Open Access Article
Open Access ArticleEmbryonic brass: pseudo two electron Cu/Zn clusters†
Hung
Banh‡
ab,
Julius
Hornung‡
ab,
Thilo
Kratz
ab,
Christian
Gemel
ab,
Alexander
Pöthig
 ab,
Franck
Gam
c,
Samia
Kahlal
c,
Jean-Yves
Saillard
*c and
Roland A.
Fischer
ab,
Franck
Gam
c,
Samia
Kahlal
c,
Jean-Yves
Saillard
*c and
Roland A.
Fischer
 *ab
*ab
aInorganic and Metalorganic Chemistry, Technical University Munich, Lichtenbergstr. 4, 85748 Garching bei München, Germany. E-mail: Roland.Fischer@tum.de
bCatalysis Research Center & Department of Chemistry, Technical University Munich, Ernst-Otto-Fischer-Str. 1, 85747 Garching bei München, Germany
cUniv Rennes, CNRS, ISCR-UMR 6226, F-35000 Rennes, France. E-mail: jean-yves.saillard@univ-rennes1.fr
First published on 1st November 2018
Abstract
The isoelectronic M7 clusters [Cu3Zn4](Cp*)5 (1) and {[Cu2Zn5](Cp*)5}+ (2) are described. While 1 can be isolated only as a minor side product from the reaction of Cu(CH3CO2) with equimolar amounts of [Zn2Cp*2] with the trigonal cluster [CuZn2](Cp*)3 as the major product, 2 is available in acceptable yields from the reaction of [CuZn2](Cp*)3 with the Cp*Zn2-transfer-reagent [Cp*Zn2(Et2O)3][BAr4F]. The trigonal bipyramidal Cu/Zn-clusters exhibit exceptional bonding situations: with formally only one skeleton electron pair they can be regarded as highly electron deficient. However, a detailed DFT analysis reveals that the cluster bonding is supported by 3d orbital contributions of the trigonal metal base unit. The data contribute to the development of an advanced tool-box for synthesis of Hume-Rothery intermetallic (e.g. brass) inspired clusters.
Introduction
Ligand stabilized metal clusters [Mn](L)m are fascinating links between molecules and bulk metals. They have attracted persistent interest since Hieber described [Co4](CO)12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 1932 up to the discovery of very high nuclearity transition-metal carbonyl clusters such as Dahl's [Pd145](CO)60(PEt3)30
1 in 1932 up to the discovery of very high nuclearity transition-metal carbonyl clusters such as Dahl's [Pd145](CO)60(PEt3)30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reported in 2000 and finally the thiolate-capped atom-precise gold clusters with the giant [Au246](p-MDT)80
2 reported in 2000 and finally the thiolate-capped atom-precise gold clusters with the giant [Au246](p-MDT)80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 as a spectacular and recent example (2016).
3 as a spectacular and recent example (2016).
A related break-through in main group metal clusters was Schnöckel's work on bottom-up synthesis based on metastable solutions of low valent group-13 species, and [Al50]Cp*12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (2004) became an icon of this chemistry (Cp* = C5(CH3)5). The cluster growth reactions are difficult to control and each case is very subtly dependent on the kinetic balance of many interlinked processes, i.e. disproportionation, agglomeration, ligand substitution and/or salt metathesis. Nevertheless, over the years a rich library of small, medium to high nuclearity clusters was successively developed.5 In this context we started out to investigate the related organometallic cluster chemistry of Zn and studied the activation of low valent [Zn2Cp*2] by oxidative cleavage of one Zn–Cp* bond to induce disproportionation of the initial intermediate [Zn2Cp*]+. From the reaction mixture {[Zn10]Cp*6(CH3)}+ was isolated as the very first example of a ligated oligonuclear Zn-cluster.6
4 (2004) became an icon of this chemistry (Cp* = C5(CH3)5). The cluster growth reactions are difficult to control and each case is very subtly dependent on the kinetic balance of many interlinked processes, i.e. disproportionation, agglomeration, ligand substitution and/or salt metathesis. Nevertheless, over the years a rich library of small, medium to high nuclearity clusters was successively developed.5 In this context we started out to investigate the related organometallic cluster chemistry of Zn and studied the activation of low valent [Zn2Cp*2] by oxidative cleavage of one Zn–Cp* bond to induce disproportionation of the initial intermediate [Zn2Cp*]+. From the reaction mixture {[Zn10]Cp*6(CH3)}+ was isolated as the very first example of a ligated oligonuclear Zn-cluster.6
The synthetic scenario becomes even more diverse and challenging for bimetallic clusters [M1aM2b](L)m, in particular when combining chemically distinctly different metals. A specific tool-box of suitable building blocks and reactions needs to be developed for each metal combination. For example, [Cu4Zn4](CNtBu)4Cp*4 is reproducibly available in moderate yields from [Zn2Cp*2] and CpCu(CNtBu) among other side products.7 Reaction steps are the reduction of Cu(I) by Zn(I) under formation of [Cp2Zn], [Cp*CpZn] and [Cp*2Zn] by involving Zn–Zn bond cleavage as well as Cp transfer from Cu to Zn as a side reaction. The [M1aM2b] cores of such bimetallic clusters often mimic structural cut-outs from of the respective M1/M2 intermetallic solid-state phases.8 Thus, the M8-core of [Cu4Zn4](CNtBu)4Cp*4 is linked to an embryonic state of γ-brass, the classic Hume-Rothery intermetallic compound.
A rational design of the synthesis procedure to yield a specific Cu/Zn-cluster also requires knowledge of potentially preferred stability islands of “magic” compositions including the role of the capping ligands. While the electronic structures of classic transition metal carbonyl-clusters are often consistent with the Wade–Mingos rules originally developed for borane clusters, the electronic situation in metal clusters in general is more complicated and needs to be addressed by computational methods in every single case.9 For example, the electron deficiency of {[Zn10](Cp*)6(CH3)}+ results mainly from the fact that Zn can behave flexible in participating to cluster skeletal bonding, sometimes providing one frontier orbital (like Group 11 metals) and sometimes providing three frontier orbitals (like Group 13 elements).6,10 Following this research guidelines towards a library of oligonuclear Cu/Zn-clusters as embryonic brass species we report here on two astonishing cases. At a first glance they completely disobey the Wade–Mingos rules for deltahedral clusters. The two isoelectronic, trigonal bipyramidal M7 clusters, namely the neutral [Cu3Zn4]Cp*5 (1) and the cation {[Cu2Zn5]Cp*5}+ (2) are teaching us an interesting lesson.
