High cetane renewable diesel fuels prepared from bio-based methyl ketones and diols†
Kale W.
Harrison
and
Benjamin G.
Harvey
 *
*
US NAVY, NAWCWD, Research Department, Chemistry Division, China Lake, California 93555, USA. E-mail: benjamin.g.harvey@navy.mil
First published on 8th November 2017
Abstract
A new class of renewable diesel fuels has been synthesized by acid catalyzed condensation of 2-tridecanone with a series of diols that can be generated from biomass. These fuels exhibit comparable net heats of combustion (NHOCs) to conventional biodiesel, while maintaining derived cetane numbers between 82-91, values which are 20–30 units higher than conventional biodiesel and 40–50 units higher than petroleum-derived diesel fuel. The short combustion delays of the dioxolane fuels make them compelling blendstocks to enhance the combustion efficiency of petroleum-derived diesel fuel by increasing the cetane number and oxygen content of the blends. In addition, careful selection of the renewable diol allows for custom tailoring of viscosity and freezing point. Unlike conventional biodiesel which is often generated from plant or animal derived triglycerides, 2-tridecanone and other methyl ketones, along with the diols, can be efficiently generated from biomass sugars or CO2/H2via fermentation with metabolically engineered microorganisms. This biosynthetic approach may allow for the generation of these fuels on industrially relevant scales while eliminating competition with food sources and promoting responsible use of land resources.
Introduction
The term “biodiesel” typically refers to a fatty acid methyl ester (FAME) prepared by transesterification of triglycerides with methanol.1,2 The resulting biodiesel fuels have suitable properties for use in conventional diesel engines, and are typically blended with petroleum diesel at levels up to 20% (B20).1 The oxygen present in FAME biodiesel leads to reduced emissions of unburned hydrocarbons, carbon monoxide, and particulate matter.3 Some studies have found increases in NOx emissions for FAME blends, but in general these increases are negligible, particularly for newer engines.1 Unfortunately, FAME biodiesel typically has a higher viscosity, lower volumetric net heat of combustion (NHOC) and increased propensity to biofouling and corrosion4,5 compared to conventional diesel fuel. In addition, FAME is derived from triglycerides which in some cases (e.g. soybean; rapeseed oil) are food sources and in other cases (palm; jatropha oil) are grown in tropical climates and provide only marginal environmental benefits due to land use changes.6 Further, the total yield of oil/hectare is rather low compared to other forms of biomass which limits the potential impact of FAME type biodiesel on supplanting petroleum-based diesel fuel.7,8Lignocellulosic biomass is the only sustainable source of hydrocarbons that has the potential to replace significant quantities of petroleum. Estimates from the US Department of Energy (DOE) suggest that in the United States, 1.2–1.5 billion tons of biomass could be available for the production of renewable energy or biofuels by 2040.9 In the short term, agricultural residues, municipal solid waste, and forest resources will make up the bulk of available biomass sources. Future projections by the DOE depend on significant contributions from energy crops including switchgrass, miscanthus, sorghum, willow, eucalyptus, poplar, and pine. Similar to efforts focused on converting lignocellulosic biomass to ethanol, a route to the production of renewable diesel fuel could focus on fermentation of biomass sugars directly to fuels or fuel precursors. In addition to sustainability, this approach affords the opportunity to design fuels that have performance advantages over conventional fuels. These “designer” biofuels could potentially be used to increase the cetane number of diesel fuels and improve emission profiles. Renewable fuels comprised of long hydrocarbon chains and containing oxygenated substituents address both challenges.
The fermentation of sugars with metabolically engineered organisms to generate methyl ketones has recently been the subject of intense interest.10–13 Medium chain methyl ketones, e.g. 2-undecanone and 2-tridecanone, have been proposed as diesel fuel blending agents11 and mixtures of medium chain methyl ketones have been produced from glucose at titers of up to 3.4 g L−1 in E. coli, while Ralstonia eutropha was shown to produce up to 180 mg L−1 from CO2 and H2.13 With the preparation of methyl ketones from sustainable carbon sources firmly established, it is of significant interest to develop new methods for the conversion of methyl ketones into fuels that can be used directly in conventional diesel engines. Although medium chain methyl ketones with straight chains have high cetane numbers,11 the saturated chains also result in high melting points that are unsuitable for diesel fuel. For example, 2-tridecanone has a melting point of 24–27 °C.
