Open Access Article
Open Access ArticleConformational selectivity and high-affinity binding in the complexation of N-phenyl amides in water by a phenyl extended calix[4]pyrrole†
L.
Escobar
 ab,
A.
Díaz-Moscoso
ab,
A.
Díaz-Moscoso
 a and
P.
Ballester
a and
P.
Ballester
 *ac
*ac
aInstitute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans, 16, 43007-Tarragona, Spain. E-mail: pballester@iciq.es
bUniversitat Rovira i Virgili, Departament de Química Analítica i Química Orgànica, c/Marcel·li Domingo, 1, 43007-Tarragona, Spain
cICREA, Pg. Lluís Companys, 23, 08018-Barcelona, Spain
First published on 7th August 2018
Abstract
We describe the synthesis of a tetrapyridinium phenyl extended calix[4]pyrrole that is soluble in neutral water solution at mM concentrations. We show that, in pure water, the synthesized calix[4]pyrrole receptor selectively binds the cis-(E) conformers of secondary N-phenyl-amides and tertiary N-methyl-N-phenyl-formamide with binding affinities larger than 103 M−1. The conformational selectivity is remarkable owing to the energetic preference of amides to adopt the trans-(Z) conformation in solution. In this respect, we used two binding models for the mathematical analyses of the titration data and calculated apparent and intrinsic binding constants. The combined action of hydrogen bonding and the hydrophobic effect that operates in the binding of the amides in water is responsible for the large affinities displayed by the receptor.
Introduction
Carboxamides (a.k.a organic amides) are important functional groups in both natural (e.g. peptides) and synthetic (e.g. nylons) polymers. The existence of many methods for their synthesis and their high thermodynamic stability under a wide range of chemical conditions render amides very useful covalent linkers in many chemical constructs. The pseudo-double-bond character of the carbon(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–nitrogen σ-bond restricts its rotation, and therefore, secondary and tertiary amides exist as two isomeric cis–trans rotamers (Fig. 1). In solution, secondary amides show a marked preference for the trans-rotamer (Z-isomer).1,2 On the one hand, the energetic difference between the two rotamers lies in the range of 0.5 to 2.5 kcal mol−1 for both secondary and tertiary amides.3 On the other hand, because the free energy barrier for the cis–trans interconversion is moderate (ΔG = 16–22 kcal mol−1)¶ the process is typically slow on the NMR time-scale but fast on the human time-scale.3 Steric contributions on either side of the amide group seem to be the most significant factors for easing the barrier crossing, as well as controlling the relative population of cis–trans isomers.
O)–nitrogen σ-bond restricts its rotation, and therefore, secondary and tertiary amides exist as two isomeric cis–trans rotamers (Fig. 1). In solution, secondary amides show a marked preference for the trans-rotamer (Z-isomer).1,2 On the one hand, the energetic difference between the two rotamers lies in the range of 0.5 to 2.5 kcal mol−1 for both secondary and tertiary amides.3 On the other hand, because the free energy barrier for the cis–trans interconversion is moderate (ΔG = 16–22 kcal mol−1)¶ the process is typically slow on the NMR time-scale but fast on the human time-scale.3 Steric contributions on either side of the amide group seem to be the most significant factors for easing the barrier crossing, as well as controlling the relative population of cis–trans isomers.
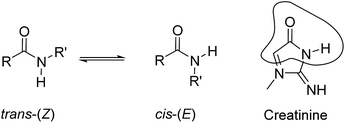 | ||
| Fig. 1 Equilibrium between the cis-(E) and trans-(Z) conformers of a secondary amide. The structural analogy of the cis-(E) isomer with the lactam tautomeric form of creatinine is highlighted. | ||
The cis–trans isomerism of the amide bond plays an important role in determining folding,4 functional5 and ligand interactions in proteins and peptides.6–10 Likewise, the photophysical behaviour of simple phenanthrene carboxamides was shown to be dependent on the amide conformation.11 The equilibrium between secondary amide rotamers is known to be solvent dependent.12 In addition, cis-amide rotamers have been stabilized by intramolecular CH–π interactions,13 lone pair–π interactions,14,15 and the hydrophobic effect.16 In organic solvents, the formation of host–guest hydrogen bonded complexes has also been used to stabilize cis-amides.15,17–20 In water, the combination of hydrogen bonding and the hydrophobic effect has provided thermodynamically stable complexes of synthetic receptors with small linear and cyclic peptides.21,22 However, to the best of our knowledge, examples of synthetic receptors displaying selective binding of the cis-rotamers of mono-amides in water and producing thermodynamically highly stable complexes are not known.
Sessler et al. described the binding of small tertiary amides (i.e. DMF and DEF) using octa-methyl calix[4]pyrrole in benzene solution. The resulting complexes displayed binding affinities of the order of 10 M−1 and were mainly stabilized by the establishment of hydrogen-bonding interactions between the pyrrole NHs and the oxygen atom of the amide's carbonyl group.23 More recently, we reported the use of phosphonate calix[4]pyrrole cavitands, featuring a polar aromatic cavity, for the efficient binding of creatinine in organic solvents.24 In solution and in the solid-state, the inclusion of creatinine in the cavity of the receptor was mainly driven by the establishment of multiple hydrogen bonds. Nevertheless, additional intermolecular CH–π interactions were also present in the above creatinine complex. To us, the lactam tautomer of the bound creatinine resembled a cis-rotamer of a secondary amide and suggested the potential use of aryl-extended calix[4]pyrroles for its selective binding in water solution (Fig. 1).