Results and discussion
Synthesis and characterization of [Cu3Zn4](Cp*)5 (1)
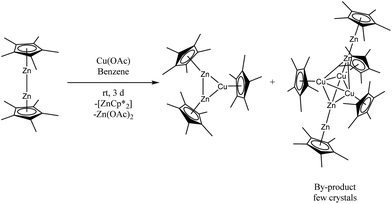
Reaction of Cu(CH3CO2) with equimolar amounts of [Zn2Cp*2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–13 in benzene at room temperature for three days leads to the formation of a dark red suspension of a mixture of unsoluble Zn(CH3CO2)2, the known cluster [CuZn2](Cp*)3 and the novel cluster [Cu3Zn4](Cp*)5 (1) as soluble components (Scheme 1).
11–13 in benzene at room temperature for three days leads to the formation of a dark red suspension of a mixture of unsoluble Zn(CH3CO2)2, the known cluster [CuZn2](Cp*)3 and the novel cluster [Cu3Zn4](Cp*)5 (1) as soluble components (Scheme 1).
From the filtered and concentrated reaction solution, [CuZn2](Cp*)3 deposits as the main product in the form of yellow crystals at 8 °C in 57% isolated yield (based on Cp*). Further workup of the remaining solution gives [Cu3Zn4](Cp*)5 (1) co-crystallizing together with [CuZn2](Cp*)3 at −30 °C in THF in form of dark red crystals. The single crystals of 1 could be isolated by manual separation from [CuZn2](Cp*)3 with the aid of an optical microscope in a glove box under argon atmosphere. It should be noted, that we were not successful in increasing the yield of 1 by the change of reaction parameters such as time, temperature or stoichiometric ratio of the reactants. A more rational approach by using Cp*Zn2-transfer reagents similar to the synthesis of 2 (vide infra), is not possible in this case due to the inexistence of the required base unit [Cu3](Cp*)3 (no skeleton bonding electrons). The formation mechanism of 1 is yet not clear. However, redox-reactions between Zn(I) and Cu(I) must play a role. While in the formation of the main product [Zn2Cu](Cp*)3 from Cu(CH3CO2) and [Zn2Cp*2] no redox-chemistry but only Cp* transfer reactions are involved, formation of 1 requires the reduction of M(I) to M(0). The accompanying oxidation process is obviously the formation of Zn(CH3CO2)2 from the Zn(I) source.
In situ 1H NMR spectroscopy of the reaction mixture indicates rather clean formation of [CuZn2](Cp*)3 under complete consumption of [Zn2Cp*2] (Fig. S4†) with [Cp*2Zn] and 1 as minor by-products. An unidentified Cp*-containing intermediate can be detected, which almost completely disappears in the course of the reaction. The mechanism involves Cp* transfer from Zn to Cu as well as disproportionation of Zn(I) to Zn(0) and Zn(II). The identity of [CuZn2](Cp*)3 has been confirmed by single crystal XRD measurement as well as NMR spectroscopic analysis. The data are in good agreement with the data reported in literature.14 The separated single crystals of [Cu3Zn4](Cp*)5 (1) are stable under inert gas atmosphere for several months at −30 °C and are fairly soluble in non-polar solvents like n-hexane and benzene, but 1 dissolves very well in toluene, THF or fluorobenzene. However, it readily decomposes in solution at room temperature within a few hours under precipitation of metallic particles. This property limits further characterization of its reactivity.
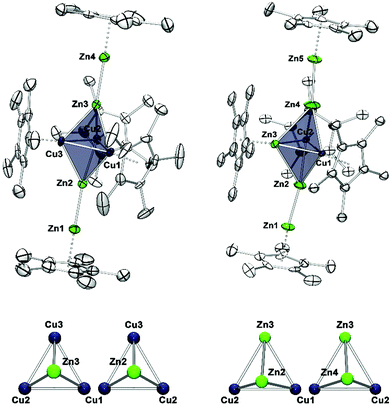
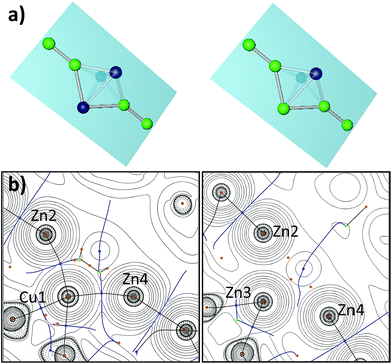
Compound 1 crystallizes in the monoclinic space group Pn with four distinct molecules in the asymmetric unit. Fig. 1 (left) shows only one of the four crystallographically distinct units for clarity. Note that Cu and Zn are not easily distinguishable by standard X-ray techniques, thus the assignment of Cu and Zn in the structure refinement has been supported by spectroscopic data (vide infra). The metal atoms are arranged in a trigonal bipyramidal fashion, with the apical positions occupied by ZnZnCp* units and the remaining equatorial triangle by CuCp* groups. Trigonal Cu3 units are rather common in molecular clusters. However, in all species known the Cu–Cu contacts are supported by μ2/μ3-bridging and chelating ligands and are not bridged by other metal atoms as it is the case for 1.15–18 The Cu–Cp*centr. distances of 1 range from 1.827–1.828 Å and are shorter than the respective distances in the terminal Zn1–Cp*centr. (1.901 Å) and Zn4–Cp*centr. (1.922 Å) units.