One potential route to upgrade the fuel properties of methyl ketones would be to form dioxolanes through condensation with diols under acidic conditions. This approach has already been investigated for the production of gasoline range fuels and solvents,14 and diesel fuel additives.15–17 The first advantage of this approach is the ability to use bio-derived diols to form the dioxolanes. These diols could include ethylene glycol,18,19 propylene glycol,20,21 and 2,3-butanediol22 which can all be made from biomass sugars via fermentation. The second advantage is the potential to lower the freezing points of the fuels by incorporating subtle chain branching, while maintaining the high inherent cetane number of the parent methyl ketone. Finally, the elimination of water during formation of the dioxolane represents a low energy pathway for deoxygenation that does not rely on energy intensive hydrogen production.
To explore alkyl dioxolanes as a new class of renewable diesel fuels, this paper describes the synthesis of a series of fuel molecules prepared from 2-tridecanone and simple diols. Some of the basic fuel properties of these molecules are then described and compared to conventional FAME biodiesel.
Experimental
General
2-Tridecanone (>96%), ethylene glycol (EG), 1,2-propanediol (PD), and p-toluenesulfonic acid (PTSA) monohydrate were purchased from Aldrich and used as received. 2,3-Butanediol (BD, mixture of stereoisomers) was purchased from TCI America. NMR spectra were collected with a Bruker Avance II 300 MHz NMR spectrometer. 1H and 13C NMR chemical shifts are reported versus the deuterated solvent peak [CDCl3: δ 7.27 ppm (1H), 77.23 ppm (13C)]. GC/MS was performed on an Agilent Technologies 6890N network GC system with a 5973 mass selective detector. The net heats of combustion of dioxolane fuels were measured at the Southwest Research Institute using ASTM D240N. Viscosity measurements were performed with a Brookfield Engineering DV-II+ Pro viscometer equipped with the small sample adapter. The dynamic viscosities of the fuel samples were converted to kinematic viscosities at −20 °C and 40 °C by calculating the densities of the fluids at those temperatures as described in a recent paper.23 The derived cetane numbers (DCNs) of fuels were measured by ignition quality testing (IQT) at the Southwest Research Institute using ASTM D6890. Differential scanning calorimetry (DSC) experiments were performed on a TA Instruments Q100 differential scanning calorimeter. Sample sizes were between 2–10 mg and the temperature was ramped from −80 °C to 50 °C, decreased to −80 °C and then increased to 50 °C, all at 10 °C min−1. Elemental analysis was performed by Atlantic Microlabs, Norcross, GA.Synthesis of 2,4,5-trimethyl-2-undecyl-1,3-dioxolane (1)
In a typical preparation, 2-tridecanone (120 g, 0.605 mol), BD (54.52 g, 0.605 mol), and PTSA monohydrate (6.00 g, 31.54 mmol) were combined in a flask with benzene (100 mL). The mixture was heated to reflux and water produced in the reaction was collected in a Dean–Stark trap. The reaction proceeded until 10.9 mL of water was collected. Upon cooling, the reaction mixture was washed with water, a 10% sodium bicarbonate solution, and brine. The organic layer was then dried over MgSO4 and the solvent was removed under reduced pressure. Fractional vacuum distillation under reduced pressure (1 Torr) yielded a first broad cut distilling between 70–100 °C. A second cut distilling at 101 °C was collected and was 97% pure 1 by GC/MS. The isolated yield of the preparation was 88.5%. 1H NMR (CDCl3) δ 4.28–4.19 (m, 1H), 3.70–3.46 (m, 1H), 1.67–1.54 (m, 2H), 1.39 (s, 1H), 1.33 (s, 3H) 1.25 (bs, 20H), 1.15–1.12 (m, 3H), 0.90–0.85 (m, 3H). Anal. calcd for C17H34O2: C, 75.50; H, 12.67 found: C, 75.41; H, 12.74.Synthesis of 2,4-dimethyl-2-undecyl-1,3-dioxolane (2)
