second plausible scenario, the presence of charged macromolecules in DES may influence the structure of the solvent surrounding the micelle, producing a segregated-solvent superstructure that will finally affect the intermicellar interactions and, thus, the structure factor contained in the scattering data. This has been previously reported for nanoparticles in DES,60,63,69 where the surface charge of the particles produce a re-arrangement of the solvent surrounding the particle through the charge imbalance at the interface. Similarly, the net charge showed by the micelles could promote a similar behaviour. However, the instrument and contrast resolution of the data presented here is insufficient to probe that.
The results from surfactant mixtures indicate that ion–ion interactions between sulfobetaine and phosphocholine headgroups affect the surface and bulk behaviour of the amphiphiles. Investigations of the aqueous behaviour of mixed phosphocholine/sulfobetaine monolayers showed a negative deviation from ideality of the APM values using the additivity rule.36 Similarly, surface tension and reflectivity demonstrates a non-ideal mixing of the surfactants, probably influenced by Coulombic attraction between different headgroups, resulting in smaller CMCs and average molecular areas. Variations in the APM calculated through reflectivity are hypothesised to be similar to those at the micelle interface, such that a smaller APM at the interface can be extrapolated to smaller areas at the micelle interface and therefore larger ARs of the micelles. The SANS results indeed showed that variations in micelle AR are present as the composition of the mixture was varied, with slightly more elongated micelles formed at ratios which correlated to smaller APMs.
Conclusions
Zwitterionic surfactants have been demonstrated to self-assemble in choline chloride:glycerol. The surface activity is retained as shown by surface tension, and the CMC of the system depends on the chain length of the surfactant and the headgroup. Increasing the chain length from C12 to C14 in the case of the sulfobetaine results in a decrease of the CMC. Furthermore, a significant difference in the CMC with different headgroups was found, showing a higher CMC for the sulfobetaine than for the phosphocholine surfactant, comparable to that which occurs in water. These differences result from changes in the headgroup structure and are confirmed by structural investigations of the monolayer by means of X-ray reflectivity. The reflectivity results demonstrate variations in the monolayer structure, with differences in the area per molecule and thickness of the layers. SB3-12 shows larger area per molecule at the CMC than C12-PC.Above the CMC, pure surfactants were found to form micelles in the bulk phase. SB3-12 forms micelles with a smaller AR than those of C12-PC. Unsurprisingly, SB3-14 was found to form bigger micelles than its C12 analogue. Intermicellar interaction appears to be stronger for C12-PC surfactant, suggesting differences in the solvation and charge screening at this surfactant headgroup. Mixtures of surfactants were found to behave similarly to the pure surfactants, also forming globular micelles. Surface tension and reflectivity results showed a subtle negative deviation from ideal behaviour of the mixture. Modelling of those results confirm that such deviations arise from electrostatic attractive interactions between neighbouring headgroups.
These investigations demonstrate the activity and aggregation of zwitterionic amphiphiles in choline chloride:glycerol DES, with certain similarities to the behaviour already known in water. Interestingly, this DES has been found to effectively solvate the headgroup of the surfactants, although the solvation mechanism of these moieties still remains unclear. Future investigations to explore such phenomena could include isotopic-substitution small-angle neutron scattering and neutron reflectivity. These results will potentially lead to the development of a framework to facilitate new methods of drug-delivery and biosensing technologies, as well as to better understand the formation of phospholipid membranes and the conformation of proteins in DES.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge Diamond Light Source and the ISIS Pulsed Neutron and Muon Source for the award of beamtime on the I07 and LOQ instruments (experiment numbers SI15584-1 and RB1710047, respectively). We further thank Diamond Light Source for the access to the drop-shape analysis instrument. A. S.-F. would like to thank the University of Bath Alumni Fund and the European Spallation Source for funding his PhD studentship. We gratefully acknowledge the University of Bath for supporting G. M., L. C. M. thanks EPSRC for a DTP studentship. This work benefited from the use of the SasView application, originally developed under NSF Award DMR-0520547. SasView also contains code developed with funding from the EU Horizon 2020 programme under the SINE2020 project Grant No. 654000.References
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef PubMed
.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef
.