However, all values are in good agreement to literature known Zn–Cp*centr. (1.83–2.19 Å) and Cu–Cp*centr. (1.82–1.96 Å) distances. The Zn1–Zn2 and Zn4–Zn5 bond lengths are almost identical with 2.318(2) and 2.317(2) Å, respectively. These values are well comparable to the Zn–Zn distance in [Cp*Zn2(THF)3]+ (2.317(7) Å) (Fig. S12†). All other M–M distances are distinctively longer with a narrow range of 2.431(2) (Cu1–Zn3)–2.458(2) Å (Cu2–Zn3). These data are similar to the Cu–Cu distance in [Cu4Zn4](CNtBu)4(Cp*)4 (2.471(4) Å)7 and the average Zn–Zn distance in {[Zn3](Cp*)3}+ (2.430 Å), but elongated compared to the Zn–Cu distances (2.381(1) Å) of [CuZn2](Cp*)3.6,19 As a consequence, the trigonal bipyramid consists of two almost perfect [Cu3Zn] tetrahedrons sharing one trigonal Cu3 face. Accordingly, the angles within the Cu1–Cu2–Cu3 triangle are all very close to 60° (59.65(5) for Cu3–Cu1–Cu2 and 60.25(6)° for Cu2–Cu3–Cu1). The Zn2–Zn1–Cp*centr. and Zn3–Zn4–Cp*centr. moieties are slightly deviated from linearity with angles of 175,74 and 176.00°, respectively.
Liquid injection field desorption mass spectrometry (LIFDI-MS) confirms the molecular composition of 1 with a well detectable [M]˙+ ion peak at m/z [a.u.] = 1128 (Fig. 2) as well as two fragment peaks [M–ZnCp*]˙+ and [M–ZnZnCp*]˙+ at m/z [a.u.] = 927 and 861, respectively (Fig. S3†). Interestingly, no loss of CuCp* is observed in the fragment ions, confirming the assignment of elements in the X-ray single crystal structure with equatorial CuCp* and apical ZnZnCp* units.
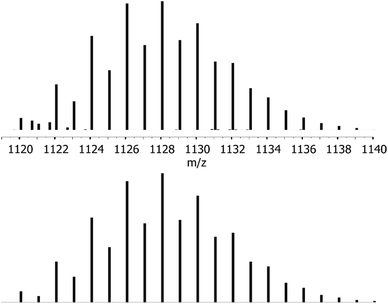 | ||
| Fig. 2 Molecular ion peak of 1 (m/z = 1128) from LIFDI-MS measurements (top) and calculated isotopic pattern for 1 (bottom). | ||
The 1H NMR spectrum of 1 in C6D6 at room temperature shows two Cp* resonances at 2.08 and 2.19 ppm in a 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 integral ratio, corresponding to the three chemically equivalent CuCp* groups as well as two equivalent apical ZnZnCp* groups. Accordingly, the 13C NMR spectrum shows the expected set of signals for two different Cp* moieties (δ = 10.90 (s, CuC5Me5), 12.17 (s, ZnZnC5Me5), 104.62 (s, CuC5Me5), 110.18 (s, ZnZnC5Me5) ppm). Note, that the only assignment of metal atoms which is in agreement with this observed D3h symmetry of the cluster in solution is the allocation of the four Zn atoms to the apical positions and the three Cu atoms to the equatorial triangle (Scheme S1†).
30 integral ratio, corresponding to the three chemically equivalent CuCp* groups as well as two equivalent apical ZnZnCp* groups. Accordingly, the 13C NMR spectrum shows the expected set of signals for two different Cp* moieties (δ = 10.90 (s, CuC5Me5), 12.17 (s, ZnZnC5Me5), 104.62 (s, CuC5Me5), 110.18 (s, ZnZnC5Me5) ppm). Note, that the only assignment of metal atoms which is in agreement with this observed D3h symmetry of the cluster in solution is the allocation of the four Zn atoms to the apical positions and the three Cu atoms to the equatorial triangle (Scheme S1†).
Synthesis and characterization of {[Cu2Zn5](Cp*)5}[BAr4F] (2[BAr4F])
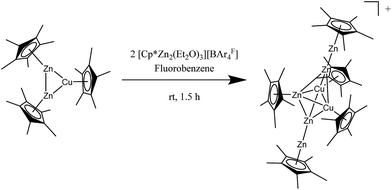
The reaction of [Zn2Cu](Cp*)3 with two equivalents of the Cp*Zn2 transfer-reagent [Zn2(Cp*)(Et2O)3][BAr4F]20 in fluorobenzene at room temperature for 1.5 h (Scheme 2) gives a dark red solution with very little amount of metallic precipitate. The product cluster {[Zn5Cu2](Cp*)5}[BAr4F]·5C6H5F (2[BAr4F]·5C6H5F) crystallizes from the concentrated an filtered reaction solution at −30 °C in the form of dark red needles.Minor amounts of an unidentified side product are removed by repeated recrystallization of the crude product in fluorobenzene at −30 °C giving 2[BAr4F] in 23% analytically pure yield (based on Cu). As described for the formation of 1, the reaction leading to 2 proceeds via a Cp* exchange mechanism. This is in nice accordance with the reactivity studies of [Cp*Zn2(THF)3]+ showing that this cation can act as both, a Cp* donor as well as a Cp* acceptor forming {[Zn2](THF)6}2+ or [Zn2Cp*2] (Scheme S4†).6 Likewise, an in situ NMR experiment of a mixture of [Cp*Zn2(Et2O)3]+ and [CuZn2](Cp*)3 at room temperature in fluorobenzene points to a rapid Cp* exchange between the metal atoms, indicated by the detection of only one coalescence signal for all Cp* groups. This signal decoalesces into two peaks at temperatures below −20 °C (see Fig. S7†). Due to incomplete separation of the signals at −50 °C, no integral ratio of the two peaks could be determined. As in the case of 1, changing reaction parameters such as temperature, time or stoichiometric ratio of the reactants did not result in any increase of the isolated yield. The complete pathway leading from the reactants to cluster 2 appears rather complex, as evident from the composition of the central trigonal M3 unit. While the product exhibits a [Cu2Zn] central unit, the starting cluster consists of a [CuZn2] triangle. However, despite the obviously involved redox chemistry, no oxidized side products as it is the case in the synthesis of 1 could be detected.
