Open Access Article
Open Access ArticleFacile synthesis of polycyclic metallaarynes†
Wenqing
Ruan‡
,
Tsz-Fai
Leung‡
,
Chuan
Shi
,
Ka Ho
Lee
,
Herman H. Y.
Sung
,
Ian D.
Williams
 *,
Zhenyang
Lin
*,
Zhenyang
Lin
 * and
Guochen
Jia
* and
Guochen
Jia
 *
*
Department of Chemistry, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong. E-mail: chwill@ust.hk; chzlin@ust.hk; chjiag@ust.hk
First published on 19th June 2018
Abstract
Metalla-analogs of polycyclic arynes represent an interesting class of metallaaromatics with a formal M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond within the ring. The first examples of a bicyclic β-metallaaryne and tricyclic metallaarynes, including a metallaanthracyne and a metallaphenanthryne, were obtained in good yields by reactions of OsCl2(PPh3)3 with alkyne-functionalized phosphorus ylides.
C bond within the ring. The first examples of a bicyclic β-metallaaryne and tricyclic metallaarynes, including a metallaanthracyne and a metallaphenanthryne, were obtained in good yields by reactions of OsCl2(PPh3)3 with alkyne-functionalized phosphorus ylides.
Introduction
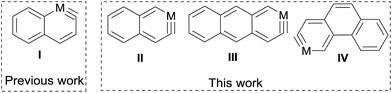
There has been much interest in the development of the chemistry of aromatic metallacycles of transition metals.1 Monocyclic six-membered metallacycles such as metallabenzenes and metallabenzynes are among the most well-studied aromatic metallacycles.2 More recently, aromatic metallacycles with a more extended structure have been attracting considerable attention.3Polycyclic arenes and arynes are an important class of aromatic organic compounds with an extended conjugation system. Thus, it is naturally desirable to develop the chemistry of metalla-analogs of such compounds. These compounds are attractive considering the interesting material properties of polycyclic aromatic hydrocarbons and hetero-arenes containing a main group element.4 However, limited progress has been made in this direction. Well-characterized polycyclic metallaarenes are confined to a few bicyclic metallanaphthalenes (of iridium5 and osmium6) and a tricyclic iridaanthracene.7 Reported polycyclic metallaarynes are even more rare and are limited to two bicyclic α-osmanaphthalynes6,8 (derivatives of α-metallanaphthalyne I, Scheme 1).
In this work, we report a versatile synthetic method for the synthesis of polycyclic metallaarynes, which enabled us to prepare the first examples of a bicyclic β-metallanaphthalyne (II) and tricyclic metallaarynes including a metallaanthracyne (III) and a metallaphenanthryne (IV) (Scheme 1).
The development of the chemistry of polycyclic metallaarynes has been hindered by the lack of efficient routes to synthesize such compounds. Although two α-osmanaphthalynes have been prepared by cyclometallation of a phenyl ring in the vinylcarbyne complexes (PPh3)2Cl3Os{![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH
CCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(o-C6H4Cl)2} (induced by Zn)8 and (PPh3)2Cl2HOs{
C(o-C6H4Cl)2} (induced by Zn)8 and (PPh3)2Cl2HOs{![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(PPh3)
CC(PPh3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh}BF4 (induced by oxygen),6a this cyclometallation strategy could not be easily extended to prepare polycyclic metallaarynes with a metal in the β-position or with a more extended structure due to the lack of generality of the cyclometallation reactions, or the difficulty in obtaining the necessary precursor complexes. We have therefore searched for alternative and more general routes to prepare polycyclic metallaarynes, and explored the possibility of synthesizing polycyclic metallaarynes from alkyne-functionalized phosphorus ylides.
CHPh}BF4 (induced by oxygen),6a this cyclometallation strategy could not be easily extended to prepare polycyclic metallaarynes with a metal in the β-position or with a more extended structure due to the lack of generality of the cyclometallation reactions, or the difficulty in obtaining the necessary precursor complexes. We have therefore searched for alternative and more general routes to prepare polycyclic metallaarynes, and explored the possibility of synthesizing polycyclic metallaarynes from alkyne-functionalized phosphorus ylides.
