Open Access Article
Open Access ArticleWriting and erasing multicolored information in diarylethene-based supramolecular gels†
Chien-Wei
Hsu
 ,
Claire
Sauvée
,
Claire
Sauvée
 ,
Henrik
Sundén
,
Henrik
Sundén
 * and
Joakim
Andréasson
* and
Joakim
Andréasson
 *
*
Chalmers University of Technology, Department of Chemistry and Chemical Engineering, Chemistry and Biochemistyry, Kemivägen 10, 412 96, Göteborg, Sweden. E-mail: sundenh@chalmers.se; a-son@chalmers.se
First published on 4th September 2018
Abstract
A facile cocktail approach implying the mixing of diarylethene (DAE) photoswitches and low molecular weight gelators (LMWG) is presented. The photoresponsive gels exhibit multicolored emission that can be precisely controlled by different light exposure schemes (wavelength and dose), applicable for fluorescence patterning/writing. Including also a blue-emitting fluorophore allows for tri-chromatic color tuning of the emission via multistep energy transfer reactions, which in turn yields a non-linear response between the emission spectra and the light dose. This feature is highly desired in data security and anti-counterfeiting contexts. The information written in the gels can be conveniently erased by light, mass diffusion, or shaking; the latter being due to the thixotropic properties of the gels.
Introduction
Molecular photoswitches from the diarylethene (DAE) photochromic family are utilized in research disciplines spanning chemical biology, material science, and molecular electronics to mention a few.1–4 Key to many of these applications is the pronounced color change that follows upon isomerization between the two isomeric forms. A small selection of the studied DAE derivatives also display dramatic concomitant changes in the fluorescence properties, adding to the appeal.5,6 It is becoming apparent that the use of systems containing two or more individual DAE derivatives broadens the application window of this class of compounds further, as multicolor switching is possible in these systems.7 This should be contrasted to the binary switching between “colorless and colored” or “non-fluorescent and fluorescent” observed for the unimolecular case. Irie and co-workers showed the first example of multicolored DAEs in a single crystal, but the challenging recrystallization limits the use in large scale application.8 Later, the same group reported on a DAE trimer that can be highly enriched in four forms, displaying vastly different solution colors.9 Full color ink-jet printing has also been demonstrated using three different DAE monomers with different absorption spectra.10Similar studies have also been undertaken to control the emission color in polymers.11–13 In all these studies, however, non-fluorescent DAE derivatives were used together with additional fluorophores. The emission from these fluorophores changes from “on” to “off” by isomerization induced excitation energy transfer quenching by the DAEs. A much more elegant way would be to instead use DAE derivatives that are intrinsically fluorescent in one of the two isomeric forms. First, it does not require auxiliary fluorophores to generate the desired emission colors. Second, no photons are being “wasted” in any energy transfer process, as fluorescence is being re-emitted again from the sensitized DAE acceptors.
We have recently shown that DAEs can be engaged in emission color tuning upon encapsulation into polymer micelles.14 However, in any context where spatial resolution matters, these energy transfer systems must be transferred into rigid or semi-rigid media. Polymers indeed offer a possibility for immobilization, but in many applications where the polarity of the environment is crucial to molecular function (see below), polymers simply fail. For these reasons we were interested in investigating supramolecular gels made from low-molecular-weight gelators (LMWGs). LMWGs are molecules that can self-assemble into three-dimensional networks within the solvent to form a gel.15–18 Supramolecular gels are typically formed at a loading ranging from 1 to 5 wt% of the gelator. Thus, bulk polarity can be expected not to experience any substantial changes when incorporated to the matrix of a gel as compared to free in solution. These properties make LMWG ideal candidates to study photochromic molecules, as isomerization rates (both thermal and photoinduced) often are dramatically changed with polarity. In our group we have recently developed a new type of LMWG based on the oxotriphenylhexanoate (OTHO) structure.19–21 These gelators are highly tunable and can, depending on the substitution pattern, form gels in a large number of solvents/solvent mixtures.