The compound 2[BAr4F]·5C6H5F crystallizes in the monoclinic space group Cc with two distinct ion pairs in the asymmetric unit. Only one of these two independent units is shown in Fig. 1 (above) for clarity. Similar to compound 1, the metal atoms of cation 2 are arranged in a trigonal bipyramidal fashion, however, with higher deviations from the ideal polyhedral structure. Again, assignment of metal atoms to the atomic positions in the crystal structure has been done with the support of spectroscopic data (vide infra). The equatorial triangle consists of a Cu2Zn unit capped by ZnZnCp* units on both sides. The apical Zn1–Zn2 (2.299(6) Å) and Zn4–Zn5 (2.323(6) Å) bond lengths are comparable to the respective apical Zn1–Zn2 (2.318(2) Å) and Zn3–Zn4 (2.317(2) Å) distances in 1. The Cu1–Cu2 bond length in 2 (2.415(3) Å) is shorter than the Cu–Cu distances in [Cu4Zn4](CNtBu)4Cp*4 (2.471(4) Å) and in 1 (2.431(2)–2.446(2) Å).7 The equatorial Cu2Zn triangle is almost perfectly equilateral (Cu1–Cu2 = 2.415(3), Cu1–Zn3 = 2.400(4), Cu2–Zn3 = 2.396(3) Å). However, the intersection of the apical Zn2–Zn4 axis does not coincide with the centre of gravity of the equatorial Cu3-plane, but is rather dislocated towards the Cu2 edge. Thus, the contacts of the apical Zn atoms are not equidistant to the three equatorial metal atoms: the Zn3–Zn2 (2.745(6) Å) and Zn3–Zn4 (2.688(7) Å) distances are considerably longer than the Cu–Zn2 (avg. 2.463 Å) and Cu–Zn4 (avg. 2.487 Å) distances.
The molecular composition of 2[BAr4F] as determined by EA (C, H) and AAS (Zn, Cu, B, F) is in good agreement with the sum formula (calculated for H87BC82F24Cu2Zn5 [%]: H 4.40, B 0.54, C 49.41, F 22.87, Cu 6.38, Zn 16.40; found: H 4.62, B 0.47, C 51.48, F 20.20, Cu 6.48, Zn 15.98). The deviations in the carbon and fluorine values are attributed to the thermal instability of the compound and the difficulty in handling the pure samples. The 1H NMR spectrum of 2[BAr4F] in CD2Cl2 at room temperature shows three resonances at 1.96 (s, 30, ZnZnC5Me5), 2.04 (s, 15H, ZnC5Me5) and 2.12 (s, 30H, CuC5Me5) ppm for the three chemically non-equivalent Cp* groups in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, which points to a C2v symmetry of 2 in solution. In addition, the expected set of signals for the [BAr4F]− anion 7.56 (s, 4H) and 7.72 ppm (s, 8H) are detected. Due to the high lability of 2[BAr4F] in solution and the moderate solubility of 2[BAr4F] at low temperatures meaningful 13C NMR spectra could not be obtained. The IR spectrum reveals typical absorption bands for the Cp* moieties at 2905 and 2860 cm−1 and the C–F vibration of the [BAr4F]− anion at 1272 cm−1.20 The only reasonable assignment of elements to the metal core of the molecular structure of 2, which is in agreement with the symmetry of the cation in solution (C2v), is the allocation of four Zn atoms in the axial MMCp* moieties, leaving a [ZnCu2] triangle in the equatorial plane (Scheme S2†).
2 ratio, which points to a C2v symmetry of 2 in solution. In addition, the expected set of signals for the [BAr4F]− anion 7.56 (s, 4H) and 7.72 ppm (s, 8H) are detected. Due to the high lability of 2[BAr4F] in solution and the moderate solubility of 2[BAr4F] at low temperatures meaningful 13C NMR spectra could not be obtained. The IR spectrum reveals typical absorption bands for the Cp* moieties at 2905 and 2860 cm−1 and the C–F vibration of the [BAr4F]− anion at 1272 cm−1.20 The only reasonable assignment of elements to the metal core of the molecular structure of 2, which is in agreement with the symmetry of the cation in solution (C2v), is the allocation of four Zn atoms in the axial MMCp* moieties, leaving a [ZnCu2] triangle in the equatorial plane (Scheme S2†).
Cluster bonding analysis of 1 and 2
The polyhedral structure types of clusters are strongly related to their electronic structure, associating specific cluster shapes with specific numbers of electrons.21–25 Stable closed-shell organometallic clusters (e.g. protected by hydrocarbon ligands via metal–carbon bonds) most often obey the Wade–Mingos electron-counting rules.23–25 These rules state that the number of skeletal electron pairs (SEPs) associated with cluster bonding is equal to the number of vertices of the deltahedron in which the cluster is inscribed, plus one. Accordingly, the trigonal bipyramidal skeletons of 1 and 2, should be associated with 5 + 1 = 6 SEPs. Cluster 1 can be formally considered as constituted of five fragments, three CuCp* and two Zn–ZnCp* units. Assuming first that, as generally admitted, the Cu and Zn 3d electrons are not significantly involved in the bonding, CuCp* and Zn–ZnCp* are 0- and 1-electron donor moieties, respectively. One thus ends up with only 1 SEP for 1, as for its isoelectronic relative 2. This makes 1 and 2 highly electron-deficient with respect to the Wade–Mingos rules. It should be however kept in mind that these rules assume that the fragments constituting the cluster participate to cluster bonding with 3 frontier orbitals, one of σ-type and two of