Herein, we report the synthesis of a water soluble tetra-cationic pyridinium aryl-extended calix[4]pyrrole receptor 1. We also describe the results of the binding studies of receptor 1 with a series of acyclic mono-amides in water. The conformational selectivity displayed by receptor 1 in the exclusive binding of the cis-rotamers of N-phenyl-amides with affinity constants larger than 103 M−1 is highlighted.‡
Results and discussion
Synthesis of tetra-pyridinium calix[4]pyrrole receptor 1
Water soluble aryl-extended calix[4]pyrroles bearing ionizable carboxylic acid functions have been previously reported in the literature.25–27 These receptors required basic media for their water solubilisation. In order to avoid these conditions, not compatible with biological standards, we designed α,α,α,α-tetra-phenyl calix[4]pyrrole 1 (Scheme 1a) featuring four meso-(3-pyridinium-propyl) substituents at its lower rim. The water solubilization of calix[4]pyrrole 1 was inspired by previous studies on water soluble deep cavitands bearing benzimidazolones at their upper rims.28 In these examples, the deep cavitands were rendered water soluble by converting the lower-rim tetra-chloride derivatives into quaternary ammonium salts by treatment with an excess of pyridine. Receptor 1 was synthesized in two steps from commercially available starting materials (Scheme 1a). To start with, the α,α,α,α-tetra-chloro calix[4]pyrrole derivative 2 was prepared using a synthetic methodology recently developed by us.29 Treatment of tetra-chloride 2 at 110 °C in an excess of pyridine provided the tetra-pyridinium calix[4]pyrrole salt 1 as a yellow precipitate in excellent yield and purity. Calix[4]pyrrole 1 was soluble in neutral water at concentrations up to 15 mM.§ A dilution experiment (1 mM to 0.4 mM) showed negligible changes in the proton signals of the acquired 1H NMR spectra. At diluted concentrations, the proton signals of 1 are sharp and well-defined and their number is in agreement with C4v symmetry (ESI†). Most likely, both alternate and cone conformations of 1 are present in solution. The two conformers are in equilibrium and display a fast chemical exchange on the chemical shift time-scale.30,31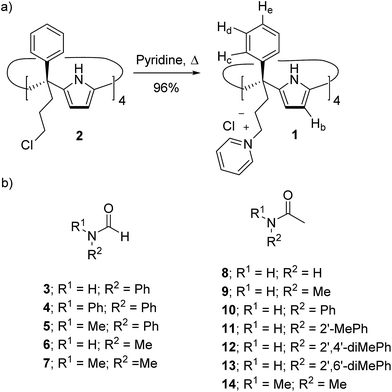 | ||
| Scheme 1 (a) Synthesis of tetra-phenyl tetra-pyridinium calix[4]pyrrole receptor 1 and (b) line-drawing structures of the formamides (3–7) and acetamides (8–14) discussed in this work. When applicable only the cis-rotamer is depicted. See Fig. 1 for the cis/trans equilibrium. | ||
Binding studies of 1 with formamides in water
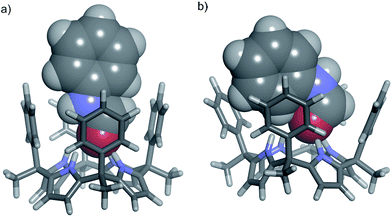
The cis-3⊂1 complex is stabilized by four hydrogen-bonding interactions established between the amide carbonyl oxygen atom and the NHs of the four pyrrole units. Additional CH–π and NH–π17,33,34 interactions are also established between the included cis-3 amide and the meso-phenyl walls of 1.35 On the other hand, the inclusion of trans-3 in the cone conformation of 1, trans-3⊂1 complex, evidenced significant steric clashes between the N-phenyl substituent and two of the four meso-phenyl groups of the receptor (Fig. 2b).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 cis/trans ratio, based on the integral values of the two singlets resonating at δ = 8.65 and 8.28 ppm, respectively, which are assigned to the hydrogen atoms of the formyl group in each rotamer (ESI†).
68 cis/trans ratio, based on the integral values of the two singlets resonating at δ = 8.65 and 8.28 ppm, respectively, which are assigned to the hydrogen atoms of the formyl group in each rotamer (ESI†).
The incremental addition of formamide 3 to a 1 mM water solution of the tetra-pyridinium receptor 1 induced significant chemical changes to several proton signals of the receptor. In particular, the triplet of the para-aromatic proton, He, of the meso-phenyl groups of 1 moved upfield (Δδmax = −0.21 ppm). In contrast, the β-pyrrole protons (Hb) moved downfield (Δδmax = +0.09 ppm).¶ These observations indicated the existence of a fast chemical exchange on the 1H NMR time-scale between free and bound receptor 1. Remarkably, after the addition of 0.6 equiv. of 3 only the sharp singlet corresponding to the hydrogen atom of the formyl group for the free trans-isomer became visible. At this point, we also observed a complex signal of aromatic protons resonating at δ = 7.5 ppm that was assigned to the phenyl group of the formamide. The signals assigned to free trans-3 grew in intensity as the concentration of 3 was increased (Fig. 3). In contrast, the signal of the formyl proton in the free cis-rotamer was not observed even when 2 equiv. of 3 were added.