Results and discussion
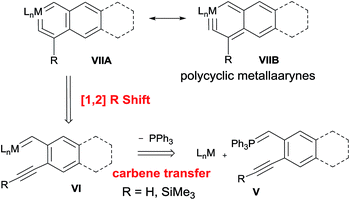
The strategy was devised on the basis of the retrosynthetic analysis shown in Scheme 2. It is known that phosphorus ylides can act as genuine carbene transfer agents9 and that alkyne complexes LnM(R′C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR) (R = H,10 SiMe3 (ref. 11)) can rearrange to give vinylidene complexes LnM{
CR) (R = H,10 SiMe3 (ref. 11)) can rearrange to give vinylidene complexes LnM{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R′)(R)} via a 1,2-R shift. We therefore envisioned that polycyclic metallaaryne complex VII might be obtained from rearrangement of carbene intermediate VI which was generated from the reaction of alkyne-functionalized phosphorus ylide V with a coordinatively unsaturated complex LnM.
C(R′)(R)} via a 1,2-R shift. We therefore envisioned that polycyclic metallaaryne complex VII might be obtained from rearrangement of carbene intermediate VI which was generated from the reaction of alkyne-functionalized phosphorus ylide V with a coordinatively unsaturated complex LnM.
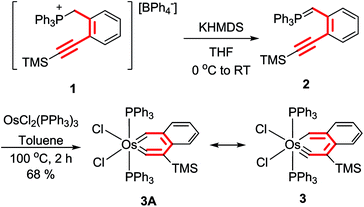
To verify the hypothesis, we have studied the reactions of OsCl2(PPh3)3 with the ylides o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)(CH
CR)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3) (R = H, SiMe3), which can be obtained from the phosphonium salt [o-C6H4(C
PPh3) (R = H, SiMe3), which can be obtained from the phosphonium salt [o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)(CH2PPh3)]BPh4 (1) (Scheme 3). The ylide o-C6H4(C
CSiMe3)(CH2PPh3)]BPh4 (1) (Scheme 3). The ylide o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)(CH
CH)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3) was obtained by treatment of 1 with two equiv. of PhCH2K followed by one equiv. of 2,6-lutidinium tetraphenylborate, and the ylide o-C6H4(C
PPh3) was obtained by treatment of 1 with two equiv. of PhCH2K followed by one equiv. of 2,6-lutidinium tetraphenylborate, and the ylide o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3)(CH
CSiMe3)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3) (2) was obtained by the reaction of 1 with one equiv. of potassium hexamethyldisilazide (KHMDS). The ylide o-C6H4(C
PPh3) (2) was obtained by the reaction of 1 with one equiv. of potassium hexamethyldisilazide (KHMDS). The ylide o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH)(CH
CH)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3) reacted with OsCl2(PPh3)3 in toluene at room temperature or 110 °C to give a mixture of highly sensitive and intractable species. To our delight, the treatment of OsCl2(PPh3)3 with the ylide o-C6H4(C
PPh3) reacted with OsCl2(PPh3)3 in toluene at room temperature or 110 °C to give a mixture of highly sensitive and intractable species. To our delight, the treatment of OsCl2(PPh3)3 with the ylide o-C6H4(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CTMS)(CH
CTMS)(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3) (2) in toluene at 110 °C for 2 h gave the expected osmanaphthalyne 3, which was isolated as a green solid in 68% yield (Scheme 3). Apparently, the SiMe3-containing osmanaphthalyne 3 has higher stability or lower reactivity than the analogous desiylated osmanaphthalyne. Indeed, early computational studies have revealed that a silyl group on the carbon adjacent to the carbyne carbon can increase the conjugation energy of metallabenzynes12 and increase the stability of the metallabenzyne with respect to the formation of the corresponding carbene complexes.13 In addition, the silyl group could also decrease the reactivity of the carbyne carbon with nucleophiles.