Here we report on a supramolecular OTHO gel containing one or more fluorescent DAE derivatives as the switching units. We show that conventional “on–off” switching of the fluorescence is achievable for the unimolecular case, allowing for one-color fluorescence patterning. Furthermore, di-chromatic and tri-chromatic color tuning is observed for systems containing two or more fluorophores. As multi-step energy transfer reactions are responsible for the color changes, there is a highly non-linear response between the concentrations of the fluorophores and the respective emission intensities, making this scheme of potential use in information security applications.22–24
Results and discussion
The structures of the gelator and the fluorophores/photoswitches are shown in Fig. 1. The synthesis of the DAEs and the sugar based OTHO gelator followed procedures in previous literature.6,19,25 The OTHO3 gelator forms gels in solvents of varying polarity such as toluene, acetonitrile, and isopropanol. As shown in Fig. 1b, OTHO3 with the open isomers of DAE-5 and DAE-8 (referred to herein as OTHO3/DAE-5o and OTHO3/DAE-8o, respectively) forms a transparent gel in toluene. After UV irradiation at 365 nm, the gels do not collapse as the photoswitches isomerize to the closed fluorescent forms, DAE-5c and DAE-8c, with concomitant color changes: yellow for OTHO3/DAE-5c and pink for OTHO3/DAE-8c (Fig. 1c, see Fig. S3–S6 in the ESI† for absorption and emission spectra of all compounds). The photophysical properties of the DAEs are very similar both within and without gel. The gels were investigated in detail as for their rheological properties using dynamic shear oscillation, and were found to be correctly defined as gels both in the absence and the presence of the fluorophores. The same is true for the thixotropic behavior (see Fig. S1 and S2 in the ESI† for characterization of the gels).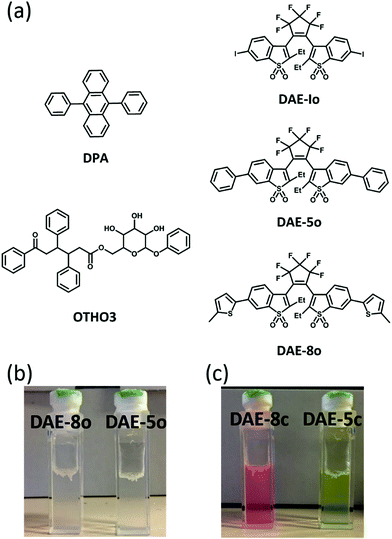 | ||
| Fig. 1 (a) The chemical structure of 9,10-diphenylanthracene (DPA), OTHO3, and the DAE derivatives. (b) Gels containing DAEs before UV irradiation. (c) Gels containing DAEs after UV irradiation. | ||
The writing and erasing of fluorescent patterns were investigated initially in the OTHO3/DAE-5 system, and the results are shown in Fig. 2. At the start, the sample contained the colorless isomer DAE-5o (a). As expected, irradiation with 365 nm UV through a mask yielded the square- and the triangle shaped patterns in the gel (b). This is seen in both the reflected color and in the fluorescence, clearly showing that isomerization to DAE-5c occurred. Exposure to 523 nm light conveniently erases the information due to isomerization of DAE-5c to DAE-5o (c). The sample can be re-written again, excluding that the 523 nm erasing step bleached the sample as a result of photodecomposition (d). An additional means of erasing the written pattern/information is to let mass diffusion act. The coded pattern starts to become vague after 30 minutes (e), and the information has disappeared after a total of 2 hours, indicating the time-range by which diffusion occurs (f).