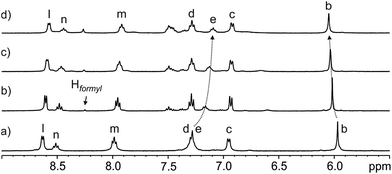 | ||
Fig. 3 Selected region of the 1H NMR (400 MHz, D2O, 298 K) spectra of the titration of calix[4]pyrrole 1 with N-phenyl-formamide 3: (a) 1; (b) 3 + 1 (0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio); (c) 3 + 1 (1 1 molar ratio); (c) 3 + 1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and (d) 3 + 1 (2 1 molar ratio) and (d) 3 + 1 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). The Hformyl signal corresponds to the hydrogen atom of the formyl group for trans-3. See Scheme 1a for proton assignments of receptor 1. 1 molar ratio). The Hformyl signal corresponds to the hydrogen atom of the formyl group for trans-3. See Scheme 1a for proton assignments of receptor 1. | ||
The titration data were mathematically analyzed using the HypNMR 2008 software Version 4.0.66.37 The fit of the chemical shift changes experienced by the selected proton signals of receptor 1 (Hb, Hc, Hd, and He, see Scheme 1 for proton assignment) to a binding isotherm of a theoretical 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model was good. We estimated an apparent binding constant value Ka > 104 M−1 for the formed complex and determined the chemical shift values of the protons of the receptor in the complex (ESI†). These latter values allowed the calculation of the corresponding complexation induced shifts (CISs).
1 binding model was good. We estimated an apparent binding constant value Ka > 104 M−1 for the formed complex and determined the chemical shift values of the protons of the receptor in the complex (ESI†). These latter values allowed the calculation of the corresponding complexation induced shifts (CISs).
At first sight, the determined magnitude of the apparent binding constant might be considered as the weighted-average of two putative 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes: cis-3⊂1 and trans-3⊂1. In order to assess the conformational selectivity exhibited by receptor 1 in the binding of 3, we decided to titrate it against tertiary N,N-diphenyl-formamide 4. The hydrogen-bonding inclusion of formamide 4 in the cavity of 1 should produce the 4⊂1 complex exhibiting a binding geometry closely resembling that of the trans-3⊂1 complex (Fig. 2b). Our idea was to use the binding constant value of 4⊂1 as the reference for the trans-3⊂1 counterpart.
1 inclusion complexes: cis-3⊂1 and trans-3⊂1. In order to assess the conformational selectivity exhibited by receptor 1 in the binding of 3, we decided to titrate it against tertiary N,N-diphenyl-formamide 4. The hydrogen-bonding inclusion of formamide 4 in the cavity of 1 should produce the 4⊂1 complex exhibiting a binding geometry closely resembling that of the trans-3⊂1 complex (Fig. 2b). Our idea was to use the binding constant value of 4⊂1 as the reference for the trans-3⊂1 counterpart.
The extensive sonication of a suspension of N,N-diphenyl-formamide 4 with a 1.7 mM water solution of 1 led to the dissolution of 2 equiv. of 4 but did not induce appreciable changes in the chemical shift values of the receptor's protons. Thus, we estimated a binding constant value Ka(4⊂1) < 10 M−1. On the one hand, this result strongly supports a conformational selectivity towards the cis-rotamer in the binding of N-phenyl-formamide 3 with receptor 1. On the other hand, it also requested a change in the mathematical analysis of the titration data in order to assess a more accurate value of the binding constant for the cis-3⊂1 complex.
Therefore, we reanalyzed the titration data of 1 with 3 using a theoretical binding model that considers the existence of the equilibrium between the two rotamers of 3 and the exclusive formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with the cis-rotamer and receptor 1.
1 complex with the cis-rotamer and receptor 1.
In doing so a theoretical binding isotherm was calculated from the simulated speciation profile produced with the Specfit Software Version 3.0.40 (differential kinetics module). We considered the equilibrium constant between the two rotamers of 3 and the determined chemical shift values for the protons in the free and bound receptor 1 as fixed values. The value of K(cis-3/trans-3) = 32/68 = 0.47 was determined from the 1H NMR spectrum of 3. The complexation induced shift values (CISs) for the protons of 1 were those calculated from the previous fit (vide supra and ESI† for details). The fit of the experimental titration data to the more elaborate theoretical binding model only served to estimate that the binding constant value Ka(cis-3⊂1) was larger than 104 M−1. The magnitude of this estimate represents a remarkable binding affinity for the complexation of a small polar molecule in water using a synthetic receptor.38,39
The fact that the cis-rotamer is present in solution to a significant extent (32%) is the main reason for the observed coincidence between the binding constant values estimated using the two bindings models, that is Ka(cis-3⊂1) ∼ Kapp. We will show below that when the cis-rotamer is present in solution at low levels, the mathematical analyses of the titration data using the two binding models will produce very different values for Ka(cis⊂1) and Kapp.
Next, we performed a reverse titration of N-phenyl-formamide 3 with receptor 1 (Fig. 4). The 1H NMR spectrum of a 1.2 mM water solution of 3 containing just 0.05 equiv. of 1 (Fig. 4b) showed the formyl proton of the trans-3 rotamer as a sharp singlet resonating at the same chemical shift as in the absence of 1. On the contrary, the formyl singlet assigned to the cis-3 rotamer was not detectable at this stage of the titration. This observation indicated that while the equilibrium involving the two free rotamers of 3 displayed slow chemical exchange on the chemical shift time-scale, the cis-3 rotamer was also involved in a binding equilibrium, probably with bound cis-3, featuring intermediate chemical exchange on the chemical shift time-scale. The intermediate kinetics of the latter chemical exchange process produced broadening beyond detection for the formyl proton in the cis-3 rotamer. This result also provides irrefutable support to the selective binding of the cis-3 rotamer by receptor 1.