PPh3) (2) in toluene at 110 °C for 2 h gave the expected osmanaphthalyne 3, which was isolated as a green solid in 68% yield (Scheme 3). Apparently, the SiMe3-containing osmanaphthalyne 3 has higher stability or lower reactivity than the analogous desiylated osmanaphthalyne. Indeed, early computational studies have revealed that a silyl group on the carbon adjacent to the carbyne carbon can increase the conjugation energy of metallabenzynes12 and increase the stability of the metallabenzyne with respect to the formation of the corresponding carbene complexes.13 In addition, the silyl group could also decrease the reactivity of the carbyne carbon with nucleophiles.
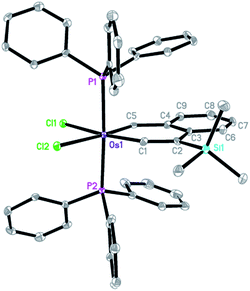
The structure of complex 3 was confirmed by X-ray diffraction analysis (Fig. 1),14 which reveals that complex 3 has an essentially planar metallacycle. The angle around the carbyne carbon (153.1(5)°) is similar to those found in reported metallabenzynes.15 The Os–C1 (carbyne) bond distance (1.826(6) Å) of 3 is appreciably longer than those of typical Os![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds (1.69–1.79 Å) and at the low end of those observed for LnOs
C bonds (1.69–1.79 Å) and at the low end of those observed for LnOs![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CRR′ complexes (1.786–1.892 Å), while the Os–C5 bond distance (1.963(6) Å) is within the range of Os–C (vinyl) bonds (1.897–2.195 Å).15 The structural data indicate that the vinylidene resonance form 3A makes a significant contribution to the overall structure of the osmanaphthalyne.
CRR′ complexes (1.786–1.892 Å), while the Os–C5 bond distance (1.963(6) Å) is within the range of Os–C (vinyl) bonds (1.897–2.195 Å).15 The structural data indicate that the vinylidene resonance form 3A makes a significant contribution to the overall structure of the osmanaphthalyne.
The solid state-structure of complex 3 is supported by the solution NMR spectroscopic data. The 31P{1H} NMR spectrum showed a singlet at −2.9 ppm for the two equivalent PPh3 ligands. The 13C{1H} NMR spectrum displayed signals of OsC1 (Os![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) and OsC5 (OsCH) at 308.0 and 277.6 ppm, respectively. In the 1H NMR spectrum, the OsCH signal was observed at 15.76 ppm.
C) and OsC5 (OsCH) at 308.0 and 277.6 ppm, respectively. In the 1H NMR spectrum, the OsCH signal was observed at 15.76 ppm.
Having succeeded in isolating the β-osmanaphthalyne 3, we tried to extend the chemistry to larger polycyclic metallaarynes, starting with tricyclic systems. There are two types of tricyclic metallaarynes: (i) metallaanthracynes and (ii) metallaphenanthrynes, which are derived respectively from anthracene and phenanthrene, the simplest ‘linear’ and ‘bent’ triarenes.
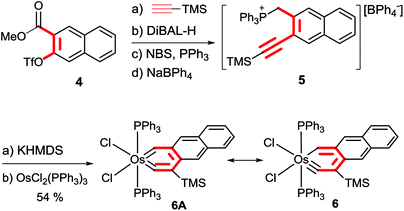
Preparation of a metallaanthracyne was first pursued. The required phosphonium salt 5 was obtained by the Sonogashira coupling reaction of ester 4 with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CTMS to give an alkyne derivative, followed by sequential treatment of the derivative with DiBAL-H, NBS/PPh3 and NaBPh4 (Scheme 4). Treatment of 5 with KHMDS generated an ylide which reacted smoothly with OsCl2(PPh3)3 in toluene at 110 °C to give the desired osmaanthracyne 6, which was isolated as a yellow solid in 54% yield.