In a typical preparation, 2-tridecanone (120 g, 0.605 mol), PD (46.04 g, 0.605 mol), and PTSA monohydrate (6.00 g, 31.54 mmol) were combined in a flask with benzene (100 mL). The reaction and workup were conducted as described above. A distillation cut was collected from 95–102 °C and was 97% pure by GC/MS. The isolated yield of the preparation was 50.8%. 1H NMR (CDCl3) δ 4.27–4.14 (m, 1H), 4.06–4.01 (m, 1H), 3.41 (dt, J = 19.1, 7.9 Hz, 1H), 1.66–1.56 (m, 2H), 1.35–1.25 (m, 24H), 0.90–0.85 (m, 3H). Anal. calcd for C16H32O2: C, 74.94; H, 12.58. Found: C, 75.16; H, 12.75.Synthesis of 2-methyl-2-undecyl-1,3-dioxolane (3)
In a typical preparation, 2-tridecanone (120 g, 0.605 mol), EG (37.55 g, 0.605 mol), and PTSA monohydrate (6.00 g, 31.54 mmol) were combined in a flask with benzene (100 mL). The reaction and workup were conducted as described above. A distillation cut was collected from 92–98 °C and was 95% pure by GC/MS. The isolated yield of the preparation was 50.5%. 1H NMR (CDCl3) δ 3.99–3.78 (m, 4H), 1.61–1.53 (m, 2H), 1.43–1.13 (bs, 21H), 0.87–0.82 (m, 3H). Anal. calcd for C15H30O2: C, 74.32; H, 12.47. Found: C, 74.55; H, 12.61.Results and discussion
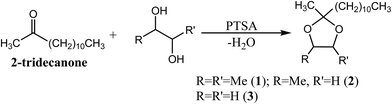
The synthesis of dioxolanes 1–3 (Fig. 1) was achieved using equimolar amounts of a given diol and 2-tridecanone. PTSA was selected as a convenient, low cost acid catalyst and utilized at relatively high loading (∼5 mol%) to reduce the reaction time. The isolated (distilled) yield of 1 was nearly 90%, while the yields of 2 and 3 were more modest (∼50%), due in part to the difficulty in efficiently separating 2-tridecanone from the lower boiling dioxolanes via distillation. It was also observed that a certain amount of the diol co-distills with the water/benzene azeotrope during the reaction. This effect was more pronounced when PD and EG were used as reagents. The high solubility of PD and EG in water resulted in significant phase transfer to the aqueous layer of the Dean–Stark trap, resulting in a change in stoichiometry that increased the concentration of tridecanone in the final reaction mixture. The difference in stoichiometry is dependent on the diol, size of the Dean–Stark trap relative to the reaction volume, and the reflux rate. Increasing the amount of diol in the original reaction mixture would be expected to increase the yield of dioxolanes 2 and 3, but it could also increase the formation of unwanted side products including low molecular weight dioxolanes and aldehydes. Due to the focus of this work on the fuel properties of the dioxolanes, no further optimization of the reaction conditions was attempted, but it is likely that the yields of 2 and 3 could be improved by controlling the diol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-tridecanone ratio and using a more efficient fractional distillation system.
2-tridecanone ratio and using a more efficient fractional distillation system.