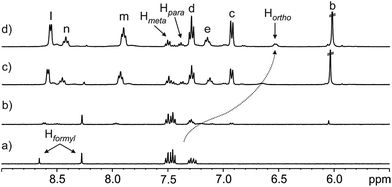 | ||
Fig. 4 Selected region of the 1H NMR (400 MHz, D2O, 298 K) spectra of the titration of N-phenyl-formamide 3 with calix[4]pyrrole 1: (a) 3; (b) 1 + 3 (0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio); (c) 1 + 3 (1 1 molar ratio); (c) 1 + 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and (d) 1 + 3 (2 1 molar ratio) and (d) 1 + 3 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). In (a) the Hformyl signals correspond to cis-3 (left) and trans-3 (right) providing a 32 1 molar ratio). In (a) the Hformyl signals correspond to cis-3 (left) and trans-3 (right) providing a 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 molar ratio. See Scheme 1a for proton assignments of receptor 1. In (d) the ortho, meta and para phenyl protons of bound 3 are also indicated. 68 molar ratio. See Scheme 1a for proton assignments of receptor 1. In (d) the ortho, meta and para phenyl protons of bound 3 are also indicated. | ||
In complete agreement with the previous statement, an increase in the concentration of 1 did not affect the appearance and chemical shift value of the formyl proton signal for the trans-3 rotamer, however, its intensity decreased. We also observed an increase in the intensity of three aromatic signals, two triplets moving downfield and one doublet that shifted upfield. We assigned these three signals to the protons of the N-phenyl group of the cis-3 rotamer, which are involved in a fast chemical exchange on the chemical shift time-scale between its free and bound states. The different kinetics of the chemical exchanges experienced by the protons of the cis-3 rotamer, that is, the formyl proton is involved in an intermediate exchange and the aromatic protons are involved in a fast exchange, result from notably different CISs. The formyl proton of bound cis-3 is included in the aromatic cavity of 1 experiencing the strong shielding exerted by the meso-phenyl substituents.||
On the other hand, the N-phenyl unit of bound cis-3 resides almost completely outside of the aromatic cavity of 1, with its ortho-protons being the only ones affected by the shielding effect (Fig. 2a and 4).
In order to evaluate the scope of the conformational selectivity featured by tetra-phenyl calix[4]pyrrole receptor 1 in the binding of other formamides, we investigated the complexation of 1 with N-methyl-N-phenyl-formamide 5. Based on the integral values of the two separate formyl protons, formamide 5 is present in water solution as an 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 cis/trans mixture of rotamers (K(cis-5/trans-5) = 4.56).
18 cis/trans mixture of rotamers (K(cis-5/trans-5) = 4.56).
The direct 1H NMR titration of 1 with incremental amounts of 5 showed an analogous behavior with respect to the chemical shift changes of the proton signals of receptor 1 to the one previously described for 3. The mathematical analysis of the titration data using the binding model that considers the equilibrium between rotamers and the exclusive formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with the cis-5 isomer allowed us to estimate that the affinity constant value Ka(cis-5⊂1) must be > 104 M−1. We also performed an ITC titration experiment for formamide 5 and calix[4]pyrrole 1 (ESI†). The calculated apparent binding constant was 8.6 ± 0.1 × 103 M−1, a value that is in line with the estimated one from the 1H NMR titration analysis. The ITC results showed that the binding process was enthalpically driven (−7.1 kcal mol−1) and entropically opposed (1.75 kcal mol−1). Clearly, this thermodynamic signature is not the expected one for purely hydrophobic binding.40
1 complex with the cis-5 isomer allowed us to estimate that the affinity constant value Ka(cis-5⊂1) must be > 104 M−1. We also performed an ITC titration experiment for formamide 5 and calix[4]pyrrole 1 (ESI†). The calculated apparent binding constant was 8.6 ± 0.1 × 103 M−1, a value that is in line with the estimated one from the 1H NMR titration analysis. The ITC results showed that the binding process was enthalpically driven (−7.1 kcal mol−1) and entropically opposed (1.75 kcal mol−1). Clearly, this thermodynamic signature is not the expected one for purely hydrophobic binding.40
We also performed a reverse titration by adding increasing amounts of 1 to a 2.9 mM water solution of 5. The proton signals assigned to the trans-5 rotamer did not experience noticeable chemical shift changes. In contrast, those of the cis-5 counterpart, especially the formyl proton and the methyl protons, moved significantly upfield. From these titration data, we could estimate the CISs experienced by these protons because, although their signals broadened beyond detection in the initial and middle phases of the titration, they became observable again in the presence of 2 equiv. of 1. In the cis-5⊂1 complex, the formyl group resonates at δ = 5.01 ppm (Δδ = −3.39 ppm) and the methyl resonates at δ = 1.25 ppm (Δδ = −2.07 ppm). The large calculated CISs confirm the deep inclusion of cis-5 in the aromatic cavity of 1.
A 2D NOESY experiment showed intermolecular cross-peaks between the singlet of the methyl protons of bound cis-5 and the aromatic protons (Hc and Hd) of the meso-phenyl substituents of the calix[4]pyrrole 1.**
We also assessed the affinity constant of calix[4]pyrrole 1 for N-methyl-formamide 6. Formamide 6 is present in water as an 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 mixture of cis/trans rotamers. Nevertheless, molecular modelling studies and the previous results obtained in the binding of the secondary and tertiary formamides 3 and 5 strongly supported the inclusion of both isomers of 6 in receptor 1. The energy minimized 1
92 mixture of cis/trans rotamers. Nevertheless, molecular modelling studies and the previous results obtained in the binding of the secondary and tertiary formamides 3 and 5 strongly supported the inclusion of both isomers of 6 in receptor 1. The energy minimized 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes, cis-6⊂1 and trans-6⊂1, featured close to isoenergetic values (ESI†).
1 inclusion complexes, cis-6⊂1 and trans-6⊂1, featured close to isoenergetic values (ESI†).
Therefore, the 1H NMR titration data for the interaction of 1 with 6 were analyzed using a simple 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model obtaining a good fit and returning an affinity constant value of Ka(6⊂1) = 4.4 × 103 M−1. The removal of the phenyl group in 6 compared to 5 slightly diminished the binding affinity for receptor 1 (Table 1). Probably, this difference is caused by a combination of reduction in hydrophobicity and the hydrogen-bonding accepting character of the oxygen atom in formamide 6 (secondary vs. tertiary).