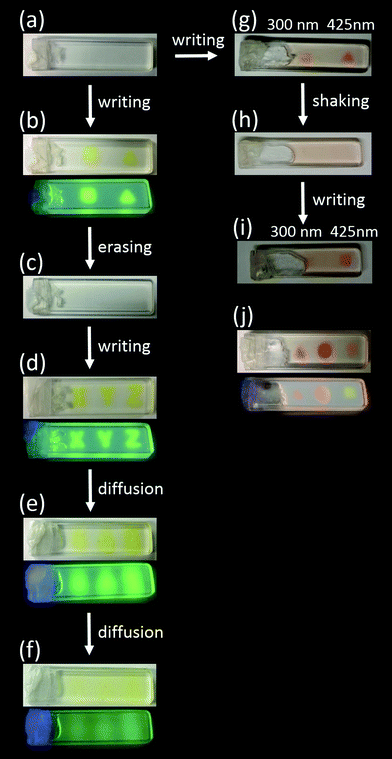 | ||
| Fig. 2 Photo-patterning of the OTHO3/DAE gels. The concentrations of DAE-5 and DAE-8 were 10−4 M, and that of the OTHO3 gelator was 20 mM, all in toluene. (a) Representative gel containing the DAE photoswitches before light irradiation. (b) OTHO3/DAE-5 gel irradiated with 365 nm light. Emission color displayed in the lower panel. (c) Erasing the information by irradiation at 523 nm. (d) Rewriting of information by irradiation at 365 nm light. (e) After 30 minutes. (f) After 2 hours. (g) OTHO3/DAE-5/DAE-8 gel irradiated with light, right spot: λex = 425 nm, left spot: λex = 300 nm. (h) Shaking and subsequent reformation of the gel. (i) Rewriting of information by irradiating with light, right spot: λex = 425 nm, left spot: λex = 300 nm. (j) OTHO3/DAE-5/DAE-8 gel irradiated with light, right spot: λex = 300 nm, middle spot: λex = 300 nm and 425 nm, left spot: λex = 425 nm. Emission color shown in the bottom panel. See ESI† for the choice of irradiation wavelengths. | ||
Next, the OTHO3/DAE-5/DAE-8 gel, containing both DAE-5 and DAE-8, was studied. For multi-component systems like this gel, selective isomerization of the respective photoswitch is key to achieve maximum color discrimination. This translates into finding regions in the absorption spectra where each of the open isomers (DAE-5o and DAE-8o) absorb light substantially stronger than the other. For the combination DAE-5o/DAE-8o, these wavelengths were chosen as 300 nm and 425 nm (see Fig. S5, S6 and Section F in the ESI†). Fig. 2 shows the changes in color after exposure at these wavelengths, and it is clear from the yellow and the pink areas in (g) that the selectivity of the respective isomerization process is high enough to clearly differentiate between the colors of the two spots. Shown in the figure is also how the written pattern can be erased by shaking (h). This is due to the thixotropic properties of the OTHO3 gel. After re-gelating, the same pattern could be written again, albeit with a clear decrease in the contrast (i). Irradiating the same sample spot with both 300 nm and 425 nm light yields an orange color in both reflection and fluorescence, as expected from the simultaneous appearance of DAE-5c and DAE-8c (j). This is the first example of DAEs showing multicolored patterned emission in supramolecular gels which allows not only multifrequency writing but also multifrequency read-out.
Until this point, the isomerization of the DAE derivatives has been described in binary terms, i.e., fully open or fully closed. Moreover, excitation energy transfer, herein referred to as EET, has not been used to initiate emission color changes (see ESI† for arguments about the EET mechanisms, i.e., FRET and radiative “trivial” EET). Below, it will be described how the inclusion of a “static” fluorophore, DPA, acting as the EET donor, together with continuous monitoring of the isomerization process allows for the emission color to proceed in a continuous fashion too. This is conveniently illustrated for the OTHO3/DPA/DAE gels shown in Fig. 3.