In addition to optimization of the reaction conditions, the use of a solid acid catalyst in place of PTSA would allow for easier isolation of the product and catalyst recyclability. Solid acid catalysts could also be used in continuous or semi-batch processes for production of these dioxolanes on a large scale. Recent work has shown that Amberlyst-15, a heterogeneous Brønsted acid catalyst, is suitable for the dehydration/condensation of BD to form 2-ethyl-2,4,5-trimethyl-1,3-dioxolane.14 The acid clay, montmorillonite K-10, has also been shown to convert 2-ethylhexane-1,2-diol to a mixture of isomeric dioxolanes.24 These results suggest that a variety of Brønsted and Lewis acid catalysts should be screened for the conversion of methyl ketones to alkyl dioxolanes.
Dioxolanes 1–3 were analyzed by 1H and 13C NMR, GC/MS, and elemental analysis. In the case of compound 1, which contains three stereocenters and a plane of symmetry that reduces the stereodiversity of the products, three diastereomeric pairs are formed. This can be most clearly observed in the 13C spectrum which contains three resonances at ∼109 ppm resulting from the quaternary carbon of the dioxolane ring (Fig. S2†). Compound 2 only has two stereocenters, and as expected, two diastereomeric pairs are observed in the 13C NMR spectrum (Fig. S4†). Finally, compound 3 has a single stereocenter and only one set of resonances is observed in the 13C NMR spectrum (Fig. S6†). The dioxolanes were isolated in 95–97% purity, as measured by GC/MS.
After establishing the purity of the dioxolanes, the key fuel properties of 1–3, including their densities, net heats of combustion (NHOC), viscosities, freezing/melting points, and derived cetane numbers (DCN) were measured (Table 1). The densities of the dioxolane fuels are in the range of 0.87–0.88 g mL−1 and they all have similar NHOCs around 115 kBtu gal−1. The dynamic viscosity of compounds 1 and 2 was measured from −20 to 40 °C, while that of compound 3 was measured from 0 to 40 °C due to its higher freezing point (Fig. 2). The lowest viscosity at 40 °C was observed for 1, due to the presence of several isomers and more chain branching than the other two dioxolanes. Interestingly, this trend was reversed at low temperatures, likely due to the higher molecular weight of 1. 1 also had the lowest freezing and melting points as measured by DSC (Fig. 3, S7 and S8†). The derived cetane numbers for all three dioxolanes were above 80. This is a remarkable result and suggests that these fuels could be used as blending agents to enhance the cetane numbers of petroleum derived diesel fuels.
| Fuel | Density (g mL−1) | NHOC (kBtu gal−1) | Viscosity (40 °C, mm2 s−1)a | Freezing/MP (°C)b | DCN |
|---|---|---|---|---|---|
| a The kinematic viscosities of 1–3 were calculated from the dynamic viscosities as described in ref. 23. b Freezing and melting points were measured by DSC and are the maxima or minima of the respective peaks. PP is short for pour point. c Taken from ref. 2. d Taken from ref. 25. e Numbers in parentheses are the standard deviations of the measurements. | |||||
| 1 | 0.868 | 114.8 | 4.44 | −51/−18 | 84(2)e |
| 2 | 0.872 | 114.8 | 5.15 | −48/−14 | 91(3)e |
| 3 | 0.883 | 115.8 | 4.98 | −25/0 | 81(2)e |
| Soy biodieselc | 0.882 | 117.1 | 4.26 | 0/−4 (PP) | 51.3 |
| Palm biodieselc | 0.873 | 116.8 | 4.61 | 14/13(PP) | 61.9 |
| Jatropha biodieselc | 0.876 | 118.5 | 4.75 | 5/0 (PP) | 55.7 |
| Winter diesel (U.S.)d | 0.826–0.859 | ∼129 | 1.9–4.1 | <−3 (PP) | 40–54 |
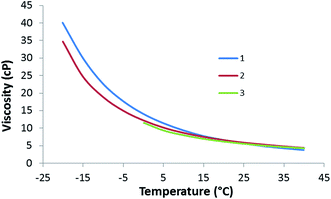 | ||
| Fig. 2 Dynamic viscosity of dioxolanes derived from 2-tridecanone. The data for compound 3 is truncated due to freezing of the liquid after equilibration in the viscometer at temperatures below 0 °C. | ||
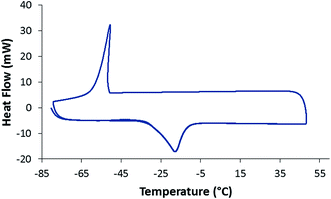 | ||
| Fig. 3 DSC data for compound 1. The temperature was ramped from −80 to 50 °C, decreased to −80 °C, and increased to 50 °C, all at 10 °C min−1. | ||