1 binding model obtaining a good fit and returning an affinity constant value of Ka(6⊂1) = 4.4 × 103 M−1. The removal of the phenyl group in 6 compared to 5 slightly diminished the binding affinity for receptor 1 (Table 1). Probably, this difference is caused by a combination of reduction in hydrophobicity and the hydrogen-bonding accepting character of the oxygen atom in formamide 6 (secondary vs. tertiary).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes with receptor 1. See Scheme 1 for the line-drawing of the molecular structures of the amides
1 inclusion complexes with receptor 1. See Scheme 1 for the line-drawing of the molecular structures of the amides
| Formamides | R1 | R2 | cis/trans | K a(cis⊂ 1 ) | K app | K a(1:1) |
|---|---|---|---|---|---|---|
a A theoretical binding model considering the cis/trans equilibrium between amide rotamers and the exclusive formation of the cis⊂1 complex was used.
b Using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 theoretical binding model.
c Weighted-average of Ka(cis⊂1) and Ka(trans⊂1). Errors (standard deviations) are estimated to be lower than 20%. n.a. = not applicable. 1 theoretical binding model.
c Weighted-average of Ka(cis⊂1) and Ka(trans⊂1). Errors (standard deviations) are estimated to be lower than 20%. n.a. = not applicable.
|
||||||
| 3 | H | Ph | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68 |
>104 | >104 | |
| 4 | Ph | Ph | n.a. | <10 | ||
| 5 | Me | Ph | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
>104 | >104 | |
| 6 | H | Me | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 92 |
4.4 × 103,c | ||
| 7 | Me | Me | n.a. | >104 | ||
In agreement with this hypothesis, the addition of an extra methyl group into N,N-dimethyl-formamide 7 increased its binding affinity for 1, compared to 6, placing it at the same level observed for the analogous but more lipophilic N-phenyl derivatives 3 and 5.
It is worth noting that in the reverse 1H NMR titration of N-methyl-formamide 6 with receptor 1, after the first addition of the receptor, the separate methyl proton signals of the two rotamers of 6 coalesce into a broad singlet that shifted upfield upon increasing the concentration of 1 (ESI†). This result clearly supports the binding of two rotamers of 6, which are involved in a chemical exchange equilibrium with the free counterparts featuring fast/intermediate dynamics on the chemical shift time-scale.
Also note that, in the examples of N-phenyl-formamides 3 and 5, only the proton signals assigned to the cis-rotamer experienced broadening and chemical shift changes. We used this observation as evidence of the conformational selectivity in the binding process. The results described above indicate that receptor 1 might be considered as a minimal synthetic chaperone selecting the cis-conformation of the bound N-phenyl-formamides and increasing their relative concentration in solution in the bound form.
Binding studies of 1 with acetamides in water
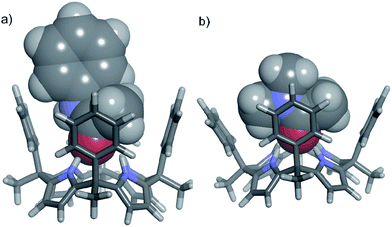
In contrast, tertiary N,N-dimethyl acetamide 14 presents an additional methyl group to be included in the cavity of 1. The energy minimized structure of the 14⊂1 complex displayed a severe distortion of the cone conformation of the receptor owing to steric clashes between the meso-phenyl substituents and the two included methyl groups of the amide (Fig. 5b).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model returning Ka(8⊂1) = 6.8 × 103 M−1. The magnitude of the binding constant is in agreement with the value determined for the isosteric N-methyl-formamide, Ka(6⊂1) = 4.4 × 103 M−1.
1 binding model returning Ka(8⊂1) = 6.8 × 103 M−1. The magnitude of the binding constant is in agreement with the value determined for the isosteric N-methyl-formamide, Ka(6⊂1) = 4.4 × 103 M−1.
In water, N-methyl-acetamide 9 and N-phenyl-acetamide 10 displayed cis/trans isomeric ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 and 1
98 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, respectively, based on the integral values of their methyl acetamide proton signals. In particular, the assignment of the methyl proton signal for the cis-10 rotamer to a small singlet resonating at δ = 1.90 ppm was confirmed by magnetization transfer experiments (ESI†). The titration data of 1 with both acetamides, 9 and 10, were analyzed in an analogous manner. For the sake of brevity, we will only describe in detail the titration experiments performed with 10 and the corresponding data analyses.
99, respectively, based on the integral values of their methyl acetamide proton signals. In particular, the assignment of the methyl proton signal for the cis-10 rotamer to a small singlet resonating at δ = 1.90 ppm was confirmed by magnetization transfer experiments (ESI†). The titration data of 1 with both acetamides, 9 and 10, were analyzed in an analogous manner. For the sake of brevity, we will only describe in detail the titration experiments performed with 10 and the corresponding data analyses.
The incremental addition of 10 (up to 15 equiv.) to a 1.2 mM solution of 1 in water produced very small changes in the chemical shift values of the diagnostic protons of the receptor (Hb, Hc, Hd and He) used for signaling of complex formation in the formamide series.
We also performed a reverse 1H NMR titration experiment adding incremental amounts of calix[4]pyrrole 1 to a 1.5 mM solution of 10 (ESI†). As expected the proton signals of trans-10 did not experience chemical shift changes and only a small reduction of their intensities was observed. Unfortunately, it was not possible to accurately quantify the amount of trans-10 rotamer that has been isomerized into the cis-10 counterpart using the integral values of selected signals in the acquired 1H NMR spectra due to its selective complexation.