CTMS to give an alkyne derivative, followed by sequential treatment of the derivative with DiBAL-H, NBS/PPh3 and NaBPh4 (Scheme 4). Treatment of 5 with KHMDS generated an ylide which reacted smoothly with OsCl2(PPh3)3 in toluene at 110 °C to give the desired osmaanthracyne 6, which was isolated as a yellow solid in 54% yield.
Complex 6 was characterized by NMR and elemental analysis. The 31P{1H} NMR spectrum showed a singlet at −7.0 ppm for the two equivalent PPh3 ligands. The 13C{1H} NMR spectrum displayed the signals of Os![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and OsCH at 304.1 and 288.4 ppm, respectively. In the 1H NMR spectrum, the OsCH signal was observed at 16.51 ppm. The chemical shifts of the 1H and 13C signals of 6 are similar to those of 3.
C and OsCH at 304.1 and 288.4 ppm, respectively. In the 1H NMR spectrum, the OsCH signal was observed at 16.51 ppm. The chemical shifts of the 1H and 13C signals of 6 are similar to those of 3.
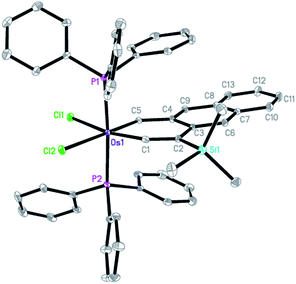
The structure of complex 6 was also confirmed by X-ray diffraction analysis (Fig. 2). Complex 6 can be viewed as a compound formed by annulation of a benzene ring to the metallanaphthalyne structural unit of 3. The X-ray data indicate that the annulation does not alter the structural parameters of the metallanaphthalyne structural unit significantly. For example, the Os–C bond distances as well as the bond angle around the carbyne carbon of 6 are similar to those observed for 3.
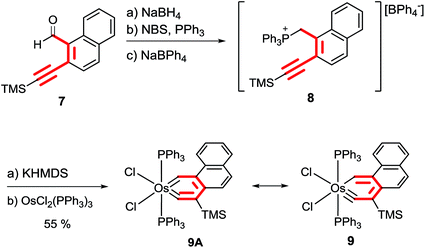
The methodology could also be extended to synthesize metallaphenanthrynes. As shown in Scheme 5, treatment of the aldehyde derivative 7 with NaBH4 followed by NBS/PPh3 and NaBPh4 produced the phosphonium salt 8. The ylide generated from the reaction of phosphonium salt 8 with KHMDS reacted smoothly with OsCl2(PPh3)3 in toluene at 110 °C to give the osmaphenanthryne 9 which was isolated as a green solid in 55% yield.
The identity of complex 9 has been confirmed by the NMR and analytical data as well as X-ray diffraction analysis (Fig. 3). The 31P{1H} NMR spectrum of complex 9 showed a singlet at −0.37 ppm. Characteristic 13C signals of Os![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and OsCH were observed respectively at 308.1 and 250.9 ppm in the 13C{1H} NMR spectrum. In the 1H NMR spectra, the OsCH signal was observed at 16.14 ppm.
C and OsCH were observed respectively at 308.1 and 250.9 ppm in the 13C{1H} NMR spectrum. In the 1H NMR spectra, the OsCH signal was observed at 16.14 ppm.
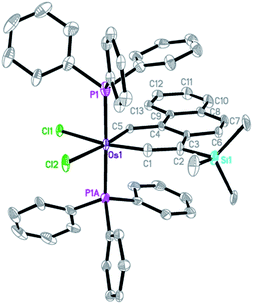
The X-ray diffraction analysis of complex 9 confirms that it has a planar metallacycle. Subtle differences were noted in the structural parameters of the osmaanthracyne 6 and the osmaphenanthryne 9, which are structural isomers. The Os–C1 (carbyne) bond distance (1.791(7) Å) of 9 is appreciably shorter than that (1.826(6) Å) of 6 while the Os–C5 bond distance (2.002(6)Å) of 9 is appreciably longer than that (1.963(6) Å) of 6.
The structural data indicate that, compared with the case of 6, the vinylidene resonance form 9A makes a lower contribution to the overall structure of the osmaphenanthryne 9.