The properties of 1–3 can be compared with several conventional biodiesel fuels and petroleum-derived winter diesel (Table 1). The density and NHOCs of the dioxolane fuels are quite comparable to the biodiesel fuels which would be expected based on the similarities in density and oxygen content between the fuels. Soy, palm, and jatropha derived biodiesels have a lower percent oxygen content compared to the dioxolanes which leads to the slightly higher NHOC values for the FAME fuels. The 40 °C viscosities of 2 and 3 are slightly higher than the FAME fuels, but the extra chain branching in 1 reduces the viscosity to a point that is lower than palm and jatropha biodiesel. The freezing points of 1–3 are in general lower than those reported for conventional biodiesel fuels (Table 1). Although 3 has a melting point comparable to the pour point of soy, palm, and jatropha-derived biodiesel, 1 and 2 have significantly lower melting points, consistent with extra chain branching.
Perhaps the most striking difference between the FAME and dioxolane fuels is the substantially higher range of DCNs observed for 1–3. The dioxolane-based fuels have DCNs ranging from 81–91 units, while the FAME fuels have DCNs ranging from 51–62, roughly 30 units lower. Some insight into this difference can be gained through an examination of the cetane numbers of various hydrocarbons and their analogous methyl esters (Table 2). For example, a comparison of hexadecane (CN = 100) to methyl palmitate (CN = 74–86 (ref. 26 and 27)) reveals a substantial decrease in the cetane number. These lower cetane numbers can be attributed to stabilization of radical species by resonance structures with the carbonyl group, formed after radical abstraction of hydrogen from the methylene alpha to the carbonyl (Cα).28 In contrast, a comparison of compounds 1–3 (DCNs = 81–91) to tridecane (CN = ∼80 (ref. 29)), suggests that the dioxolane functionality does not negatively impact the cetane number despite the presence of additional chain branching (compounds 1 and 2). This is not surprising given that ether linkages decrease the bond dissociation energy of Cα–H bonds, resulting in shorter ignition delays and higher cetane numbers.28 For example, diethyl ether has an estimated cetane number greater than 125.30 The dioxolanes also have higher cetane numbers than the parent methyl ketone. The DCN of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 blend of 2-undecanone and 2-tridecanone has been reported as 58.4, while the cetane number of 2-undecanone was reported as 56.6.11 This suggests that the cetane number of 2-tridecanone is approximately 60, which is 20–30 units lower than dioxolanes 1–3.
50 blend of 2-undecanone and 2-tridecanone has been reported as 58.4, while the cetane number of 2-undecanone was reported as 56.6.11 This suggests that the cetane number of 2-tridecanone is approximately 60, which is 20–30 units lower than dioxolanes 1–3.
In addition to the properties of the dioxolane fuels, the long term sustainable production of these materials is a critical consideration. Use of lignocellulosic biomass as a feedstock could allow for large scale production of these fuels with significant greenhouse gas reductions. A recent study by Biddy et al.31 suggests that in the near term, diesel fuels generated from lignocellulosic feedstocks by hydrolysis, fermentation of biomass sugars to fatty acids, and chemical upgrading (hydrotreating), could be produced for $9.55/(gallon of gasoline equivalent; GGE). In the long term, these prices could be as low as $2–3/GGE, assuming generation of high value co-products and transformational improvements in carbon efficiency and purification. Translating this model to commercial production of methyl ketones and alkyl dioxolanes, suggests the potential for eventual cost parity with petroleum derived fuels, particularly in regulatory environments that provide incentives for reducing carbon emissions. Another intriguing approach would be to couple methyl ketone production to solar powered water hydrolysis for the storage of solar energy in the form of alkyl dioxolanes. Work by Nocera has shown that photosynthetic systems based on Ralstonia eutropha can be used for the production of fuels and chemicals from CO2 and sunlight.32 Nocera's system is capable of up to 10% CO2 reduction energy efficiency, greatly outperforming natural photosynthesis. Further, Beller has recently reported that methyl ketones can be generated by Ralstonia eutropha from CO2 and H2.13 The use of Beller's organisms in Nocera's system would be a compelling route to the sustainable production of dioxolane fuels.