We rationalized these results assuming that, as expected, receptor 1 does not bind the trans-10 rotamer and that, owing to the low concentration of the cis-10 rotamer in solution, the formation of the cis-10⊂1 complex (in the direct and reverse titrations) takes place to a reduced extent. To verify our hypothesis, we simulated the speciation profile of a direct titration considering K(cis-10/trans-10) = 0.01 and Ka(cis-10⊂1) = 1 × 104 M−1. Surprisingly to us, the simulated profile indicated a significant formation of the cis-10⊂1 complex in the concentration range used for the experimental titration (ESI†). Owing to the low saturation levels of complex formation attained during the direct and reverse titration of 1 with 10 we could not determine an accurate binding constant value for the cis-10⊂1 complex. Nevertheless, we obtained a reasonable fit of the experimental data using the theoretical binding model that considers the equilibrium between rotamers and the exclusive formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with the cis-isomer. The fit was performed by fixing the values of K(cis-10/trans-10), δfree and δbound†† (0.01, 5.97 ppm, 6.08 ppm). The only variable to refine was Ka(cis-10⊂1). The best fit was obtained assuming Ka(cis-10⊂1) = 5.0 × 103 M−1. This result suggests a reduction in the binding constant of the inclusion complex of the cis-rotamer of acetamide 10 compared to the isosteric N-methyl-N-phenyl-formamide 5. Remarkably, the mathematical analysis of the same titration data using the simple 1
1 complex with the cis-isomer. The fit was performed by fixing the values of K(cis-10/trans-10), δfree and δbound†† (0.01, 5.97 ppm, 6.08 ppm). The only variable to refine was Ka(cis-10⊂1). The best fit was obtained assuming Ka(cis-10⊂1) = 5.0 × 103 M−1. This result suggests a reduction in the binding constant of the inclusion complex of the cis-rotamer of acetamide 10 compared to the isosteric N-methyl-N-phenyl-formamide 5. Remarkably, the mathematical analysis of the same titration data using the simple 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model provided an apparent binding constant of 51 M−1.
1 binding model provided an apparent binding constant of 51 M−1.
This example showcases the significant difference in the calculated values of Ka(cis-10⊂1) and Kapp when the cis-rotamer is present in solution at very low concentration. It also serves to justify the use of the elaborate binding model in the estimation or accurate determination of Ka(cis⊂1) values.
We also determined the cis/trans ratios for the N-aryl acetamide derivatives 11–13 in water. We expected to observe an increase in the amount of the cis-rotamer compared to 10 as was previously described in organic solvents.18 Unfortunately, in water, the percentage of the cis-isomers for the series of acetamides 11–13 did not increase over 3%. This limitation precluded the undertaking of the experimental quantification of the corresponding Ka(cis⊂1) values for these N-aryl acetamides.
Finally, the affinity constant value of receptor 1 for N,N-dimethyl-acetamide 14 was determined to be Ka(14⊂1) = 27 M−1 using a simple 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. This value supports the conformational selectivity of the receptor in the binding of cis-9. It also serves to quantify in three orders of magnitude the binding selectivity expressed by receptor 1 in the binding of N,N-dimethyl-formamide 7 compared to the homologated and more hydrophobic N,N-dimethyl-acetamide 14.
1 binding model. This value supports the conformational selectivity of the receptor in the binding of cis-9. It also serves to quantify in three orders of magnitude the binding selectivity expressed by receptor 1 in the binding of N,N-dimethyl-formamide 7 compared to the homologated and more hydrophobic N,N-dimethyl-acetamide 14.
Conclusions
In summary, we report the synthesis of a tetra-α aryl-extended calix[4]pyrrole receptor 1 bearing four meso-(3-pyridinium-propyl) groups providing water solubility. We studied the binding of a series of primary, secondary and tertiary formamides and acetamides with receptor 1 in water. The mathematical analyses of the 1H NMR titrations of N-phenyl-formamides 3 and 5 using a simple 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, Kapp, or a more elaborate theoretical model including the cis/trans isomerization and the exclusive formation of the cis⊂1 complex, Kcis⊂1, returned similar values, which were typically larger than 103 M−1. We demonstrated that calix[4]pyrrole 1 selectively binds the cis-rotamers of these two formamides. In contrast, both rotamers of formamides 6 and 7, the N-methyl analogues of 3 and 5, are bound by receptor 1 without substantial changes in affinity values. Remarkably, primary (8) and secondary (9 and 10) acetamides are also bound in water by receptor 1 with high affinities. For the secondary acetamides (9 and 10), receptor 1 also features conformational selectivity for their cis-rotamers. In these examples, the mathematical analyses of the 1H NMR titration data for the secondary acetamides provided very different binding constant values depending on the binding model used. We showed that this result is a direct consequence of the low level of cis-rotamers present in solution. N,N-Diphenyl-formamide 4 and N,N-dimethyl-acetamide 14 show a reduced affinity for 1 owing to size complementary issues with the receptor's polar cavity. Taken together, the reported findings demonstrate that calix[4]pyrrole 1 functions as a minimal chaperone analogue increasing the amount of amide cis-rotamers in solution through selective binding. The reported association constant values for most of the amide⊂1 complexes are among the highest reported to date for the binding of small polar molecules38 and even small peptides41–46 in water using synthetic receptors. The amides are bound in the functionalized aromatic cavity of calix[4]pyrrole 1 by a combination of hydrogen-bonding, NH–π and CH–π interactions and the hydrophobic effect. We foresee that further elaboration of the aromatic cavity of water soluble meso-aryl extended calix[4]pyrroles could have an impact on improving their recognition properties, such as achieving higher binding constants or selectivity. Studies to further develop and understand these receptors are currently ongoing in our laboratory.