Complexes 6 and 9 are interesting as they represent the first examples of tricyclic metallaarynes. In fact, even well-characterized tricyclic metallaarenes are rare. Wright and his co-workers recently reported the isolation and structural characterization of the first metallaanthracene, an iridaanthracene.7 Jones and his co-workers generated a thermally unstable lithiated ruthenaphenanthrene oxide that could only be characterized spectroscopically at low temperature and reacted with Et3OBF4 to give an η3-benzyl complex.16 In related work, Bettinger and his co-workers found that thermolysis of 9-azido-9-borafluorene produced a reactive 9,10-BN-phenanthryne which undergoes a self-trapping reaction to give a cyclic tetramer.17
Our complexes 3, 6 and 9 are metalla-analogs of β-naphthalyne, anthracyne and phenanthryne, respectively. Similarities in the structural properties of the metallacycles and their organic counterparts are noted. In particular, they have the same bond alternation pattern and similar corresponding C–C bond distances when the C–Os–C fragment in the metallacycles and the C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C fragment in the organic counterparts are excluded (see Fig. S28–30 in the ESI†). Naphthalyne, anthracyne and phenanthryne are aromatic. Our DFT calculations confirm that all the rings of complexes 3, 6 and 9 have negative NICS(1) values18 (see Fig. S31 in the ESI†), indicating that the aromatic character is retained when a carbon atom in a polycyclic aryne is replaced by an isolobal metal fragment.
C fragment in the organic counterparts are excluded (see Fig. S28–30 in the ESI†). Naphthalyne, anthracyne and phenanthryne are aromatic. Our DFT calculations confirm that all the rings of complexes 3, 6 and 9 have negative NICS(1) values18 (see Fig. S31 in the ESI†), indicating that the aromatic character is retained when a carbon atom in a polycyclic aryne is replaced by an isolobal metal fragment.
It is interesting to note that polycyclic arynes and their metalla-analogs show different thermal stabilities. Naphthalynes,19 anthracynes20 and phenanthrynes21 are too reactive to be isolated in the free-state and have only been detected in argon matrices at low temperature. In contrast, complexes 3, 6 and 9 are air-stable species which can be stored in the solid form for months without appreciable decomposition. The higher stability of 3, 6 and 9 could be related to the fact that the ring strains of 3, 6 and 9 are smaller than those of their organic counterparts as significant bond bending occurs at only one carbon in 3, 6 and 9 (from 180° to ca. 153°), but at two carbons in the organic compounds with an even larger bending angle (from 180° to ca. 127°).
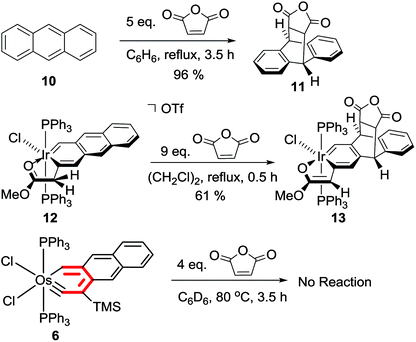
Preliminary reactivity studies reveal that polycyclic metallaarynes can also possess chemical properties different from those of polycyclic arenes and metallaarenes. For example, anthracene22 and iridaanthracene 12 (ref. 7) have been reported to undergo [4 + 2] cycloaddition reactions with maleic anhydride at the central ring (in refluxing benzene or 1,2-dichloroethane, respectively) (Scheme 6). In contrast, essentially no reaction was observed when a mixture of the osmaanthracyne 6 and maleic anhydride (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio) was heated in benzene at 80 °C for 3.5 h, suggesting that the central ring of the osmaanthracyne is less reactive than those of anthracene and the iridaanthracene.
4 molar ratio) was heated in benzene at 80 °C for 3.5 h, suggesting that the central ring of the osmaanthracyne is less reactive than those of anthracene and the iridaanthracene.