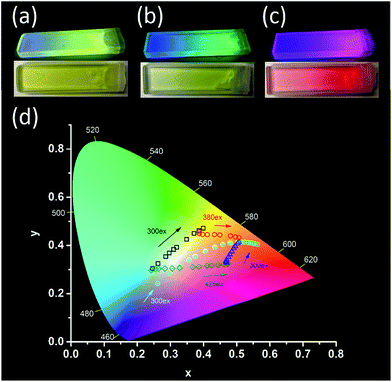
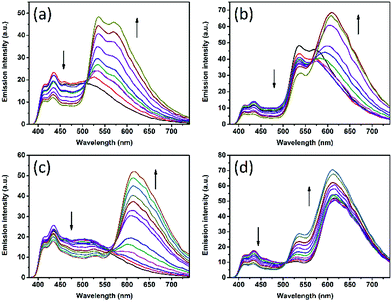
Before irradiation at 365 nm, the gels consisted of DPA together with, respectively, DAE-5o 3(a), DAE-Io 3(b), and DAE-8o 3(c). These three gels were chosen to demonstrate the flexibility in the observed color change offered by the use of different DAE derivatives. As there is no spectral overlap between the fluorescence of DPA and the absorption of the open DAE isomers, EET is not active. Thus, blue emission from DPA is observed. If a small fraction of the DAEs are isomerized to the closed fluorescent form, part of the DPA fluorophores will be quenched in an EET process as the absorption of the closed forms of DAE overlaps the blue emission. At the same time, sensitized emission from the DAE acceptors appear. The larger the isomerized fraction, the more efficient is the EET process, resulting in a gradual shift toward almost pure emission from the DAEs. If a graduated ND-filter is used as a mask for the 365 nm isomerization light, a DAE-c gradient from 0% to 100% results (from left to right in Fig. 3a–c). A similar gradient is observed for the reflected color as well as for the fluorescence color. This is a very rare example of an “energy transfer meter”, allowing for naked eye estimation of the EET efficiencies along the gradient gels.
The di-chromatic color tuning is expanded to the tri-chromatic version by introducing two DAE derivatives in addition to DPA, here demonstrated for the OTHO3/DPA/DAE-5/DAE-8 gels. This system displays direct as well as consecutive EET reactions involving DPA, DAE-5c, and DAE-8c. The following processes must be considered: DPA → DAE-5c, DPA → DAE-8c, and DAE-5c → DAE-8c. As described above, selective isomerization of both DAE-5o and DAE-8o to the respective closed isomers is key to maximizing the attainable changes in the emission color. These changes are conveniently illustrated in the CIE-diagram (see Fig. 3d). Exposing the gel to 300 nm UV light triggers mainly the DAE-5o → DAE-5c isomerization, whereas 425 nm was chosen for DAE-8o → DAE-8c (see ESI† for the choice of irradiation wavelengths). Assuming these isomerizations to be selective to 100%, 300 nm exposure would result in a pure gradual change in the emission color from blue (DPA) to yellow (DAE-5c), resulting from the same EET arguments as described above. This would be manifested by a straight trajectory in the CIE diagram from blue to yellow. The corresponding blue to red trajectory would result upon isomerization with 425 nm light. From the experimentally observed trajectories shown in Fig. 3d (black and green hollow squares, respectively, see Fig. 4 for emission spectra), it is seen that virtually selective isomerization of DAE-5 and DAE-8 is indeed possible. A larger range of attainable emission colors is achieved by isomerization of both DAE derivatives to the fluorescent form, demonstrated in Fig. 3d by subsequent exposure to 300 nm (blue triangles) and 380 nm (red circles), respectively. Note that all emission colors contained in the area defined by these four trajectories are attainable by carefully choosing the wavelength and the dose of the irradiations.
For certain applications, it is desirable to isomerize the DAE derivatives simultaneously (non-selectively) using one and the same irradiation wavelength. This procedure yields instead curved trajectories in the CIE diagram, due to the multiple EET processes involved in the overall color change, resulting in a highly non-linear response of the outputs (e.g., the CIE coordinate, or other spectroscopically attainable features in the emission spectra) to the inputs (isomerization wavelength and duration). This is demonstrated by the cyan circles in Fig. 3d (see Fig. S7† for emission spectra) for a gel with a slightly different solvent composition (100% toluene), upon isomerizing the sample at 300 nm. In addition, the emission spectra are also depending on the excitation wavelength for emission readout, adding to the complexity. Similar schemes are highly desired in information security applications, such as authentication and encryption.22–24
Conclusions
In summary, we have demonstrated examples of how to control the fluorescence properties of DAE-based supramolecular gels. Fluorescence patterning is easily achieved, and the written information can conveniently be erased by several means. For gels containing two or more fluorophores, di-chromatic as well as tri-chromatic color tuning are possible, based on consecutive EET reactions within the gels. This results in non-linear emission color changes in response to the photonic inputs, an observation that is desired in systems for information security.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Swedish Research Council VR and Formas (VR grant No 2016-03601 for JA and No 2014-04664 for HS and Formas grant No 2016-00484 for HS) for funding, Carl Trygger Foundation for Scientific Research, and Wenner-Gren Foundations for providing postdoc fellowships for C-WH and CS, respectively. Chalmers Excellence Initiative in Nanoscience and Nanotechnology is also acknowledged for financial support.Notes and references
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef PubMed.