π-type.23–25 This is obviously the case for the Zn–ZnCp* moiety, the external Zn atom having two available non-bonding 4pπ frontier orbitals as well as one 4s/4p hybrid of σ-type (the other sp hybrid is involved in the Zn–Zn single bond). On the other hand, the case of the CuCp* (or [ZnCp*]+) fragment is different since it is generally considered as participating to the bonding with only one frontier orbital of σ-type (a 4s/4p hybrid), the occupied low-lying 3d-block being, as said above, discarded. Thus, the Wade–Mingos standard “reference” should be considered with caution when dealing with 1 and 2. To clear out this situation, we have performed density functional (DFT) calculations on these two clusters as well as on several cluster models (BP86/STO-TZ2P level with Grimme D3BJ dispersion corrections, see Computational details in the ESI†). For the sake of simplicity we have first replaced the pentamethylcyclopentadienyl (Cp*) ligands in 1 and 2 by simple cyclopentadienyls (Cp). The optimized structures of the resulting models, namely 1′ and 2′, were found to be energy minima for Cs symmetry, with their symmetry plane containing the Cu3 and ZnCu2 triangle, respectively. The optimized metrical data of 1′ and 2′ (Table 1) are consistent with their experimental counterparts in 1 and 2. The [Cu3Zn4] metal core of 1′ is very close to D3h symmetry. Its Kohn–Sham orbital diagram is shown in Fig. 3.| (avg) | 1 (X-ray) | 1′ (DFT) | 1 (DFT) |
|---|---|---|---|
| Cu–Cu | 2.440 | 2.414 (0.270) | 2.426 |
| Cu–Zn | 2.443 | 2.470 (0.091) | 2.432 |
| Zn–Zn′ | 2.318 | 2.345 (0.600) | 2.314 |
| (avg) | 2 (X-ray) | 2′ (DFT) | 2 (DFT) |
|---|---|---|---|
| Cu–Cu | 2.417 | 2.385 (0.339) | 2.401 |
| Cu–Zn | 2.475 | 2.490 (0.074) | 2.437 |
| Cu–Zn′′ | 2.399 | 2.399 (0.268) | 2.401 |
| Zn–Zn′ | 2.311 | 2.338 (0.606) | 2.299 |
| Zn–Zn′′ | 2.717 | 2.872 (0.025) | 2.682 |
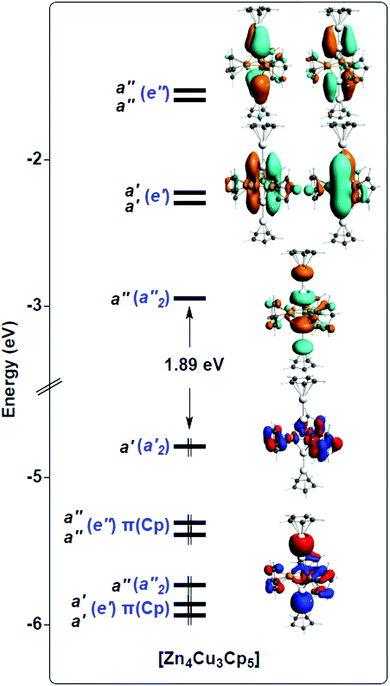 | ||
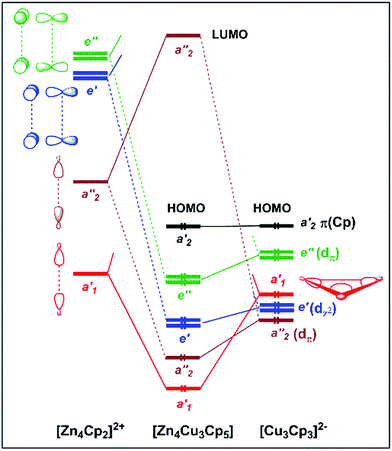
| Fig. 3 Kohn–Sham orbital diagram of [Cu3Zn4](Cp)5 (1′). The levels are labelled according to Cs symmetry (in black) and D3h pseudo-symmetry (in blue). The Cs symmetry plane contains the Cu3 triangle. | ||
The large HOMO–LUMO gap is consistent with the stability of 1. In order to get a better insight into its bonding, a Morokuma–Ziegler energy decomposition analysis (EDA)26–28 was carried out, considering the interaction between two fragments: A {[Cu3](Cp)3}2− triangular unit and a bi-capping [CpZn2⋯Zn2Cp]2+ “dimeric” unit. The formal fragment charges were chosen for closed-shell simplicity and also considering that {[Cu3](Cp)3}2− is isostructural and isoelectronic to the known [CuZn2](Cp*)3 (see above),14 which can be viewed as an isolobal29,30 analogue of [H3]+ (σ-aromatic 2-electron/3-center bonding). A similar fragmentation has been suggested for the cluster [{Pd(C6H4F)}3(μ2-SC6H4Cl)3Ag(H2O)2][BF4]2 implying that an aromatic [Pd3]+ triangle acts as a donor ligand to a Ag+ ion.31 The same approach has also been used for treating a related [Pd4]2+ system.32 The EDA results are given in Table 2.
| 1′ (Cs) | 2′ (Cs) | |||
|---|---|---|---|---|
| Fragments | [Cu3Cp3]2− + [CpZn2⋯Zn2Cp]2+ | [Cu2ZnCp3]− + [CpZn2⋯Zn2Cp]2+ | ||
| E Pauli | 14.92 | 10.37 | ||
| E elstat | −24.53 | −14.15 | ||
| E orb,decomposition | a′ | −6.30 | a′ | −4.30 |
| a′′ | −4.55 | a′′ | −3.48 | |
| E orb | −10.85 | −7.78 | ||
| E disp | −1.24 | −1.16 | ||
| TBE | −21.70 | −12.72 | ||
Unsurprisingly, with such a formal fragment partitioning in 1′, the electrostatic interaction component dominates the total bonding energy. Representing 44% of the former, the orbital interaction component is, however, not negligible. Its a′ and a′′ components, respectively of σ- and π-type with respect to the Cu3 plane, are of similar order, the a′ interaction being the strongest. A detailed analysis of the 1′ Kohn–Sham orbital compositions based on its fragment orbitals allowed us to describe the covalent component of the bonding interaction through the simplified qualitative interaction orbital diagram sketched in Fig. 4, considering D3h pseudo-symmetry.
The [CpZn2⋯Zn2Cp]2+ “dimer” has six accepting frontier orbitals, the in-phase and out-of-phase combinations of the three accepting orbitals on the terminal Zn of each [CpZn2]+ “monomer” (see above). In the D3h pseudo-symmetry of 1′, the sp(Zn) hybrids give rise to a′1 and a′′2 combinations, whereas the 4pπ(Zn) AO's lead to e′ and e′′ combinations (see left side of Fig. 4). On the other hand, the {[Cu3](Cp)3}2− unit has no accepting orbitals. Its highest occupied levels are the π(Cp) and 3d(Cu) combinations, among which is buried the strongly bonding a′1 orbital, principally of 4s/4p composition, which contains the 3-center bonding electron pair of this fragment. This Cu3 a′1 orbital interacts strongly with the a′1 LUMO of the [CpZn2⋯Zn2Cp]2+ “dimeric” fragment, giving rise to a fully in-phase, strongly bonding, combination which contains the unique SEP that one would consider for cluster bonding if no 3d(Cu) orbitals were involved (see above).