- Y. Dai, J. van Spronsen, G. J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef PubMed
.
- A. Hayyan, F. S. Mjalli, I. M. AlNashef, T. Al-Wahaibi, Y. M. Al-Wahaibi and M. A. Hashim, Thermochim. Acta, 2012, 541, 70–75 CrossRef
.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC
.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Green Chem., 2016, 18, 2736–2744 RSC
.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef PubMed
.
- D. V. Wagle, G. A. Baker and E. Mamontov, J. Phys. Lett., 2015, 6, 2924–2928 Search PubMed
.
- C. F. Araujo, J. A. P. Coutinho, M. M. Nolasco, S. F. Parker, P. J. A. Ribeiro-Claro, S. Rudic, B. I. G. Soares and P. D. Vaz, Phys. Chem. Chem. Phys., 2017, 19, 17998–18009 RSC
.
- B. Tang and K. H. Row, Monatsh. Chem., 2013, 144, 1427–1454 CrossRef
.
- R. Esquembre, J. M. Sanz, J. G. Wall, F. del Monte, C. R. Mateo and M. L. Ferrer, Phys. Chem. Chem. Phys., 2013, 15, 11248–11256 RSC
.
- S. J. Bryant, R. Atkin and G. G. Warr, Langmuir, 2017, 33, 6878–6884 CrossRef PubMed
.
- A. Sanchez-Fernandez, K. J. Edler, T. Arnold, D. Alba Venero and A. J. Jackson, Phys. Chem. Chem. Phys., 2017, 19, 8667–8670 RSC
.
- B.-P. Wu, Q. Wen, H. Xu and Z. Yang, J. Mol. Catal. B: Enzym., 2014, 101, 101–107 CrossRef
.
- A. A. Papadopoulou, E. Efstathiadou, M. Patila, A. C. Polydera and H. Stamatis, Ind. Eng. Chem. Res., 2016, 55, 5145–5151 CrossRef
.
- A. R. Harifi-Mood, R. Ghobadi and A. Divsalar, Int. J. Biol. Macromol., 2017, 95, 115–120 CrossRef PubMed
.
- R. P. Xin, S. J. Qi, C. X. Zeng, F. I. Khan, B. Yang and Y. H. Wang, Food Chem., 2017, 217, 560–567 CrossRef PubMed
.
- I. Mamajanov, A. E. Engelhart, H. D. Bean and N. V. Hud, Angew. Chem., 2010, 122, 6454–6458 CrossRef
.
- S. J. Bryant, R. Atkin and G. G. Warr, Soft Matter, 2016, 12, 1645–1648 RSC
.
- F. Milano, L. Giotta, M. R. Guascito, A. Agostiano, S. Sblendorio, L. Valli, F. M. Perna, L. Cicco, M. Trotta and V. Capriati, ACS Sustainable Chem. Eng., 2017, 5, 7768–7776 CrossRef
.
- H. Mohwald, Annu. Rev. Phys. Chem., 1990, 41, 441–476 CrossRef PubMed
.
- R. Ekerdt and D. Papahadjopoulos, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 2273–2277 CrossRef
.
- O. Choksupmanee, K. Hodge, G. Katzenmeier and S. Chimnaronk, Biochemistry, 2012, 51, 2840–2851 CrossRef PubMed
.
- X. Wang, K. Corin, C. Rich and S. Zhang, Sci. Rep., 2011, 1, 102 CrossRef PubMed
.
- B. L. Cook, D. Steuerwald, L. Kaiser, J. Graveland-Bikker, M. Vanberghem, A. P. Berke, K. Herlihy, H. Pick, H. Vogel and S. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 11925–11930 CrossRef PubMed
.
- J. Lipfert, L. Columbus, V. B. Chu, S. A. Lesley and S. Doniach, J. Phys. Chem. B, 2007, 111, 12427–12438 CrossRef PubMed
.
- J. P. Priebe, M. L. Satnami, D. W. Tondo, B. S. Souza, J. M. Priebe, G. A. Micke, A. C. O. Costa, H. D. Fiedler, C. A. Bunton and F. Nome, J. Phys. Chem. B, 2008, 112, 14373–14378 CrossRef PubMed
.