1 binding model, Kapp, or a more elaborate theoretical model including the cis/trans isomerization and the exclusive formation of the cis⊂1 complex, Kcis⊂1, returned similar values, which were typically larger than 103 M−1. We demonstrated that calix[4]pyrrole 1 selectively binds the cis-rotamers of these two formamides. In contrast, both rotamers of formamides 6 and 7, the N-methyl analogues of 3 and 5, are bound by receptor 1 without substantial changes in affinity values. Remarkably, primary (8) and secondary (9 and 10) acetamides are also bound in water by receptor 1 with high affinities. For the secondary acetamides (9 and 10), receptor 1 also features conformational selectivity for their cis-rotamers. In these examples, the mathematical analyses of the 1H NMR titration data for the secondary acetamides provided very different binding constant values depending on the binding model used. We showed that this result is a direct consequence of the low level of cis-rotamers present in solution. N,N-Diphenyl-formamide 4 and N,N-dimethyl-acetamide 14 show a reduced affinity for 1 owing to size complementary issues with the receptor's polar cavity. Taken together, the reported findings demonstrate that calix[4]pyrrole 1 functions as a minimal chaperone analogue increasing the amount of amide cis-rotamers in solution through selective binding. The reported association constant values for most of the amide⊂1 complexes are among the highest reported to date for the binding of small polar molecules38 and even small peptides41–46 in water using synthetic receptors. The amides are bound in the functionalized aromatic cavity of calix[4]pyrrole 1 by a combination of hydrogen-bonding, NH–π and CH–π interactions and the hydrophobic effect. We foresee that further elaboration of the aromatic cavity of water soluble meso-aryl extended calix[4]pyrroles could have an impact on improving their recognition properties, such as achieving higher binding constants or selectivity. Studies to further develop and understand these receptors are currently ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Gobierno de España MINECO and FEDER funds for the project (CTQ2017-84319-P), the CERCA Programme/Generalitat de Catalunya, AGAUR (2017 SGR 1123) and the ICIQ Foundation for funding. L. E. thanks MECD for a predoctoral fellowship (FPU14/01016) and A.D.-M. thanks MINECO for a postdoctoral fellowship (FPDI-2013-15632). We also thank Peter Gans (Protonic Software) for assistance in the fit of the NMR titration data.Notes and references
- G. Fischer, Chem. Soc. Rev., 2000, 29, 119–127 RSC.
- C. Dugave and L. Demange, Chem. Rev., 2003, 103, 2475–2532 CrossRef PubMed.
- W. E. Stewart and T. H. Siddall, Chem. Rev., 1970, 70, 517–551 CrossRef.
- G. E. S. Schulz and R. H. Schirmer, Principles of Protein Structure, Springer-Verlag, New York, 1979 Search PubMed.
- O. Tchaicheeyan, FASEB J., 2004, 18, 783–789 CrossRef PubMed.
- H. Kagechika, T. Himi, E. Kawachi and K. Shudo, J. Med. Chem., 1989, 32, 2292–2296 CrossRef PubMed.
- W. L. Cody, J. X. He, M. D. Reily, S. J. Haleen, D. M. Walker, E. L. Reyner, B. H. Stewart and A. M. Doherty, J. Med. Chem., 1997, 40, 2228–2240 CrossRef PubMed.
- M. Keller, C. Boissard, L. Patiny, N. N. Chung, C. Lemieux, M. Mutter and P. W. Schiller, J. Med. Chem., 2001, 44, 3896–3903 CrossRef PubMed.
- J. Chatterjee, D. Mierke and H. Kessler, J. Am. Chem. Soc., 2006, 128, 15164–15172 CrossRef PubMed.
- E. Biron, J. Chatterjee, O. Ovadia, D. Langenegger, J. Brueggen, D. Hoyer, H. A. Schmid, R. Jelinek, C. Gilon, A. Hoffman and H. Kessler, Angew. Chem., Int. Ed., 2008, 47, 2595–2599 CrossRef PubMed.
- F. D. Lewis and E. L. Burch, J. Am. Chem. Soc., 1994, 116, 1159–1160 CrossRef.
- E. Bairaktari, D. F. Mierke, S. Mammi and E. Peggion, J. Am. Chem. Soc., 1990, 112, 5383 CrossRef.
- A. Jabs, M. S. Weiss and R. Hilgenfeld, J. Mol. Biol., 1999, 286, 291–304 CrossRef PubMed.
- B. C. Gorske, J. R. Stringer, B. L. Bastian, S. A. Fowler and H. E. Blackwell, J. Am. Chem. Soc., 2009, 131, 16555–16567 CrossRef PubMed.
- C. C. Forbes, A. M. Beatty and B. D. Smith, Org. Lett., 2001, 3, 3595–3598 CrossRef PubMed.
- R. R. Gardner, S. L. McKay and S. H. Gellman, Org. Lett., 2000, 2, 2335–2338 CrossRef PubMed.
- F. H. Beijer, R. P. Sijbesma, J. A. J. M. Vekemans, E. W. Meijer, H. Kooijman and A. L. Spek, J. Org. Chem., 1996, 61, 6371–6380 CrossRef PubMed.
- M. J. Deetz, J. E. Fahey and B. D. Smith, J. Phys. Org. Chem., 2001, 14, 463–467 CrossRef.
- G. J. Pernía, J. D. Kilburn, J. W. Essex, R. J. Mortishire-Smith and M. Rowley, J. Am. Chem. Soc., 1996, 118, 10220–10227 CrossRef.
- C. Vicent, S. C. Hirst, F. Garciatellado and A. D. Hamilton, J. Am. Chem. Soc., 1991, 113, 5466–5467 CrossRef.