But in fact 3d(Cu) combinations of proper symmetry interact with the other accepting orbitals of the [CpZn2⋯Zn2Cp]2+ fragment. This can be traced by the occupation of it's a′′2, e′ and e′′ frontier orbitals, which is 0.54, 2 × 0.10 and 2 × 0.11, respectively. For comparison, the a′1 occupation is 1.02. The 3d(Cu) counterparts are of 3dπ (a′′2 and e′′) and 3dz2 (e′) character. Counting the resulting bonding pairs, one ends up with 6 SEPs, all of the proper symmetry (a′1, a′′2, e′ and e′′) as in clusters satisfying the classical Wade–Mingos rules. Thus, to some extent, 1 satisfies the Wade–Mingos rules, despite not all, but only specific, 3dπ and 33dz2 combinations are involved. Moreover, the e′ and e′′ interactions are not very strong, because of the relatively poor energy match between 4p(Zn) and 3d(Cu). On the other hand, some second-order participation to the bonding (not represented in Fig. 4) and involving the a1′ and a′′2 combinations associated with the Zn–Zn bonding pairs can also be traced. This tends to weaken somewhat the strength of the Zn–Zn bonds at the expenses of Zn–Cu bonding, as exemplified by the computed Zn–Zn Wiberg index in 1′ (0.600), significantly weaker than in CpZnZnCp (0.811).
To summarize the above MO analysis, the bonding within the [Cu3Zn2] trigonal bipyramidal skeleton in 1 can be viewed as intermediate between that of a hypothetical electron-deficient 1-SEP cluster and that of a regular Wade–Mingos 6-SEP species. It should be pointed out that, although not preponderant, the role of the 4pπ(Zn) AOs in the framework stability is crucial. This can be shown by replacing in 1′ the [CpZnZn]+ capping units by [CpZn]+ fragments which, assuming η5-Cp coordination, do not possess 4pπ accepting frontier orbitals. Indeed, the optimized geometry of the resulting M5 cluster [Cu3Zn2](Cp)5 (see ESI†) exhibits (η1-Cp)Zn units, rather than (η5-Cp)Zn, thus leaving the 4pπ(Zn) orbitals available for bonding within the [Zn2Cu3] trigonal bipyramidal skeleton. With such a low coordination mode associated with the rather fragile electron-poor character of the [Cu3Zn2] skeleton, the hypothetical [Cu3Zn2](Cp)5 cluster appears unlikely to be isolable.
The optimized structure of the model 2′ is also in good agreement with the X-ray structure of 2 (see Table 1). In particular it shows a rather long Zn(apical)–Zn′′(equatorial) bond of 2.872 Å. It is noteworthy that when the dispersion corrections are not included in the calculations, the Zn–Zn′′ distance is elongated to 3.019 Å, a no-bond value. Thus, despite the small Edisp component in the fragment energy decomposition of Table 2, the dispersion forces appear to play a significant role in the overall cluster structure. The weak covalent Zn–Zn′′ bonding is also exemplified by its low Wiberg index (0.025). It can be explained by the fact that the highest 3d-type combinations of the {[Cu2Zn](Cp)3}− fragment have little Zn participation (3d(Zn) levels are lower) and therefore interact with the 4pπ(Zn) combinations of the [CpZn2⋯Zn2Cp]2+ “dimer” primarily through the copper atoms.
The structures of the real methylated compounds 1 and 2 were also optimized and were found to be of C1 symmetry, slightly distorted away from Cs due to the steric effect of the methyl groups. Their selected metrical data reported in Table 1 are in good agreement with that of the X-ray structures. For these compounds, it was not possible to perform a 2-fragment EDA analysis similar to that carried out for the 1′ and 2′ models. As a matter of fact, it was not possible to converge the [Cp*Zn2⋯Zn2Cp*]2+ unit in the proper closed-shell configuration. However, a 3-fragment analysis in which this “dimeric” unit is split into two [Cp*Zn2]+ fragments was possible. The corresponding results, together with that obtained in a similar way for 1′ and 2′ are provided in Table S2.† It appears clearly that the 2- and 3-fragment EDA analyses of 1′ and 2′ are fully consistent and indicate negligible interaction between the two capping [Cp*Zn2]+ units. Comparing in Table S2,† the results obtained for 1 and 2 with that of 1′ and 2′ indicate that the bonding analysis carried out for the non-methylated models applies to the real methylated compounds. Among the changes upon ligand methylation (i.e. Cp vs. Cp*), one can note an increase of the absolute value of the Eorb component by ∼1.6/1.8 eV due a destabilization of the [Cu3] or [Cu2Zn] 3d(Cu) block. The Edisp component is also doubled upon methylation, due to methyl⋯methyl van der Waals interactions. However, with an absolute value increase of ∼1.3/1.4 eV, its contribution to the total bonding interaction remains relatively small (7–10% of the sum of the stabilizing components).
Going back to the long Zn–Zn′′ bond in 2 (exp: 2.717 Å, calc: 2.872 Å; averaged values), it is interesting to note that, within the series of pseudo-homoleptic [TM(ZnR)n] compounds (TM = d-block metal) the shortest intramolecular Zn–Zn contact was found to be 2.676(1) Å in [Ru(ZnCp*)4(ZnMe)6]33 being only slightly shorter than the corresponding contacts in 2. Investigations of the bonding in these compounds revealed that the Zn–Zn interactions should not be interpreted as strong Zn–Zn covalent bonds but rather as weak Zn⋯Zn interactions. Therefore, the rather small Wiberg bond index of 0.16 found for the Zn3–Zn2/Zn3–Zn4 contacts in 2 can also be interpreted as rather weak interactions. In this respect a complementary view is obtained from Quantum Theory of Atoms in Molecules (QTAIM, see Computational details in the ESI†) analysis of 1 and 2. Bond critical points and bond paths are found for all M–M interactions, except for the long Zn–Zn′′ contacts in 2 (see Fig. 5 where they are labelled Zn3–Zn2 and Zn3–Zn4 as in the X-ray structures). In accordance to these observations, ring and cage critical points can be found for all M3 triangles and M4 pyramides in 1, whereas in 2 ring critical points are only found for the Cu2Zn triangles. Alternatively, especially cluster 2 can be viewed as a copper triple bonded moiety [Cp*CuCuCp*]2− (with occupied π-like orbitals),34 which is “ligated” by three groups [ZnR]+ (R = Cp*, ZnCp*). This picture is in accordance with the results of the QTAIM analysis, which suggests isolated [ZnR]+ fragments (see ESI†).