Conclusions
Dioxolanes prepared via the acid catalyzed condensation of renewable diols with bio-derived methyl ketones show great promise as next generation renewable diesel fuels. The viscosity, freezing point, and cetane number of these fuels can be controlled by judicious choice of the methyl ketone and diol. The dioxolane fuels have similar volumetric net heats of combustion to conventional biodiesel fuels, but significantly higher cetane numbers and lower melting points. Although additional engine and emissions testing will need to be conducted, the dioxolanes described in this study would be expected to enhance the combustion efficiency of conventional petroleum distillates by increasing both the oxygen content and cetane number of blends. The potential to derive both methyl ketones and diols from lignocellulosic biomass or atmospheric CO2 may allow for sustainable production of these fuels on an industrial scale.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Naval Air Warfare Center, Weapons Division for funding this work.References
- R. L. McCormick, A. Williams, J. Ireland, M. Brimhall and R. R. Hayes, Effects of Biodiesel Blends on Vehicle Emissions, Milestone Report NREL/MP-540-40554, National Renewable Energy Laboratory, 2006 Search PubMed.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros and M. Natarajan, Renewable Sustainable Energy Rev., 2012, 16, 143–169 CrossRef CAS.
- United States Environmental Protection Agency, A Comprehensive Analysis of Biodiesel Impacts on Exhaust Emissions, Draft Technical Report, EPA420-P-02001, 2002 Search PubMed.
- B. S. Sazzad, M. A. Fazal, A. S. M. A. Haseeb and H. H. Masjuki, RSC Adv., 2016, 6, 60244–60263 RSC.
- D. F. Aktas, J. S. Lee, B. J. Little, R. L. Ray, I. A. Davidova, C. N. Lyles and J. M. Suflita, Energy Fuels, 2010, 24, 2924–2928 CrossRef CAS.
- W. M. J. Achten and L. V. Verchot, Ecol. Soc., 2011, 16, 14 Search PubMed.
- C. Somerville, Curr. Biol., 2007, 17, R115–R119 CrossRef CAS PubMed.
- R. J. Henry, Plant Biotechnol. J., 2010, 8, 288–293 CrossRef CAS PubMed.
- U.S. Department of Energy, Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks, ed. M. H. Langholtz, B. J. Stokes and L. M. Eaton, ORNL/TM-2016/160, Oak Ridge National Laboratory, Oak Ridge, TN, 2016, 448p, DOI:10.2172/1271651.
- H. R. Beller, T. S. Lee and L. Katz, Nat. Prod. Rep., 2015, 32, 1508–1526 RSC.
- E. B. Goh, E. E. Baidoo, J. D. Keasling and H. R. Beller, Appl. Environ. Microbiol., 2012, 78, 70–80 CrossRef CAS PubMed.
- E. B. Goh, E. E. Baidoo, H. Burd, T. S. Lee, J. D. Keasling and H. R. Beller, Metab. Eng., 2014, 26, 67–76 CrossRef CAS PubMed.
- J. Müller, D. MacEachran, H. Burd, N. Sathitsuksanoh, C. Bi, Y. C. Yeh, T. S. Lee, N. J. Hillson, S. R. Chhabra, S. W. Singer and H. R. Beller, Appl. Environ. Microbiol., 2013, 79, 4433–4439 CrossRef PubMed.