- W. C. Still, Acc. Chem. Res., 1996, 29, 155–163 CrossRef.
- C. Allott, H. Adams, C. A. Hunter, J. A. Thomas, P. L. Bernad Jr and C. Rotger, Chem. Commun., 1998, 2449–2450 RSC.
- W. E. Allen, P. A. Gale, C. T. Brown, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 12471–12472 CrossRef.
- T. Guinovart, D. Hernández-Alonso, L. Adriaenssens, P. Blondeau, M. Martínez-Belmonte, F. X. Rius, F. J. Andrade and P. Ballester, Angew. Chem., Int. Ed., 2016, 55, 2435–2440 CrossRef PubMed.
- B. Verdejo, G. Gil-Ramírez and P. Ballester, J. Am. Chem. Soc., 2009, 131, 3178–3179 CrossRef PubMed.
- D. Hernandez-Alonso, S. Zankowski, L. Adriaenssens and P. Ballester, Org. Biomol. Chem., 2015, 13, 1022–1029 RSC.
- K. D. Bhatt, D. J. Vyas, B. A. Makwana, S. M. Darjee and V. K. Jain, Spectrochim. Acta, Part A, 2014, 121, 94–100 CrossRef PubMed.
- K.-D. Zhang, D. Ajami and J. Rebek, J. Am. Chem. Soc., 2013, 135, 18064–18066 CrossRef PubMed.
- A. Díaz-Moscoso, D. Hernández-Alonso, L. Escobar, F. A. Arroyave and P. Ballester, Org. Lett., 2017, 19, 226–229 CrossRef PubMed.
- J. R. Blas, J. M. Lopez-Bes, M. Marquez, J. L. Sessler, F. J. Luque and M. Orozco, Chem. - Eur. J., 2007, 13, 1108–1116 CrossRef PubMed.
- J. R. Blas, M. Marquez, J. L. Sessler, F. J. Luque and M. Orozco, J. Am. Chem. Soc., 2002, 124, 12796–12805 CrossRef PubMed.
- G. Gil-Ramirez, E. C. Escudero-Adan, J. Benet-Buchholz and P. Ballester, Angew. Chem., Int. Ed., 2008, 47, 4114–4118 CrossRef PubMed.
- H. Adams, F. J. Carver, C. A. Hunter and N. J. Osborne, Chem. Commun., 1996, 2529–2530 RSC.
- I. Alfonso, M. I. Burguete, F. Galindo, S. V. Luis and L. Vigara, J. Org. Chem., 2007, 72, 7947–7956 CrossRef PubMed.
- L. M. Salonen, M. Ellermann and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 4808–4842 CrossRef PubMed.
- V. P. Manea, K. J. Wilson and J. R. Cable, J. Am. Chem. Soc., 1997, 119, 2033–2039 CrossRef.
- C. Frassineti, S. Ghelli, P. Gans, A. Sabatini, M. S. Moruzzi and A. Vacca, Anal. Biochem., 1995, 231, 374–382 CrossRef PubMed.
- G.-B. Huang, S.-H. Wang, H. Ke, L.-P. Yang and W. Jiang, J. Am. Chem. Soc., 2016, 138, 14550–14553 CrossRef PubMed.
- L.-L. Wang, Z. Chen, W.-E. Liu, H. Ke, S.-H. Wang and W. Jiang, J. Am. Chem. Soc., 2017, 139, 8436–8439 CrossRef PubMed.
- P. S. Cremer, A. H. Flood, B. C. Gibb and D. L. Mobley, Nat. Chem., 2018, 10, 8–16 CrossRef PubMed.
- X. Cha, K. Ariga and T. Kunitake, J. Am. Chem. Soc., 1996, 118, 9545–9551 CrossRef.
- A. T. Wright and E. V. Anslyn, Org. Lett., 2004, 6, 1341–1344 CrossRef PubMed.
- H. Imai, H. Munakata, Y. Uemori and N. Sakura, Inorg. Chem., 2004, 43, 1211–1213 CrossRef PubMed.
- C. P. Mandl and B. König, J. Org. Chem., 2005, 70, 670–674 CrossRef PubMed.
- C. Schmuck and U. Machon, Chem. - Eur. J., 2005, 11, 1109–1118 CrossRef PubMed.
- M. E. Bush, N. D. Bouley and A. R. Urbach, J. Am. Chem. Soc., 2005, 127, 14511–14517 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General methods, synthetic procedures, characterisation data and association studies by NMR and ITC. See DOI: 10.1039/c8sc03034k |
| ‡ The results of the binding studies of related water soluble aryl-extended calix[4]pyrroles with creatinine and a homologous series of lactams will be reported elsewhere. |
| § Some foam appeared in the solutions after vigorous shaking at high concentrations indicating the amphiphilic nature of tetra-pyridinium calix[4]pyrrole 1. In order to avoid misleading results, we performed our experiments at concentrations below 5 mM. |
| ¶ The pyridinium proton signals also displayed chemical shift changes upon addition of incremental amounts of the guest. However, we decided not to use these nuclei to determine binding constants from the 1H NMR titration data because they are not close to the receptor's binding site. |
| || A broad signal resonating at δ = 5.32 ppm was assigned to the formyl proton of bound cis-3. The computationally determined chemical shift value (DFT) of this proton in the inclusion complex cis-3⊂1 is in complete agreement with the assignment. |
| ** Using DFT calculations we computed the chemical shift values of the protons for bound cis-5. We were gratefully surprised to find a nice agreement with the experimental ones (ESI†). |
| †† The chemical shift value of the beta-pyrrole protons in the cis-5⊂1 complex was considered to be a good estimate for the δbound value in cis-10⊂1. |
| This journal is © The Royal Society of Chemistry 2018 |