Summary and perspectives
In summary, the homoleptic all-hydrocarbon Cp*-protected M7 Cu/Zn-clusters [Cu3Zn4](Cp*)5 (1) and {[Cu2Zn5](Cp*)5}+ (2) have been synthesized and characterized. They expand the unique series {[CuaZnb](Cp*)c}n+ with varying Cu/Zn ratios a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b and Cp* content. The series starts with the M3 clusters {[Zn3](Cp*)3}+ and [CuZn2](Cp*)3, it includes the (heteroleptic) M8 cluster [Cu4Zn4](Cp*)4(CNtBu)4. The series so far ends with the M9 and M10 all-zinc clusters [Zn9](Cp*)6 and {[Zn10](Cp*)6(CH3)}+ (see the Introduction).2–4 The new M7 clusters 1 and 2 demonstrate the feasibility to substitute Zn and Cu on a molecular level in small nuclearity clusters while keeping the cluster's polyhedral structural type. As to that the preparation of further isoelectronic structural pairs of {[CuaZnb](L)c}n+ (a + b = 4–6) and expanded versions of such clusters (i.e. with higher nuclearity a + b > 10), which only differ in the Zn
b and Cp* content. The series starts with the M3 clusters {[Zn3](Cp*)3}+ and [CuZn2](Cp*)3, it includes the (heteroleptic) M8 cluster [Cu4Zn4](Cp*)4(CNtBu)4. The series so far ends with the M9 and M10 all-zinc clusters [Zn9](Cp*)6 and {[Zn10](Cp*)6(CH3)}+ (see the Introduction).2–4 The new M7 clusters 1 and 2 demonstrate the feasibility to substitute Zn and Cu on a molecular level in small nuclearity clusters while keeping the cluster's polyhedral structural type. As to that the preparation of further isoelectronic structural pairs of {[CuaZnb](L)c}n+ (a + b = 4–6) and expanded versions of such clusters (i.e. with higher nuclearity a + b > 10), which only differ in the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio appears feasible. The trigonal bipyramidal clusters 1 and 2 are at first glance with 1 SEP highly electron deficient with regard to the Wade–Mingos rules, owing to the fact that the basal MCp* units lack 4p frontier orbitals. However, according to EDA analysis of the model 1′ based on the fragments {[Cu3](Cp)3}2− and [CpZn2⋯Zn2Cp]2+ not only 4s/p orbitals of the trigonal M3 basal unit are involved in skeletal bonding, but even if to a lower extend, also 3dπ and 3dz2 combinations. The latter provide frontier orbitals of proper symmetry and 5 additional SEPs to satisfy the Wade–Mingos rules for trigonal bipyramidal clusters. In spite of the fact that some of these additional SEPs have only moderate bonding character, the metal skeletons of 1 and 2 do not need the help of μ2/μ3 ligands for maintaining their unbridged bipyramidal trigonal structures. Dispersion interactions between the Cp* protecting ligands provide additional stabilization to the structures. This is a specific property of Cp* as a ligand,19,35 among others such as its flexible coordination mode (η1⋯η5), its transferability between Cu and Zn centres, its steric bulk and the stabilization of elimination products such as [Cp*2Zn] (related to disproportionation reactions). It can be expected that other oligonuclear Cu/Zn clusters on the borderline between electron deficient and regular Wade–Mingos species are likely to be highly reactive and thus should allow further cluster expansion reactions, eventually reaching stability islands of larger clusters predicted by the superatom model. Our ultimate goal along these lines would be the demonstration of high nuclearity atom-precise Hume-Rothery intermetallics inspired superatom clusters solely protected by all-hydrocarbon ligands, such as the recently discovered M55 magic number Al/Cu cluster [Cu43Al12](Cp*)12.35
Cu ratio appears feasible. The trigonal bipyramidal clusters 1 and 2 are at first glance with 1 SEP highly electron deficient with regard to the Wade–Mingos rules, owing to the fact that the basal MCp* units lack 4p frontier orbitals. However, according to EDA analysis of the model 1′ based on the fragments {[Cu3](Cp)3}2− and [CpZn2⋯Zn2Cp]2+ not only 4s/p orbitals of the trigonal M3 basal unit are involved in skeletal bonding, but even if to a lower extend, also 3dπ and 3dz2 combinations. The latter provide frontier orbitals of proper symmetry and 5 additional SEPs to satisfy the Wade–Mingos rules for trigonal bipyramidal clusters. In spite of the fact that some of these additional SEPs have only moderate bonding character, the metal skeletons of 1 and 2 do not need the help of μ2/μ3 ligands for maintaining their unbridged bipyramidal trigonal structures. Dispersion interactions between the Cp* protecting ligands provide additional stabilization to the structures. This is a specific property of Cp* as a ligand,19,35 among others such as its flexible coordination mode (η1⋯η5), its transferability between Cu and Zn centres, its steric bulk and the stabilization of elimination products such as [Cp*2Zn] (related to disproportionation reactions). It can be expected that other oligonuclear Cu/Zn clusters on the borderline between electron deficient and regular Wade–Mingos species are likely to be highly reactive and thus should allow further cluster expansion reactions, eventually reaching stability islands of larger clusters predicted by the superatom model. Our ultimate goal along these lines would be the demonstration of high nuclearity atom-precise Hume-Rothery intermetallics inspired superatom clusters solely protected by all-hydrocarbon ligands, such as the recently discovered M55 magic number Al/Cu cluster [Cu43Al12](Cp*)12.35
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deutsche Forschungs-gemeinschaft (grant Fi-502/23-2). H. B. is grateful for a PhD scholarship donated by the German Chemical Industry Fund. We thank Prof. T. Marder and his co-workers at the Julius-Maximilians-Universität Würzburg and Linden CMS GmbH for support in mass spectrometry. The GENCI French national computer resource center is acknowledged (grant x2016-087367). F. G. thanks the Région Bretagne for a PhD studentship grant (ARED NANOCLU 9334).Notes and references
- W. Hieber, F. Mühlbauer and E. A. Ehmann, Ber. Dtsch. Chem. Ges. A/B, 1932, 65, 1090–1101 CrossRef.
- N. T. Tran, D. R. Powell and L. F. Dahl, Angew. Chem., Int. Ed., 2000, 39, 4121–4125 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS PubMed.