- D. Vomasta, C. Hogner, N. R. Branda and B. König, Angew. Chem., Int. Ed., 2008, 47, 7644–7647 CrossRef PubMed.
- J. Zhang and H. Tian, Adv. Opt. Mater., 2018, 6, 1701278 CrossRef.
- M. Fukagawa, I. Kawamura, T. Ubukata and Y. Yokoyama, Chem. - Eur. J., 2013, 19, 9434–9437 CrossRef PubMed.
- Y.-C. Jeong, S. I. Yang, K.-H. Ahn and E. Kim, Chem. Commun., 2005, 2503–2505 RSC.
- K. Uno, H. Niikura, M. Morimoto, Y. Ishibashi, H. Miyasaka and M. Irie, J. Am. Chem. Soc., 2011, 133, 13558–13564 CrossRef PubMed.
- A. Fihey, A. Perrier, W. R. Browne and D. Jacquemin, Chem. Soc. Rev., 2015, 44, 3719–3759 RSC.
- M. Morimoto, S. Kobatake and M. Irie, J. Am. Chem. Soc., 2003, 125, 11080–11087 CrossRef PubMed.
- K. Higashiguchi, K. Matsuda, N. Tanifuji and M. Irie, J. Am. Chem. Soc., 2005, 127, 8922–8923 CrossRef PubMed.
- W. Jeong, M. I. Khazi, D.-H. Park, Y.-S. Jung and J.-M. Kim, Adv. Funct. Mater., 2016, 26, 5230–5238 CrossRef.
- S. Kim, S.-J. Yoon and S. Y. Park, J. Am. Chem. Soc., 2012, 134, 12091–12097 CrossRef PubMed.
- J. Bu, K. Watanabe, H. Hayasaka and K. Akagi, Nat. Commun., 2014, 5, 3799 CrossRef PubMed.
- S. Ishida, T. Fukaminato, D. Kitagawa, S. Kobatake, S. Kim, T. Ogata and S. Kurihara, Chem. Commun., 2017, 53, 8268–8271 RSC.
- M. Bälter, S. Li, M. Morimoto, S. Tang, J. Hernando, G. Guirado, M. Irie, F. M. Raymo and J. Andréasson, Chem. Sci., 2016, 7, 5867–5871 RSC.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef PubMed.
- P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699–2715 RSC.
- J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3160 CrossRef PubMed.
- A. Axelsson, L. Ta and H. Sundén, Eur. J. Org. Chem., 2016, 3339–3343 CrossRef.
- C. Sauvée, A. Ström, M. Haukka and H. Sundén, Chem. - Eur. J., 2018, 24, 8071–8075 CrossRef PubMed.
- L. Ta, A. Axelsson, J. Bijl, M. Haukka and H. Sundén, Chem. - Eur. J., 2014, 20, 13889–13893 CrossRef PubMed.
- J. Andréasson and U. Pischel, Chem. Soc. Rev., 2018, 47, 2266–2279 RSC.
- T. Sarkar, K. Selvakumar, L. Motiei and D. Margulies, Nat. Commun., 2016, 7, 11374 CrossRef PubMed.
- V. Nellore, S. Xi and C. Dwyer, ACS Nano, 2015, 9, 11840–11848 CrossRef PubMed.
- F. Gillanders, L. Giordano, S. A. Diaz, T. M. Jovin and E. A. Jares-Erijman, Photochem. Photobiol. Sci., 2014, 13, 603–612 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The SEM and rheology data of gel, absorption and emission spectra of DPA and DAEs in solution and in gel, energy transfer mechanisms, irradiation wavelength selection, and isomerization efficiencies. See DOI: 10.1039/c8sc03127d. |
| This journal is © The Royal Society of Chemistry 2018 |