Conclusions
In summary, we have developed a convenient synthetic route to polycyclic metallaarynes. Through reactions of OsCl2(PPh3)3 with alkyne-functionalized phosphorus ylides, we have successfully prepared the first examples of β-metallanaphthalyne, metallaanthracyne and metallaphenanthryne complexes. Reactivity studies of the new metallacycles as well as the preparation of polycyclic metallaarynes with other metals or with a more extended structure utilizing the phosphorus-ylide strategy are now underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Hong Kong Research Grants Council (Project No. 602113 and 16321516).Notes and references
- (a) B. J. Frogley and L. J. Wright, Chem.–Eur. J., 2018, 24, 2025–2038 CrossRef PubMed; (b) J. Wei, W. X. Zhang and Z. Xi, Chem. Sci., 2018, 9, 560–568 RSC.
- For a recent review, see: L. J. Wright, Metallabenzenes: An Expert View, John Wiley & Sons Ltd, Oxford, 2017 Search PubMed.
- For examples of recent work, see: (a) C. Zhu, J. Zhu, X. Zhou, Q. Zhu, Y. Yang, T. B. Wen and H. Xia, Angew. Chem., Int. Ed., 2018, 57, 3154–3157 CrossRef PubMed; (b) R. Li, Z. Lu, Y. Cai, F. Jiang, C. Tang, Z. Chen, J. Zheng, J. Pi, R. Zhang, J. Liu, Z. Chen, Y. Yang, J. Shi, W. Hong and H. Xia, J. Am. Chem. Soc., 2017, 139, 14344–14347 CrossRef PubMed; (c) M. Luo, L. Long, H. Zhang, Y. Yang, Y. Hua, G. Liu, Z. Lin and H. Xia, J. Am. Chem. Soc., 2017, 139, 1822–1825 CrossRef PubMed; (d) C. Zhu, J. Wu, S. Li, Y. Yang, J. Zhu, X. Lu and H. Xia, Angew. Chem., Int. Ed., 2017, 56, 9067–9071 CrossRef PubMed; (e) Y. Zhang, J. Wei, Y. Chi, X. Zhang, W. X. Zhang and Z. Xi, J. Am. Chem. Soc., 2017, 139, 5039–5042 CrossRef PubMed; (f) J. Wei, Y. Zhang, Y. Chi, L. Liu, W. X. Zhang and Z. Xi, J. Am. Chem. Soc., 2016, 138, 60–63 CrossRef PubMed; (g) M. Batuecas, R. Castro-Rodrigo, M. A. Esteruelas, C. Garcia-Yebra, A. M. Lopez and E. Onate, Angew. Chem., Int. Ed., 2016, 55, 13749–13753 CrossRef PubMed.
- (a) M. Stepien, E. Gonka, M. Zyla and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed; (b) Polycyclic Arenes and Heteroarenes: Synthesis Properties, and Applications, ed. Q. Miao, Wiley-VCH, Weinheim, 2016 Search PubMed.
- (a) M. Paneque, C. M. Posadas, M. L. Poveda, N. Rendón, V. Salazar, E. Oñate and K. Mereiter, J. Am. Chem. Soc., 2003, 125, 9898–9899 CrossRef PubMed; (b) M. Talavera, S. Bolaño, J. Bravo, J. Castro, S. García-Fontán and J. M. Hermida-Ramón, Organometallics, 2013, 32, 4058–4060 CrossRef; (c) A. Vivancos, Y. A. Hernández, M. Paneque, M. L. Poveda, V. Salazar and E. Álvarez, Organometallics, 2015, 34, 177–188 CrossRef.
- (a) B. Liu, H. Xie, H. Wang, L. Wu, Q. Zhao, J. Chen, T. B. Wen, Z. Cao and H. Xia, Angew. Chem., Int. Ed., 2009, 48, 5461–5464 CrossRef PubMed; (b) For theoretical studies, see: J. l. Fan, X. R. Wang and J. Zhu, Organometallics, 2014, 33, 2336–2340 CrossRef.