- G. Qu, J. Cheng, J. Wei, T. Yu, W. Ding and H. Luan, J. Surfactants Deterg., 2011, 14, 31–35 CrossRef
.
- M. Yaseen, Y. Wang, T. J. Su and J. R. Lu, J. Colloid Interface Sci., 2005, 288, 361–370 CrossRef PubMed
.
- E. Pambou, J. Crewe, M. Yaseen, F. N. Padia, S. Rogers, D. Wang, H. Xu and J. R. Lu, Langmuir, 2015, 31, 9781–9789 CrossRef PubMed
.
- T. Arnold, A. J. Jackson, A. Sanchez-Fernandez, D. Magnone, A. E. Terry and K. J. Edler, Langmuir, 2015, 31, 12894–12902 CrossRef PubMed
.
- A. Sanchez-Fernandez, K. J. Edler, T. Arnold, R. K. Heenan, L. Porcar, N. J. Terrill, A. E. Terry and A. J. Jackson, Phys. Chem. Chem. Phys., 2016, 18, 14063–14073 RSC
.
- A. Sanchez-Fernandez, T. Arnold, A. J. Jackson, S. L. Fussell, R. K. Heenan, R. A. Campbell and K. J. Edler, Phys. Chem. Chem. Phys., 2016, 18, 33240–33249 RSC
.
- A. P. Abbott, R. C. Harris, K. S. Ryder, C. D'Agostino, L. F. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82–90 RSC
.
- A. Sanchez-Fernandez, O. S. Hammond, K. J. Edler, T. Arnold, J. Doutch, R. M. Dalgliesh, P. Li, K. Ma and A. J. Jackson, Phys. Chem. Chem. Phys., 2018, 20, 13952–13961 RSC
.
- T. Aikawa, K. Yokota, T. Kondo and M. Yuasa, Langmuir, 2016, 32, 10483–10490 CrossRef PubMed
.
- T. Arnold, C. Nicklin, J. Rawle, J. Sutter, T. Bates, B. Nutter, G. McIntyre and M. Burt, J. Synchrotron Radiat., 2012, 19, 408–416 CrossRef PubMed
.
- A. Nelson, J. Appl. Crystallogr., 2006, 39, 273–276 CrossRef
.
- F. Abelès, J. Phys. Radium, 1950, 11, 307–309 CrossRef
.
- G. Hazell, A. P. Gee, T. Arnold, K. J. Edler and S. E. Lewis, J. Colloid Interface Sci., 2016, 474, 190–198 CrossRef PubMed
.
- L. Braun, M. Uhlig, R. von Klitzing and R. A. Campbell, Adv. Colloid Interface Sci., 2017, 247, 130–148 CrossRef PubMed
.
- R. K. Heenan, J. Penfold and S. M. King, J. Appl. Crystallogr., 1997, 30, 1140–1147 CrossRef
.
- O. Arnold, J. C. Bilheux, J. M. Borreguero, A. Buts, S. I. Campbell, L. Chapon, M. Doucet, N. Draper, R. Ferraz Leal, M. A. Gigg, V. E. Lynch, A. Markvardsen, D. J. Mikkelson, R. L. Mikkelson, R. Miller, K. Palmen, P. Parker, G. Passos, T. G. Perring, P. F. Peterson, S. Ren, M. A. Reuter, A. T. Savici, J. W. Taylor, R. J. Taylor, R. Tolchenov, W. Zhou and J. Zikovsky, Nucl. Instrum. Methods Phys. Res., Sect. A, 2014, 764, 156–166 CrossRef
.
- G. D. Wignall and F. S. Bates, J. Appl. Crystallogr., 1987, 20, 28–40 CrossRef
.
-
M. Doucet, J. H. Cho, G. Alina, J. Bakker, W. Bouwman, P. Butler, K. Campbell, M. Gonzales, R. Heenan, A. Jackson, P. Juhas, S. King, P. Kienzle, J. Krzywon, A. Markvardsen, T. Nielsen, L. O'Driscoll, W. Potrzebowski, R. Ferraz Leal, T. Richter, P. Rozycko and A. Washington, 2017, DOI:10.5281/zenodo.438138
.