- B. G. Harvey, W. W. Merriman and R. L. Quintana, ChemSusChem, 2016, 9, 1814–1819 CrossRef CAS PubMed.
- E. García, M. Laca, E. Pérez, A. Garrido and J. Peinado, Energy Fuels, 2008, 22, 4274–4280 CrossRef.
- I. Agirre, V. L. Barrio, M. B. Guemez, J. F. Cambra and P. L. Arias. Acetals as possible diesel additives in Economic effects of biofuel production, ed. M. A. Bernardes, InTech, 2011, pp. 299–316 Search PubMed.
- X.-B. Fan, N. Yan, Z.-Y. Tao, D. Evans, C.-X. Xiao and Y. Kou, ChemSusChem, 2009, 2, 941–943 CrossRef CAS PubMed.
- N. Ji, T. Zhang, M. Zheng, A. Wang, H. Wang, X. Wang and J. G. Chen, Angew. Chem., Int. Ed., 2008, 47, 8510–8513 CrossRef CAS PubMed.
- M.-Y. Zheng, A.-Q. Wang, N. Ji, J.-F. Pang, X.-D. Wang and T. Zhang, ChemSusChem, 2010, 3, 63–66 CrossRef CAS PubMed.
- J. Van Haveren, E. L. Scott and J. Sanders, Biofuels, Bioprod. Biorefin., 2008, 2, 41–57 CrossRef CAS.
- J. H. Clark, T. J. Farmer, A. J. Hundt and J. Sherwood, Int. J. Mol. Sci., 2015, 16, 17101–17159 CrossRef CAS PubMed.
- X.-J. Ji, H. Huang and P.-K. Ouyang, Biotechnol. Adv., 2011, 29, 351–364 CrossRef CAS PubMed.
- B. G. Harvey, W. W. Merriman and T. A. Koontz, Energy Fuels, 2015, 29, 2431–2436 CrossRef CAS.
- B. G. Harvey, H. A. Meylemans and R. L. Quintana, Green Chem., 2012, 14, 2450–2456 RSC.
- Infineum International, Ltd, Worldwide Winter Diesel Fuel Quality Survey 2014, Infineum International, Ltd., Abingdon, U.K., 2014, https://www.infineum.com/media/80722/wdfs-2014-fullscreen.pdf, accessed July 27, 2017 Search PubMed.
- J. Yanowitz, M. A. Ratcliff, R. L. McCormick, J. D. Taylor and M. J. Murphy, Compendium of Experimental Cetane Numbers, National Renewable Energy Laboratory (NREL), Golden, CO, 2014, NREL/TP-5400–61693. The cetane number of methyl palmitate is reported as 74.3-74.5 when measured by a CFR engine Search PubMed.
- G. Knothe, Energy Fuels, 2008, 22, 1358–1364 CrossRef CAS.
- M. D. Boot, M. Tian, E. J. M. Hensen and S. M. Sarathy, Prog. Energy Combust. Sci., 2017, 60, 1–25 CrossRef.
- The cetane number of tridecane as measured by an ASTM method is not included in ref. 26, and to our knowledge, has not been reported in the literature. An estimate of the cetane number as determined by IQT was calculated by averaging the DCN (taken from ref. 26) of n-dodecane (73.5) and n-tetradecane (85.1).
- K. Górski and M. Przedlacki, Energy Fuels, 2014, 28, 2608–2616 CrossRef.
- M. J. Biddy, R. Davis, D. Humbird, L. Tao, N. Dowe, M. T. Guarnieri, J. G. Linger, E. M. Karp, D. Salvachúa, D. R. Vardon and G. T. Beckham, ACS Sustainable Chem. Eng., 2016, 4, 3196–3211 CrossRef CAS.
- C. Liu, B. C. Colon, M. Ziesack, P. A. Silver and D. G. Nocera, Science, 2016, 352, 1210–1213 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00415j |
| This journal is © The Royal Society of Chemistry 2018 |