- J. Vollet, J. R. Hartig and H. Schnöckel, Angew. Chem., Int. Ed., 2004, 43, 3186–3189 CrossRef CAS PubMed.
- H. Schnöckel, Chem. Rev., 2010, 110, 4125–4163 CrossRef PubMed.
- H. Banh, K. Dilchert, C. Schulz, C. Gemel, R. W. Seidel, R. Gautier, S. Kahlal, J.-Y. Saillard and R. A. Fischer, Angew. Chem., Int. Ed., 2016, 55, 3285–3289 CrossRef CAS PubMed.
- K. Freitag, H. Banh, C. Gemel, R. W. Seidel, S. Kahlal, J. Y. Saillard and R. A. Fischer, Chem. Commun., 2014, 50, 8681–8684 RSC.
- K. Mayer, J. Weßing, T. F. Fässler and R. A. Fischer, Angew. Chem., Int. Ed., 2018, 57, 14372–14393 CrossRef CAS PubMed.
- T. Fehlner, J. F. Halet and J. Y. Saillard, Molecular Clusters: A Bridge to Solid-State Chemistry, Cambridge University Press, 2013 Search PubMed.
- K. Freitag, C. Gemel, P. Jerabek, I. M. Oppel, R. W. Seidel, G. Frenking, H. Banh, K. Dilchert and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 4370–4374 CrossRef CAS PubMed.
- I. Resa, E. Carmona, E. Gutierrez-Puebla and A. Monge, Science, 2004, 305, 1136–1138 CrossRef CAS PubMed.
- M. Carrasco, R. Peloso, I. Resa, A. Rodriguez, L. Sanchez, E. Alvarez, C. Maya, R. Andreu, J. J. Calvente, A. Galindo and E. Carmona, Inorg. Chem., 2011, 50, 6361–6371 CrossRef CAS PubMed.
- M. Carrasco, R. Peloso, A. Rodriguez, E. Alvarez, C. Maya and E. Carmona, Chem.–Eur. J., 2010, 16, 9754–9757 CrossRef CAS PubMed.
- K. Freitag, C. Gemel, P. Jerabek, I. M. Oppel, R. W. Seidel, G. Frenking, H. Banh, K. Dilchert and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 4370–4374 CrossRef CAS PubMed.
- M. Zhang, B.-C. Su, C.-L. Li, Y. Shen, C.-K. Lam, X.-L. Feng and H.-Y. Chao, J. Organomet. Chem., 2011, 696, 2654–2659 CrossRef CAS.
- J. Li, J. M. White, R. J. Mulder, G. E. Reid, P. S. Donnelly and R. A. O'Hair, Inorg. Chem., 2016, 55, 9858–9868 CrossRef CAS PubMed.
- W.-Y. Lo, C.-H. Lam, V. W.-W. Yam, N. Zhu, K.-K. Cheung, S. Fathallah, S. Messaoudi, B. Le Guennic, S. Kahlal and J.-F. Halet, J. Am. Chem. Soc., 2004, 126, 7300–7310 CrossRef CAS PubMed.
- M. I. Bruce, J.-F. Halet, B. Le Guennic, B. W. Skelton, A. N. Sobolev, C. J. Sumby and A. H. White, Coord. Chem. Rev., 2018, 375, 2–12 CrossRef CAS.
- K. Freitag, H. Banh, C. Gemel, P. Jerabek, R. W. Seidel, G. Frenking and R. A. Fischer, Inorg. Chem., 2015, 54, 352–358 CrossRef CAS PubMed.
- K. Freitag, H. Banh, C. Ganesamoorthy, C. Gemel, R. W. Seidel and R. A. Fischer, Dalton Trans., 2013, 42, 10540–10544 RSC.
- H. A. Jahn and E. Teller, Proc. Roy. Soc. Lond. Math. Phys. Sci., 1937, 161, 220 CrossRef CAS.
- A. C. Reber and S. N. Khanna, Acc. Chem. Res., 2017, 50, 255–263 CrossRef CAS PubMed.
- D. M. Mingos and D. J. Wales, in Introduction to cluster chemistry, Prentice-Hall, Englewood Cliffs, 1990 Search PubMed.
- D. M. P. Mingos, Nat. Phys. Sci., 1972, 236, 99–102 CrossRef CAS.
- K. Wade, in Transition Metal Clusters, ed. B. F. G. Johnhson, John Wiley & Sons, Chichester, 1980, pp. 193–264 Search PubMed.
- K. Morokuma, J. Chem. Phys., 1971, 55, 1236–1244 CrossRef CAS.
- T. Ziegler and A. Rauk, Inorg. Chem., 1979, 18, 1558–1565 CrossRef CAS.
- F. M. Bickelhaupt and E. J. Baerends, J. Rev. Comput. Chem., Wiley, New York, 2000 Search PubMed.
- M. Elian, M. M. L. Chen, D. M. P. Mingos and R. Hoffmann, Inorg. Chem., 1976, 15, 1148–1155 CrossRef CAS.
- R. Hoffmann, Angew. Chem., Int. Ed., 1982, 21, 711–724 CrossRef.
- Y. Wang, A. Monfredini, P. A. Deyris, F. Blanchard, E. Derat, G. Maestri and M. Malacria, Chem. Sci., 2017, 8, 7394–7402 RSC.
- A. Muñoz-Castro, D. M.-L. Carey and R. Arratia-Pérez, J. Chem. Phys., 2010, 132, 164308 CrossRef.
- T. Cadenbach, T. Bollermann, C. Gemel, M. Tombul, I. Fernandez, H. M. van, G. Frenking and R. A. Fischer, J. Am. Chem. Soc., 2009, 131, 16063–16077 CrossRef CAS PubMed.
- Y. Xie, H. F. Schaefer III and R. B. King, J. Am. Chem. Soc., 2005, 127, 2818–2819 CrossRef CAS.
- J. Wessing, C. Ganesamoorthy, S. Kahlal, R. Marchal, C. Gemel, O. Cador, A. C. H. Da Silva, J. L. F. Da Silva, J. Y. Saillard and R. A. Fischer, Angew. Chem., Int. Ed., 2018, 57, 14630–14634 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: CCDC 1854851 and 1854852. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc03902j |
| ‡ Hung Banh and Julius Hornung equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2018 |