- J. S. Pedersen, Adv. Colloid Interface Sci., 1997, 70, 171–210 CrossRef
.
- A. Pandey, R. Rai, M. Pal and S. Pandey, Phys. Chem. Chem. Phys., 2014, 16, 1559–1568 RSC
.
- A. Sanchez-Fernandez, O. S. Hammond, A. J. Jackson, T. Arnold, J. Doutch and K. J. Edler, Langmuir, 2017, 33, 14304–14314 CrossRef PubMed
.
- J. A. Barker and D. Henderson, Rev. Mod. Phys., 1976, 48, 587–671 CrossRef
.
- D. J. Kinning and E. L. Thomas, Macromolecules, 1984, 17, 1712–1718 CrossRef
.
- Y. C. Liu, S. H. Chen and R. Itri, J. Phys.: Condens. Matter, 1996, 8, A169–A187 CrossRef
.
- V. K. Aswal and P. S. Goyal, Chem. Phys. Lett., 2003, 368, 59–65 CrossRef
.
- M. del Mar Graciani, A. Rodríguez, M. Muñoz and M. L. Moyá, Langmuir, 2005, 21, 7161–7169 CrossRef PubMed
.
- P. Li, K. Ma, R. K. Thomas and J. Penfold, J. Phys. Chem. B, 2016, 120, 3677–3691 CrossRef PubMed
.
- M. Yaseen, J. R. Lu, J. R. P. Webster and J. Penfold, Biophys. Chem., 2005, 117, 263–273 CrossRef PubMed
.
- A. Ben-Shaul, I. Szleifer and W. M. Gelbart, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 4601–4605 CrossRef
.
-
J. A. Holdaway, PhD thesis, University of Bath, 2013
.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525 RSC
.
- M. d. S. Baptista, I. Cuccovia, H. Chaimovich, M. J. Politi and W. F. Reed, J. Phys. Chem., 1992, 96, 6442–6449 CrossRef
.
- J. A. Hammons, F. Zhang and J. Ilavsky, J. Colloid Interface Sci., 2018, 520, 81–90 CrossRef PubMed
.
- J. H. Clint, J. Chem. Soc., Faraday Trans. 1, 1975, 71, 1327–1334 RSC
.
- P. M. Holland and D. N. Rubingh, J. Phys. Chem., 1983, 87, 1984–1990 CrossRef
.
- J. A. Hammons, T. Muselle, J. Ustarroz, M. Tzedaki, M. Raes, A. Hubin and H. Terryn, J. Phys. Chem. C, 2013, 117, 14381–14389 CrossRef
.
- O. S. Hammond, K. J. Edler, D. T. Bowron and L. Torrente-Murciano, Nat. Commun., 2017, 8, 14150 CrossRef PubMed
.
- S. Kewalramani, H. Hlaing, B. M. Ocko, I. Kuzmenko and M. Fukuto, J. Phys. Lett., 2010, 1, 489–495 Search PubMed
.
- E. K. Perttu and F. C. Szoka, Chem. Commun., 2011, 47, 12613–12615 RSC
.
- J.-P. Hansen and J. B. Hayter, Mol. Phys., 1982, 46, 651–656 CrossRef
.
- M. A. Gebbie, A. M. Smith, H. A. Dobbs, A. A. Lee, G. G. Warr, X. Banquy, M. Valtiner, M. W. Rutland, J. N. Israelachvili, S. Perkin and R. Atkin, Chem. Commun., 2017, 53, 1214–1224 RSC
.
- J. A. Hammons, J. Ustarroz, T. Muselle, A. A. J. Torriero, H. Terryn, K. Suthar and J. Ilavsky, J. Phys. Chem. C, 2016, 120, 1534–1545 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Surfactant synthesis and characterization, X-ray reflectivity model test, SANS model test and plots from isotopic mixtures not shown in the main text. Data supporting this article has been made freely available via the University of Bath Research Data Archive system at DOI: 10.15125/BATH-00523. See DOI: 10.1039/c8sm00755a |
| ‡ A. S.-F., G. L. M. and L. M. have equally contributed to the work presented here. |
| This journal is © The Royal Society of Chemistry 2018 |