- B. J. Frogley and L. J. Wright, Angew. Chem., Int. Ed., 2017, 56, 143–147 CrossRef PubMed.
- G. He, J. Zhu, W. Y. Hung, T. B. Wen, H. H. Y. Sung, I. D. Williams, Z. Lin and G. Jia, Angew. Chem., Int. Ed., 2007, 46, 9065–9068 CrossRef PubMed.
- See for example: (a) J. Vignolle, B. Donnadieu, D. Bourissou, M. Soleilhavoup and G. Bertrand, J. Am. Chem. Soc., 2006, 128, 14810–14811 CrossRef PubMed; (b) L. R. Falvello, R. Llusar, M. E. Margalejo, R. Navarro and E. P. Urriolabeitia, Organometallics, 2003, 22, 1132–1144 CrossRef; (c) L. K. Johnson, M. Frey, T. A. Ulibarri, S. C. Virgil, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1993, 115, 8167–8177 CrossRef.
- (a) J. A. Varela, C. González-Rodríguez and C. Saá, Top. Organomet. Chem., ed. C. Bruneau and P. Dixneuf, Springer, Cham, 2014, vol. 48, pp. 237–287 Search PubMed; (b) J. M. Lynam, Chem.–Eur. J., 2010, 16, 8238–8247 CrossRef PubMed.
- See for example: (a) K. Ilg, M. Paneque, M. L. Poveda, N. Rendón, L. L. Santos, E. Carmona and K. Mereiter, Organometallics, 2006, 25, 2230–2236 CrossRef; (b) M. V. Jiménez, E. Sola, F. J. Lahoz and L. A. Oro, Organometallics, 2005, 24, 2722–2729 CrossRef; (c) D. Huang, W. E. Streib, O. Eisenstein and K. G. Caulton, Organometallics, 2000, 19, 1967–1972 CrossRef.
- S. Ng, X. Huang, T. Wen, G. Jia and Z. Lin, Organometallics, 2003, 22, 3898–3904 CrossRef.
- J. Fan, K. An, X. Wang and J. Zhu, Organometallics, 2013, 32, 6271–6276 CrossRef.
- CCDC 1589937, 1589938, and 1831065 contain the supplementary crystallographic data for this paper..
- G. Jia, Coord. Chem. Rev., 2007, 251, 2167–2187 CrossRef.
- J. Yang, W. M. Jones, J. K. Dixon and N. T. Allison, J. Am. Chem. Soc., 1995, 117, 9776–9777 CrossRef.
- M. Müller, C. Maichle-Mössmer and H. F. Bettinger, Angew. Chem., Int. Ed., 2014, 53, 9380–9383 CrossRef PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef PubMed.
- (a) A. Comandini, S. Abid and N. Chaumeix, J. Phys. Chem. A, 2017, 121, 5921–5931 CrossRef PubMed; (b) T. Sato, H. Niino and A. Yabe, J. Phys. Chem. A, 2001, 105, 7790–7798 CrossRef; (c) J. Cioslowski, P. Piskorz and D. Moncrieff, J. Am. Chem. Soc., 1998, 120, 1695–1700 CrossRef; (d) T. Kitamura, N. Fukatsu and Y. Fujiwara, J. Org. Chem., 1998, 63, 8579–8581 CrossRef.
- (a) A. Comandini, S. Abid and N. Chaumeix, J. Phys. Chem. A, 2017, 121, 5921–5931 CrossRef PubMed; (b) J. Cioslowski, P. Piskorz and D. Moncrieff, J. Am. Chem. Soc., 1998, 120, 1695–1700 CrossRef; (c) R. Camenzind and B. Rickborn, J. Org. Chem., 1986, 51, 1914–1916 CrossRef.
- H. Tomioka, A. Okuno, T. Sugiyama and S. Murata, J. Org. Chem., 1995, 60, 2344–2352 CrossRef.
- W. E. Bachmann and M. C. Kloetzel, J. Am. Chem. Soc., 1938, 60, 481–485 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1589937, 1589938, and 1831065. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc02086h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |