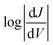Open Access Article
Open Access ArticleControlling destructive quantum interference in tunneling junctions comprising self-assembled monolayers via bond topology and functional groups†
Yanxi
Zhang‡
 ab,
Gang
Ye‡
ab,
Gang
Ye‡
 ab,
Saurabh
Soni
ab,
Saurabh
Soni
 ab,
Xinkai
Qiu
ab,
Theodorus L.
Krijger
ab,
Harry T.
Jonkman
b,
Marco
Carlotti
ab,
Eric
Sauter
c,
Michael
Zharnikov
ab,
Xinkai
Qiu
ab,
Theodorus L.
Krijger
ab,
Harry T.
Jonkman
b,
Marco
Carlotti
ab,
Eric
Sauter
c,
Michael
Zharnikov
 c and
Ryan C.
Chiechi
c and
Ryan C.
Chiechi
 *ab
*ab
aStratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: r.c.chiechi@rug.nl
bZernike Institute for Advanced Materials, Nijenborgh 4, 9747 AG Groningen, The Netherlands
cApplied Physical Chemistry, Heidelberg University, Im Neuenheier Feld 253, Heidelberg 69120, Germany
First published on 23rd April 2018
Abstract
Quantum interference effects (QI) are of interest in nano-scale devices based on molecular tunneling junctions because they can affect conductance exponentially through minor structural changes. However, their utilization requires the prediction and deterministic control over the position and magnitude of QI features, which remains a significant challenge. In this context, we designed and synthesized three benzodithiophenes based molecular wires; one linearly-conjugated, one cross-conjugated and one cross-conjugated quinone. Using eutectic Ga–In (EGaIn) and CP-AFM, we compared them to a well-known anthraquinone in molecular junctions comprising self-assembled monolayers (SAMs). By combining density functional theory and transition voltage spectroscopy, we show that the presence of an interference feature and its position can be controlled independently by manipulating bond topology and electronegativity. This is the first study to separate these two parameters experimentally, demonstrating that the conductance of a tunneling junction depends on the position and depth of a QI feature, both of which can be controlled synthetically.
Introduction
Molecular electronics is concerned with the transport of charge through molecules spanning two electrodes,1 the fabrication of which is a challenging area of nanotechnology.2–4 In such junctions, π-conjugated molecules influence transport more than a simple, rectangular tunneling barrier; when a tunneling electron traverses the region of space occupied by orbitals localized on these molecules, its wave function can undergo constructive or destructive interference, enhancing or suppressing conductance. When the presence of different pathways in molecular system affects conductance, it is typically described as quantum interference (QI),5 which was originally adapted from the Aharonov–Bohm effect6 to substituted benzenes.7,8 The concept “quantum interference effect transistor” was also proposed using meta-benzene structures for device application.9 Solomon et al. further refined the concept in the context of molecular electronics where it is now well established that destructive QI leads to lower conductance in tunneling junctions.10–15 We previously demonstrated QI in SAM-based junctions using a series of compounds based on an anthracene core; AC, which is linearly-conjugated; AQ, which is cross-conjugated via a quinone moiety; and AH, in which the conjugation is interrupted by saturated methylene bridges (Fig. S1†).16 Subsequent studies verified these findings in a variety of experimental platforms and a consensus emerged that, provided the destructive QI feature (anti-resonances in transmission) is sufficiently close to the Fermi level, EF, cross-conjugation leads to QI.17–24 However, experimental studies on conjugation patterns other than AC/AQ are currently limited to ring substitutions such as meta-substituted phenyl rings,25–32 or varied connectivities in azulene,33–35 which differ fundamentally5,11,36–38 from cross-conjugated bond topologies23,39,40 because they change tunneling pathways, molecular-lengths and bond topology simultaneously (Table S1†). Isolating these variables is however important because the only primary observable is conductance, which varies exponentially with molecular length. More recent work has focused on “gating” QI effects by controlling the alignment of π-systems through-space37,41,42 and affecting the orbital symmetry of aromatic rings with heteroatoms.43–45 These studies exclusively study the effects of the presence and absence of QI features; to date—and despite recent efforts46—the specific effects of bond topology and electronegativity on the depth and position of QI features have not been isolated experimentally.To address this issue, we designed and synthesized the series of benzodithiophene derivatives (BDT-n); benzo[1,2-b:4,5-b′]dithiophene (BDT-1, linearly-conjugated), benzo[1,2-b:4,5-b′]dithiophene-4,8-dione (BDT-2, cross-conjugated with quinone), and benzo[1,2-b:5,4-b′]dithiophene (BDT-3, cross-conjugated and an isomer of BDT-1). These compounds separate the influence of cross-conjugation (bond topology) from that of the electron-withdrawing effects of the quinone functionality while controlling for molecular formula and length. We investigated the charge transport properties of these molecules in tunneling junctions comprising self-assembled monolayers (SAMs), which are relevant for solid-state molecular-electronic devices.47–49 Through a combination of density functional theory (DFT) and transition voltage spectroscopy (TVS) we show that cross-conjugation produces QI features near occupied molecular states and that the position and depth of the QI feature is strongly influenced by the strongly electron-withdrawing quinone functionality, which places these features near unoccupied states while simultaneously bringing those states close to EF. Thus, by controlling bond topology and electronegativity separately, the conductance can be tuned independently of length and connectivity via the relative positions of the QI features and molecular states and not just the presence or absence of such features.
Results and discussion
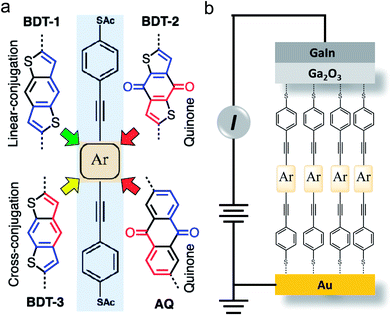
To isolate molecular effects on transport, it is important to control for changes to the width of the tunneling barrier which, in SAMs, is typically defined by the end-to-end lengths of the molecules. Conductance G generally varies exponentially with the barrier-width d such that G = G0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(–βd), where G0 is the theoretical value of G when d = 0, and β is the tunneling decay coefficient. Since β depends on the positions of molecular states relative to EF and we are comparing compounds with very different redox potentials (orbital energies) we can only ascribe changes to G if d is invariant across the series. Furthermore, to isolate the variable of bond topology experimentally, the electronic properties of the linear- and cross-conjugated compounds must be nearly identical. Fig. 1a shows the structures of the BDT-n series and AQ; the “arms” are linearly-conjugated phenylacetylenes (highlighted in the light blue background) and the cores (Ar, highlighted in the brown background) are substituted by the structures indicated. The variation in the end-to-end lengths of these compounds is within 1 Å and the linear- and cross-conjugated compounds BDT-1 and BDT-3 differ only by the relative position of sulfur atoms; they have the same molecular formula. The synthesis, full characterization and a detailed discussion of their properties are provided in the ESI.† Note that we include AQ in the series as a benchmark for destructive QI effects.
exp(–βd), where G0 is the theoretical value of G when d = 0, and β is the tunneling decay coefficient. Since β depends on the positions of molecular states relative to EF and we are comparing compounds with very different redox potentials (orbital energies) we can only ascribe changes to G if d is invariant across the series. Furthermore, to isolate the variable of bond topology experimentally, the electronic properties of the linear- and cross-conjugated compounds must be nearly identical. Fig. 1a shows the structures of the BDT-n series and AQ; the “arms” are linearly-conjugated phenylacetylenes (highlighted in the light blue background) and the cores (Ar, highlighted in the brown background) are substituted by the structures indicated. The variation in the end-to-end lengths of these compounds is within 1 Å and the linear- and cross-conjugated compounds BDT-1 and BDT-3 differ only by the relative position of sulfur atoms; they have the same molecular formula. The synthesis, full characterization and a detailed discussion of their properties are provided in the ESI.† Note that we include AQ in the series as a benchmark for destructive QI effects.
We measured tunneling charge transport through metal–molecule–metal junctions comprising BDT-1, BDT-2, BDT-3 and AQ using conformal eutectic Ga–In (EGaIn) contacts as top electrodes.50 We utilized an established procedure of the in situ deprotection of thioacetates41,51 to form well-defined SAMs on Au substrates; these substrates served then as bottom electrodes. We refer to the assembled junctions as Au/SAM//EGaIn where “/” and “//” denote a covalent and van der Waals interfaces, respectively. The geometry of the junctions is shown in Fig. 1b. To verify that the structural similarities of the compounds carry over into the self-assembly process, we characterized the SAMs of BDT-n by several complementary techniques, including (high-resolution) X-ray photoelectron spectroscopy (HRXPS/XPS) and angle-resolved near-edge X-ray absorption fine structure (NEXAFS) spectroscopy. These data are discussed in detail in the ESI† and summarized in Table 1. The characterization of SAMs of AQ is reported elsewhere.16,41 The XPS and NEXAFS data suggest that the molecules in the BDT-n SAMs are assembled upright with the tilt angle of approximately 35°. The molecules are packed densely on the order of 1014 molecules per cm2 as are similar conjugated molecular wire compounds.41
| Compound | BDT-1 | BDT-2 | BDT-3 | C18 reference |
|---|---|---|---|---|
| XPS thickness (Å) | 17 ± 3 | 18 ± 4 | 19 ± 4 | n.d. |
| HRXPS thickness (Å) | 19.81 ± 0.40 | 22.30 ± 0.45 | 17.17 ± 0.34 | 20.9 |
| Averaged XPS thickness (Å) | 18.4 | 20.2 | 18.4 | n.d. |
| Water contact angle (°) | 68.3 ± 4.8 | 65.8 ± 4.0 | 62.8 ± 4.6 | 104.2 ± 2.2 |
| Density (1014 molecules per cm2) | 2.05 | 3.30 | 2.33 | 4.63 |
| Area molecules per Å2 | 48.8 ± 2 | 30.3 ± 2 | 43.0 ± 2 | 21.6 |
| log|J|@0.5 V (A cm−2) | −2.34 ± 0.17 | −4.09 ± 0.23 | −3.53 ± 0.20 | −4.96 ± 0.87 (ref. 41) |
| Yield of working junctions (%) | 88.9 | 93.8 | 84.2 | 79 (ref. 41) |
| Num. working EGaIn junctions | 32 | 30 | 32 | 28 (ref. 41) |
| Total J/V traces | 643 | 626 | 666 | 280 (ref. 41) |
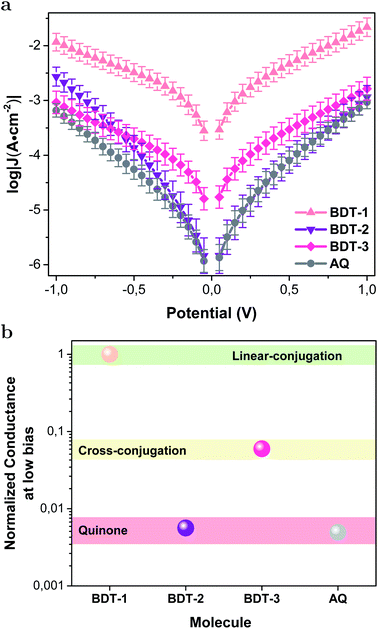
Fig. 2a shows the current–density versus voltage (J/V) curves for the BDT-n series and AQ using EGaIn top contacts. BDT-1 is the most conductive across the entire bias window. The conductance of linearly-conjugated BDT-1 and AC (Fig. S1;† a linearly-conjugated analog of AQ), are almost identical (Fig. S21†), meaning that the low-bias conductivity and/or values of J are directly comparable between the AC/AQ and BDT-n series. As expected, the cross-conjugated BDT-2, BDT-3 and AQ are all less conductive than BDT-1 (and AC). The low-bias conductivity (from the ohmic region, −0.1 V to 0.1 V) of the quinones (BDT-2 and AQ), however, is even more suppressed than the cross-conjugated BDT-3, while the magnitudes of J for BDT-2, BDT-3 and AQ are similar beyond −0.5 V. We observed similar behavior in QI mediated by through-space conjugation in which the compound with an interference feature very close to EF exhibited a sharp rise in J, eventually crossing J/V curve of the compound with a feature further from EF.41 This observation suggests that, as the junction is biased, the transmission probability “climbs” the interference feature rapidly, bringing highly transmissive conduction channels into the bias window at sufficiently low values of V to meet and exceed the total transmission of the compound for which the interference feature is far from EF at zero bias. Further discussion on the asymmetry of J/V curves is included in the ESI.†
To better compare the conductance of the molecules, we calculated the low-bias conductivities and normalized them to BDT-1. These values are plotted in Fig. 2b, showing that cross-conjugation lowers the conductance of BDT-3 by an order of magnitude compared to BDT-1 and the quinone functionality of BDT-2 and AQ lowers it by two orders of magnitude, in agreement with the analogous behavior of AC and AQ.20 To control for large-area effects (e.g., if there are defects in the SAM), we measured BDT-n series by conducting-probe atomic force microscopy (CP-AFM) with Au electrodes and found the same trend: BDT-1 > BDT-3 > BDT-2, however, a direct comparison of low-bias conductivities was precluded by the extremely high resistance of BDT-2 and AQ at low bias. These data are discussed in detail in the ESI.† Thus, we conclude that quinones suppress conductance more than cross-conjugation alone, irrespective of the measurement/device platform.
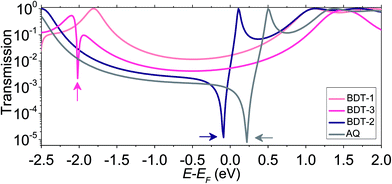
For insight into the shapes of the J/V curves and the conductance, we simulated the transmission spectra, T(E) vs. E − EF (EF value of −4.3 eV, see Experimental section), of the BDT-n series using density functional theory (DFT) and compared the resulting curves with AQ (Fig. 3). These calculations, which are discussed in more detail in the Computational methodology section of the ESI,† simulate the transmission spectra through isolated molecules in vacuum at zero bias and are useful for predicting trends in conductance. There are three important features of these curves: (1) only the compounds with cross-conjugation (including quinones) show sharp dips (anti-resonances or QI features)13,18 in the frontier orbital gap; (2) the dips occur near EF only for the two quinones; and (3) the QI features are more pronounced for the molecules in which the cross-conjugation is caused by a quinone moiety as opposed to the carbon–carbon bond topology. When bias is applied to a junction, the x-axis of the transmission plot shifts and EF broadens such that an integral starting at E − EF = 0 eV and widening to larger ranges of E − EF is a rough approximation of how T(E) translates into current, I(V). This relationship is apparent in the slightly lower conductance of AQ compared to BDT-2 (Fig. 2b) and the slightly lower values of T(E) for AQ compared to BDT-2 across the entire range of E − EF. The proximities of the QI features to EF are also apparent in the J/V curves (Fig. 2a). As the junction is biased, the minimum of the QI feature shifts such that, by 0.5 V, the transmission probabilities are roughly equal for BDT-n and AQ.
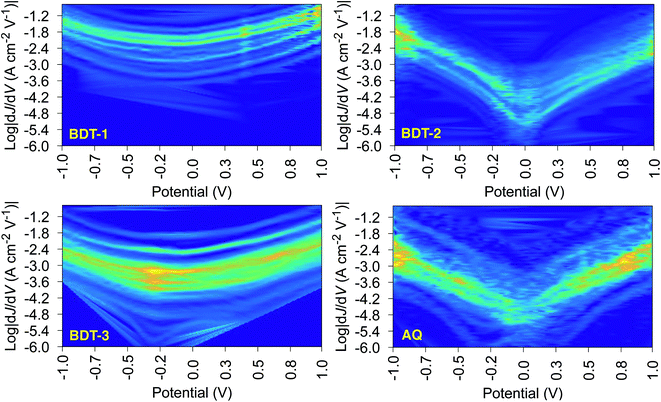
The shape of T(E) near E − EF = 0 eV is roughly traced by differential conductance plots of 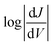 vs. V, allowing QI features near EF to be resolved experimentally.18,41,52Fig. 4 shows heatmap plots of differential conductance of Au/SAM//EGaIn constructed from histograms binned to
vs. V, allowing QI features near EF to be resolved experimentally.18,41,52Fig. 4 shows heatmap plots of differential conductance of Au/SAM//EGaIn constructed from histograms binned to 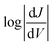 for each value of V (note that these are histograms of J/V curves with no data-selection, thus, brighter colors correspond to mean values of J and are not related to conductance histograms of single-molecule break-junctions; see ESI† for details). Both BDT-1 and BDT-3 exhibit ordinary, U-shaped plots characteristic of non-resonant tunneling. By contrast, both AQ and BDT-2—the two compounds bearing quinone functionality—show V-shaped plots with negative curvature. These results are in agreement with Fig. 3, which places the QI features for the quinone moieties, AQ and BDT-2, much closer to EF than for BDT-3. The positions of these features are related to the positions of highest-occupied and lowest-unoccupied π-states (HOPS and LUPS), which are in good agreement between DFT and experiment (Tables S2 and S3†). Thus, the differential conductance heatmaps (experiment) and DFT (simulation) both indicate that cross-conjugation suppresses conductance because it creates a dip in T(E) in the frontier orbital gap, but that the electron-withdrawing nature of the quinone functionality simultaneously pulls the LUPS and the interference features close to EF such that the J/V characteristics and transmission plots of AQ and BDT-2 are nearly indistinguishable despite the presence of two thienyl groups in BDT-2. These results also suggest that tunneling transport is mediated by the HOPS (hole-assisted tunneling) for BDT-1 and BDT-3 and by the LUPS (electron-assisted tunneling) for BDT-2 and AQ because tunneling current is dominated by the resonance(s) closest to EF.
for each value of V (note that these are histograms of J/V curves with no data-selection, thus, brighter colors correspond to mean values of J and are not related to conductance histograms of single-molecule break-junctions; see ESI† for details). Both BDT-1 and BDT-3 exhibit ordinary, U-shaped plots characteristic of non-resonant tunneling. By contrast, both AQ and BDT-2—the two compounds bearing quinone functionality—show V-shaped plots with negative curvature. These results are in agreement with Fig. 3, which places the QI features for the quinone moieties, AQ and BDT-2, much closer to EF than for BDT-3. The positions of these features are related to the positions of highest-occupied and lowest-unoccupied π-states (HOPS and LUPS), which are in good agreement between DFT and experiment (Tables S2 and S3†). Thus, the differential conductance heatmaps (experiment) and DFT (simulation) both indicate that cross-conjugation suppresses conductance because it creates a dip in T(E) in the frontier orbital gap, but that the electron-withdrawing nature of the quinone functionality simultaneously pulls the LUPS and the interference features close to EF such that the J/V characteristics and transmission plots of AQ and BDT-2 are nearly indistinguishable despite the presence of two thienyl groups in BDT-2. These results also suggest that tunneling transport is mediated by the HOPS (hole-assisted tunneling) for BDT-1 and BDT-3 and by the LUPS (electron-assisted tunneling) for BDT-2 and AQ because tunneling current is dominated by the resonance(s) closest to EF.
To further investigate the mechanism of transport, we measured transition voltages, Vtrans (Table S3, Fig. S17 and S18†), which provide information about the energy offset between EF and the dominant frontier orbital.53,54Fig. 5a shows the levels for the BDT-n series calculated by DFT with respect to EF (−4.3 eV), clearly predicting LUPS-mediated tunneling for BDT-2 and AQ. Fig. 5b compares the experimental values of Vtrans to the energy differences between EF and the frontier orbitals. The salient feature of Fig. 5b is that the trend in |EHOPS − EF| opposes the trend in Vtrans such that the trend in experimental values of Vtrans agrees with DFT only when we compare Vtrans with |EHOPS − EF| for BDT-1 and BDT-3, and with |ELUPS − EF| for BDT-2 and AQ. Thus, DFT calculations combined with experimental values of Vtrans predict electron-assisted tunneling for BDT-2 and AQ. This degree of internal consistency between the experiment and theory is important because, ultimately, the only primary observable is conductance, which we plot as J/V curves, differential conductance heatmaps and Fowler–Nordheim plots (from which we extract Vtrans). And we find remarkable agreement between these direct and indirect observations and DFT calculations on model junctions comprising single molecules.
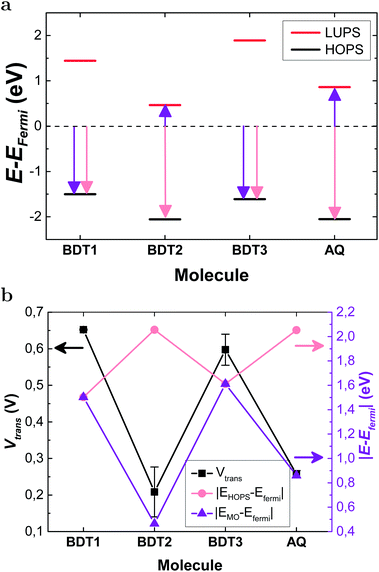 | ||
| Fig. 5 (a) Energy offsets of the frontier orbitals calculated using DFT with respect to EF value of −4.3 eV. (b) The energy offsets (salmon and purple lines, right axis) plotted with the measured values of Vtrans (black line, left axis). The salmon line plots the energy offsets of the HOPS. The purple line plots the smallest energy difference (purple arrows in (a)); |EHOPS – EFermi| for BDT-1 and BDT-3, |ELUPS − EFermi| for BDT-2 and AQ. The exact values of Vtrans and the orbital energies are shown in Tables S3 and S5.† | ||
Conclusion
The key question of this work is how cross-conjugation and electronegativity affect QI features.11,20,52,55,56 Based on our experimental observations and calculations, we assert that destructive QI induced by cross-conjugation is highly sensitive to the functional groups that induce the cross-conjugation and that quinones are, therefore, a poor testbed for tuning QI effects (beyond switching them on and off57) because their strong electron-withdrawing nature places a deep, destructive feature near EF irrespective of other functional groups (in our case, two fused thiophene rings barely make a difference). Comparing a quinone to a hydrocarbon also compares HOPS-mediated tunneling to LUPS-mediated tunneling between molecules with significantly different band-gaps and absolute frontier orbital energies. In contrast, BDT-1 and BDT-3 are heterocyclic isomers with no functional groups, identical molecular formulas, nearly-identical HOPS, identical lengths that translate into SAMs of identical thicknesses, and transport is dominated by the HOPS. They isolate the single variable of conjugation patterns, allowing us to separate bond topology (cross-conjugation) from electronic properties (functional groups), giving experimental and theoretical insight into the relationship between bond topology and QI. Our results suggest that there is a lot of room to tune the conductance of moieties derived from BDT-3 by including pendant groups (e.g., halogens, CF3 groups or acidic/basic sites) that shift the QI feature gradually towards EF synthetically and/or in response to chemical signals.Experimental
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Upon completion, the reaction was quenched with water (10 mL). The crude solid was purified by column chromatography to give BDT-2 (50 mg, 53%). 1HNMR (400 MHz, CDC13) δ: 7.73 (s, 2H), 7.59 (d, J = 8.2, 4H), 7.45 (d, J = 8.2, 4H), 2.46 (s, 6H). 13CNMR (100 MHz, CDC13) δ: 195.59, 175.96, 145.20, 136.95, 134.87, 134.20, 133.15, 132.57, 132.50, 125.24, 100.42, 85.49, 33.01. HRMS (ESI) calcd for C30H17O4S4 [M + H]+: 569.00042, found: 568.99887.
1). Upon completion, the reaction was quenched with water (10 mL). The crude solid was purified by column chromatography to give BDT-2 (50 mg, 53%). 1HNMR (400 MHz, CDC13) δ: 7.73 (s, 2H), 7.59 (d, J = 8.2, 4H), 7.45 (d, J = 8.2, 4H), 2.46 (s, 6H). 13CNMR (100 MHz, CDC13) δ: 195.59, 175.96, 145.20, 136.95, 134.87, 134.20, 133.15, 132.57, 132.50, 125.24, 100.42, 85.49, 33.01. HRMS (ESI) calcd for C30H17O4S4 [M + H]+: 569.00042, found: 568.99887.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Upon completion the reaction was quenched with water (10 mL). The crude solid was purified by column chromatography to give BDT-3 (25 mg, 26%). 1HNMR (400 MHz, CDC13) δ: 8.17 (s, 1H), 8.15 (s, 1H), 7.59 (d, J = 7.2, 4H), 7.58 (s, 2H), 7.43 (d, J = 8.2, 4H), 2.45 (s, 3H). 13CNMR (100 MHz, CDC13) δ: 195.88, 141.43, 140.03, 136.90, 134.76, 131.51, 131.48, 126.27, 126.50, 120.94, 117.42, 97.22, 87.15, 32.97. HRMS(ESI) calcd for C30H18O2S4 [M + H]+: 539.02624, found: 539.02476.
1). Upon completion the reaction was quenched with water (10 mL). The crude solid was purified by column chromatography to give BDT-3 (25 mg, 26%). 1HNMR (400 MHz, CDC13) δ: 8.17 (s, 1H), 8.15 (s, 1H), 7.59 (d, J = 7.2, 4H), 7.58 (s, 2H), 7.43 (d, J = 8.2, 4H), 2.45 (s, 3H). 13CNMR (100 MHz, CDC13) δ: 195.88, 141.43, 140.03, 136.90, 134.76, 131.51, 131.48, 126.27, 126.50, 120.94, 117.42, 97.22, 87.15, 32.97. HRMS(ESI) calcd for C30H18O2S4 [M + H]+: 539.02624, found: 539.02476.
Self-assembled monolayers
The SAMs of BDT-n were formed via in situ deprotection41,51 on template-stripped Au substrates.63 Freshly template-stripped substrates were immersed into 3 mL of 50 μM solutions of the thioacetate precursors in freshly distilled toluene inside a nitrogen-filled glovebox and sealed under a nitrogen atmosphere. The sealed vessels were kept inside a nitrogen flow box64 (O2 below 3%, RH below 15%) overnight; all subsequent handling and EGaIn measurements were performed inside the flowbox. 1.5 h prior to measurement, 0.05 mL of 17 mM diazabicycloundec-7-ene (DBU) in toluene was added to the precursor/substrate solution. The substrates were then rinsed with toluene and allowed to dry for 30 min before performing the measurements.Characterization
The SAMs of BDT-n were characterized by XPS (laboratory and synchrotron), NEXAFS spectroscopy, UPS and water contact angle goniometry. In some cases, SAMs of CH3(CH2)15SH or CH3(CH2)17SH on Au were used as a reference. See ESI† for details.Transport measurements
DFT calculations
Calculation were performed using the ORCA 4 software package65,66 and the ARTAIOS-030417 software package.67,68 The molecules terminating with thiols were first minimized to find the gas-phase geometry and then attached to two 18-atom Au(111) clusters via the terminal sulfur atoms with a distance of 1.75 Å at hexagonal close-pack hollow sites (hydrogen atom from the thiol was deleted before attaching the electrodes). Single-point energy calculations were performed on this model junction using B3LYP/G and LANL2DZ basis sets according to literature procedures to compute the energy levels.67 Transmission curves and isoplots of the central molecular orbitals, for isolated molecules without electrodes and terminal hydrogen atoms, were generated using the ARTAIOS-030417 software package and the energy axis was scaled using the EF of −4.3 eV. The use of this EF value for comparing transmission trends to the experimental tunneling conduction in Au/SAM//EGaIn junction is supported by the UPS measurements that also give a similar EF value (see ESI Section 1.3.3†). It is has also been established experimentally that SAMs of aliphatic and conjugated molecules on Au shift the EF values by 0.85 and 0.98 eV, respectively, (i.e., to −4.2 eV to −4.4 eV) from −5.2 eV for a clean gold surface.69–71 This value of EF was used for all DFT calculations. Further rational for choosing this value of EF and the detailed step-wise procedure for all the calculations involved is further described in ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. C. C., Y. Z. and M. C. acknowledge the European Research Council for the ERC Starting Grant 335473 (MOLECSYNCON). G. Y. acknowledges financial support from the China Scholarship Council (CSC): No. 201408440247. X. Q. acknowledges the Zernike Institute for Advanced Materials “Dieptestrategie.” E. S. and M. Z. thank the Helmholtz Zentrum Berlin for the allocation of synchrotron radiation beamtime at BESSY II and A. Nefedov and Ch. Wöll for the technical cooperation during the experiments there; a financial support of the German Research Society (Deutsche Forschungsgemeinschaft; DFG) within the grant ZH 63/22-1 is appreciated. We thank the Center for Information Technology of the University of Groningen for their support and for providing access to the Peregrine high performance computing cluster.References
- A. Vilan, D. Aswal and D. Cahen, Large-Area, Ensemble Molecular Electronics: Motivation and Challenges, Chem. Rev., 2017, 117, 4248–4286 CrossRef CAS PubMed.
- N. Xin and X. Guo, Catalyst: The Renaissance of Molecular Electronics, Chem, 2017, 3, 373–376 CAS.
- E. Lörtscher, Reaction: Technological Aspects of Molecular Electronics, Chem, 2017, 3, 376–377 Search PubMed.
- L.-Y. Hsu, B.-Y. Jin, C.-h. Chen and S.-M. Peng, Reaction: New Insights Into Molecular Electronics, Chem, 2017, 3, 378–379 CAS.
- C. J. Lambert, Basic Concepts of Quantum Interference and Electron Transport in Single-Molecule Electronics, Chem. Soc. Rev., 2015, 44, 875–888 RSC.
- R. a. Webb, S. Washburn, C. P. Umbach and R. B. Laibowitz, Observation of He Aharonov-Bohm Oscillations in Normal-Metal Rings, Phys. Rev. Lett., 1985, 54, 2696–2699 CrossRef CAS PubMed.
- P. Sautet and C. Joachim, Electronic Interference Produced by a Benzene Embedded in a Polyacetylene Chain, Chem. Phys. Lett., 1988, 153, 511–516 CrossRef CAS.
- L.-Y. Hsu and B.-Y. Jin, An investigation of quantum transport by the free-electron network model: resonance and interference effects, Chem. Phys., 2009, 355, 177–182 CrossRef CAS.
- D. M. Cardamone, C. A. Stafford and S. Mazumdar, Controlling Quantum Transport through a Single Molecule, Nano Lett., 2006, 6, 2422–2426 CrossRef CAS PubMed.
- G. C. Solomon, D. Q. Andrews, R. P. Van Duyne and M. A. Ratner, When Things Are Not as They Seem: Quantum Interference Turns Molecular Electron Transfer “Rules” Upside Down, J. Am. Chem. Soc., 2008, 130, 7788–7789 CrossRef CAS PubMed.
- G. C. Solomon, D. Q. Andrews, R. H. Goldsmith, T. Hansen, M. R. Wasielewski, R. P. Van Duyne and M. a. Ratner, Quantum Interference in Acyclic Systems: Conductance of Cross-Conjugated Molecules, J. Am. Chem. Soc., 2008, 130, 17301–17308 CrossRef CAS PubMed.
- D. Q. Andrews, G. C. Solomon, R. H. Goldsmith, T. Hansen, M. R. Wasielewski, R. P. V. Duyne and M. A. Ratner, Quantum Interference: The Structural Dependence of Electron Transmission Through Model Systems and Cross-Conjugated Molecules, J. Phys. Chem. C, 2008, 112, 16991–16998 CAS.
- G. C. Solomon, C. Herrmann, T. Hansen, V. Mujica and M. A. Ratner, Exploring Local Currents in Molecular Junctions, Nat. Chem., 2010, 2, 223–228 CrossRef CAS PubMed.
- E. Maggio, G. C. Solomon and A. Troisi, Exploiting Quantum Interference in Dye Sensitized Solar Cells, ACS Nano, 2014, 8, 409–418 CrossRef CAS PubMed.
- K. G. L. Pedersen, A. Borges, P. Hedegård, G. C. Solomon and M. Strange, Illusory Connection Between Cross-Conjugation and Quantum Interference, J. Phys. Chem. C, 2015, 119, 26919–26924 CAS.
- D. Fracasso, H. Valkenier, J. C. Hummelen, G. C. Solomon and R. C. Chiechi, Evidence for Quantum Interference in SAMs of Arylethynylene Thiolates in Tunneling Junctions With Eutectic Ga-In (EGaIn) Top-Contacts, J. Am. Chem. Soc., 2011, 133, 9556–9563 CrossRef CAS PubMed.
- W. Hong, H. Valkenier, G. Mészáros, D. Z. Manrique, A. Mishchenko, A. Putz, P. M. García, C. J. Lambert, J. C. Hummelen and T. Wandlowski, An MCBJ Case Study: The Influence of Π-Conjugation on the Single-Molecule Conductance at a Solid/Liquid Interface, Beilstein J. Nanotechnol., 2011, 2, 699–713 CrossRef PubMed.
- C. M. Guedon, H. Valkenier, T. Markussen, K. S. Thygesen, J. C. Hummelen and S. J. Van Der Molen, Observation of Quantum Interference in Molecular Charge Transport, Nat. Nanotechnol., 2012, 7, 305–309 CrossRef CAS PubMed.
- V. Kaliginedi, P. Moreno-García, H. Valkenier, W. Hong, V. M. García-Suárez, P. Buiter, J. L. H. Otten, J. C. Hummelen, C. J. Lambert and T. Wandlowski, Correlations Between Molecular Structure and Single-Junction Conductance: A Case Study With Oligo(phenylene-Ethynylene)-Type Wires, J. Am. Chem. Soc., 2012, 134, 5262–5275 CrossRef CAS PubMed.
- H. Valkenier, C. M. Guedon, T. Markussen, K. S. Thygesen, S. J. van der Molen and J. C. Hummelen, Cross-Conjugation and Quantum Interference: A General Correlation?, Phys. Chem. Chem. Phys., 2014, 16, 653–662 RSC.
- M. Koole, J. M. Thijssen, H. Valkenier, J. C. Hummelen and H. S. J. van der Zant, Electric-Field Control of Interfering Transport Pathways in a Single-Molecule Anthraquinone Transistor, Nano Lett., 2015, 15, 5569–5573 CrossRef CAS PubMed.
- J. P. Bergfield, H. M. Heitzer, C. Van Dyck, T. J. Marks and M. A. Ratner, Harnessing Quantum Interference in Molecular Dielectric Materials, ACS Nano, 2015, 9, 6412–6418 CrossRef CAS PubMed.
- T. Markussen, R. Stadler and K. S. Thygesen, The Relation Between Structure and Quantum Interference in Single Molecule Junctions, Nano Lett., 2010, 10, 4260–4265 CrossRef CAS PubMed.
- C. Salhani, M. L. Della Rocca, C. Bessis, R. Bonnet, C. Barraud, P. Lafarge, A. Chevillot, P. Martin and J. C. Lacroix, Inelastic Electron Tunneling Spectroscopy in Molecular Junctions Showing Quantum Interference, Phys. Rev. B, 2017, 95, 165431 CrossRef.
- M. Mayor, H. B. Weber, J. Reichert, M. Elbing, C. von Hänisch, D. Beckmann and M. Fischer, Electric Current Through a Molecular Rod—Relevance of the Position of the Anchor Groups, Angew. Chem., Int. Ed., 2003, 42, 5834–5838 CrossRef CAS PubMed.
- M. Taniguchi, M. Tsutsui, R. Mogi, T. Sugawara, Y. Tsuji, K. Yoshizawa and T. Kawai, Dependence of Single-Molecule Conductance on Molecule Junction Symmetry, J. Am. Chem. Soc., 2011, 133, 11426–11429 CrossRef CAS PubMed.
- J. S. Meisner, S. Ahn, S. V. Aradhya, M. Krikorian, R. Parameswaran, M. Steigerwald, L. Venkataraman and C. Nuckolls, Importance of Direct Metal π Coupling in Electronic Transport Through Conjugated Single-Molecule Junctions, J. Am. Chem. Soc., 2012, 134, 20440–20445 CrossRef CAS PubMed.
- C. R. Arroyo, S. Tarkuc, R. Frisenda, J. S. Seldenthuis, C. H. M. Woerde, R. Eelkema, F. C. Grozema and H. S. J. van der Zant, Signatures of Quantum Interference Effects on Charge Transport Through a Single Benzene Ring, Angew. Chem., Int. Ed., 2013, 52, 3152–3155 CrossRef CAS PubMed.
- J. R. Quinn, F. W. Foss, L. Venkataraman, M. S. Hybertsen and R. Breslow, Single-Molecule Junction Conductance Through Diaminoacenes, J. Am. Chem. Soc., 2007, 129, 6714–6715 CrossRef CAS PubMed.
- M. Kiguchi, H. Nakamura, Y. Takahashi, T. Takahashi and T. Ohto, Effect of Anchoring Group Position on Formation and Conductance of a Single Disubstituted Benzene Molecule Bridging Au Electrodes: Change of Conductive Molecular Orbital and Electron Pathway, J. Phys. Chem. C, 2010, 114, 22254–22261 CAS.
- S. V. Aradhya, J. S. Meisner, M. Krikorian, S. Ahn, R. Parameswaran, M. L. Steigerwald, C. Nuckolls and L. Venkataraman, Dissecting Contact Mechanics From Quantum Interference in Single-Molecule Junctions of Stilbene Derivatives, Nano Lett., 2012, 12, 1643–1647 CrossRef CAS PubMed.
- D. Z. Manrique, C. Huang, M. Baghernejad, X. Zhao, O. a. Al-Owaedi, H. Sadeghi, V. Kaliginedi, W. Hong, M. Gulcur and T. Wandlowski, et al., A Quantum Circuit Rule for Interference Effects in Single-Molecule Electrical Junctions, Nat. Commun., 2015, 6, 6389 CrossRef CAS PubMed.
- J. Xia, B. Capozzi, S. Wei, M. Strange, A. Batra, J. R. Moreno, R. J. Amir, E. Amir, G. C. Solomon and L. Venkataraman, et al., Breakdown of Interference Rules in Azulene, a Nonalternant Hydrocarbon, Nano Lett., 2014, 14, 2941–2945 CrossRef CAS PubMed.
- F. Schwarz, M. Koch, G. Kastlunger, H. Berke, R. Stadler, K. Venkatesan and E. Lörtscher, Charge Transport and Conductance Switching of Redox-Active Azulene Derivatives, Angew. Chem., Int. Ed., 2016, 55, 11781–11786 CrossRef CAS PubMed.
- G. Yang, S. Sangtarash, Z. Liu, X. Li, H. Sadeghi, Z. Tan, R. Li, J. Zheng, X. Dong and J.-Y. Liu, et al., Protonation Tuning of Quantum Interference in Azulene-Type Single-Molecule Junctions, Chem. Sci., 2017, 8, 7505–7509 RSC.
- R. Baer and D. Neuhauser, Phase Coherent Electronics: A Molecular Switch Based on Quantum Interference, J. Am. Chem. Soc., 2002, 124, 4200–4201 CrossRef CAS PubMed.
- G. C. Solomon, C. Herrmann, J. Vura-Weis, M. R. Wasielewski and M. A. Ratner, The Chameleonic Nature of Electron Transport Through Π-Stacked Systems, J. Am. Chem. Soc., 2010, 132, 7887–7889 CrossRef CAS PubMed.
- A. A. Kocherzhenko, F. C. Grozema and L. D. A. Siebbeles, Charge Transfer Through Molecules With Multiple Pathways: Quantum Interference and Dephasing, J. Phys. Chem. C, 2010, 114, 7973–7979 CAS.
- N. F. Phelan and M. Orchin, Cross Conjugation, J. Chem. Educ., 1968, 45, 633–637 CrossRef CAS.
- P. a. Limacher and H. P. Lüthi, Cross-Conjugation, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 477–486 CrossRef CAS.
- M. Carlotti, A. Kovalchuk, T. Wächter, X. Qiu, M. Zharnikov and R. C. Chiechi, Conformation-Driven Quantum Interference Effects Mediated by Through-Space Conjugation in Self-Assembled Monolayers, Nat. Commun., 2016, 7, 13904 CrossRef CAS PubMed.
- A. Borges, J. Xia, S. H. Liu, L. Venkataraman and G. C. Solomon, The Role of Through-Space Interactions in Modulating Constructive and Destructive Interference Effects in Benzene, Nano Lett., 2017, 17, 4436–4442 CrossRef CAS PubMed.
- S. Sangtarash, H. Sadeghi and C. J. Lambert, Exploring Quantum Interference in Heteroatom-Substituted Graphene-Like Molecules, Nanoscale, 2016, 8, 13199–13205 RSC.
- X. Liu, S. Sangtarash, D. Reber, D. Zhang, H. Sadeghi, J. Shi, Z.-Y. Xiao, W. Hong, C. J. Lambert and S.-X. Liu, Gating of Quantum Interference in Molecular Junctions by Heteroatom Substitution, Angew. Chem., Int. Ed., 2017, 56, 173–176 CrossRef CAS PubMed.
- Y. Tsuji, T. Stuyver, S. Gunasekaran and L. Venkataraman, The Influence of Linkers on Quantum Interference: A Linker Theorem, J. Phys. Chem. C, 2017, 121, 14451–14462 CAS.
- H. Lissau, R. Frisenda, S. T. Olsen, M. Jevric, C. R. Parker, A. Kadziola, T. Hansen, H. S. J. van der Zant, M. B. Nielsen and K. V. Mikkelsen, Tracking molecular resonance forms of donor–acceptor push–pull molecules by single-molecule conductance experiments, Nat. Commun., 2015, 6, 10233 CrossRef PubMed.
- Z. Wang, H. Dong, T. Li, R. Hviid, Y. Zou, Z. Wei, X. Fu, E. Wang, Y. Zhen and K. Norgaard, et al., Role of redox centre in charge transport investigated by novel self-assembled conjugated polymer molecular junctions, Nat. Commun., 2015, 6, 7478 CrossRef CAS PubMed.
- P. Pourhossein, R. K. Vijayaraghavan, S. C. J. Meskers and R. C. Chiechi, Optical Modulation of Nano-Gap Tunnelling Junctions Comprising Self-Assembled Monolayers of Hemicyanine Dyes, Nat. Commun., 2016, 7, 11749 CrossRef CAS PubMed.
- S. Seo, E. Hwang, Y. Cho, J. Lee and H. Lee, Functional Molecular Junctions Derived From Double Self-Assembled Monolayers, Angew. Chem., Int. Ed., 2017, 56, 12122–12126 CrossRef CAS PubMed.
- R. C. Chiechi, E. A. Weiss, M. D. Dickey and G. M. Whitesides, Eutectic Gallium–Indium (EGaIn): A Moldable Liquid Metal for Electrical Characterization of Self-Assembled Monolayers, Angew. Chem., Int. Ed., 2008, 120, 148–150 CrossRef.
- H. Valkenier, E. H. Huisman, P. A. van Hal, D. M. de Leeuw, R. C. Chiechi and J. C. Hummelen, Formation of High-Quality Self-Assembled Monolayers of Conjugated Dithiols on Gold: Base Matters, J. Am. Chem. Soc., 2011, 133, 4930–4939 CrossRef CAS PubMed.
- J. Lykkebo, A. Gagliardi, A. Pecchia and G. C. Solomon, IETS and Quantum Interference: Propensity Rules in the Presence of an Interference Feature, J. Chem. Phys., 2014, 141, 124119 CrossRef PubMed.
- J. M. Beebe, B. Kim, C. D. Frisbie and J. G. Kushmerick, Measuring Relative Barrier Heights in Molecular Electronic Junctions With Transition Voltage Spectroscopy, ACS Nano, 2008, 2, 827–832 CrossRef CAS PubMed.
- Y. Zhang, X. Qiu, P. Gordiichuk, S. Soni, T. L. Krijger, A. Herrmann and R. C. Chiechi, Mechanically and Electrically Robust Self-Assembled Monolayers for Large-Area Tunneling Junctions, J. Phys. Chem. C, 2017, 121, 14920–14928 CAS.
- K. G. L. Pedersen, M. Strange, M. Leijnse, P. Hedegard, G. C. Solomon and J. Paaske, Quantum Interference in Off-Resonant Transport Through Single Molecules, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 125413 CrossRef.
- G. C. Solomon, Cross Conjugation, Wiley-VCH Verlag GmbH & Co. KGaA, 2016, pp. 397–412 Search PubMed.
- M. Baghernejad, X. Zhao, K. Baruël Ørnsø, M. Füeg, P. Moreno-García, A. V. Rudnev, V. Kaliginedi, S. Vesztergom, C. Huang and W. Hong, et al., Electrochemical Control of Single-Molecule Conductance by Fermi-Level Tuning and Conjugation Switching, J. Am. Chem. Soc., 2014, 136, 17922–17925 CrossRef CAS PubMed.
- C.-Y. Kuo, W. Nie, H. Tsai, H.-J. Yen, A. D. Mohite, G. Gupta, A. M. Dattelbaum, D. J. William, K. C. Cha and Y. Yang, et al., Structural Design of Benzo[1,2-b:4,5-b]dithiophene-Based 2D Conjugated Polymers With Bithienyl and Terthienyl Substituents Toward Photovoltaic Applications, Macromolecules, 2014, 47, 1008–1020 CrossRef CAS.
- R. Rieger, D. Beckmann, A. Mavrinskiy, M. Kastler and K. Müllen, Backbone Curvature in Polythiophenes, Chem. Mater., 2010, 22, 5314–5318 CrossRef CAS.
- Z.-F. Shi, L.-J. Wang, H. Wang, X.-P. Cao and H.-L. Zhang, Synthesis of Oligo(phenylene Ethynylene)s With Dendrimer “Shells” for Molecular Electronics, Org. Lett., 2007, 9, 595–598 CrossRef CAS PubMed.
- E. H. van Dijk, D. J. T. Myles, M. H. van der Veen and J. C. Hummelen, Synthesis and Properties of an Anthraquinone-Based Redox Switch for Molecular Electronics, Org. Lett., 2006, 8, 2333–2336 CrossRef CAS PubMed.
- T. C. Pijper, J. Robertus, W. R. Browne and B. L. Feringa, Mild Ti-Mediated Transformation of T-Butyl Thio-Ethers Into Thio-Acetates, Org. Biomol. Chem., 2015, 13, 265–268 CAS.
- E. A. Weiss, G. K. Kaufman, J. K. Kriebel, Z. Li, R. Schalek and G. M. Whitesides, Si/SiO2-Templated Formation of Ultraflat Metal Surfaces on Glass, Polymer, and Solder Supports: Their Use as Substrates for Self-Assembled Monolayers, Langmuir, 2007, 23, 9686–9694 CrossRef CAS PubMed.
- M. Carlotti, M. Degen, Y. Zhang and R. C. Chiechi, Pronounced Environmental Effects on Injection Currents in EGaIn Tunneling Junctions Comprising Self-Assembled Monolayers, J. Phys. Chem. C, 2016, 120, 20437–20445 CAS.
- F. Neese, The ORCA Program System, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CrossRef CAS.
- F. Neese, Software update: the ORCA program system, version 4.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2017, 8, e1327 CrossRef.
- C. Herrmann, L. Gross, T. Steenbock, M. Deffner, B. A. Voigt and G. C. Solomon, ARTAIOS - A Transport Code for Postprocessing Quantum Chemical Electronic Structure Calculations, https://www.chemie.uni-hamburg.de/ac/herrmann/software/index.html. 2016.
- C. Herrmann, G. C. Solomon, J. E. Subotnik, V. Mujica and M. A. Ratner, Ghost Transmission: How Large Basis Sets Can Make Electron Transport Calculations Worse, J. Chem. Phys., 2010, 132, 024103 CrossRef PubMed.
- O. M. Cabarcos, S. Schuster, I. Hehn, P. P. Zhang, M. M. Maitani, N. Sullivan, J.-B. Gigure, J.-F. Morin, P. S. Weiss and E. Zojer, et al., Effects of Embedded Dipole Layers on Electrostatic Properties of Alkanethiolate Self-Assembled Monolayers, J. Phys. Chem. C, 2017, 121, 15815–15830 CAS.
- T. AbuHusein, S. Schuster, D. A. Egger, M. Kind, T. Santowski, A. Wiesner, R. Chiechi, E. Zojer, A. Terfort and M. Zharnikov, The Effects of Embedded Dipoles in Aromatic SelfAssembled Monolayers, Adv. Funct. Mater., 2015, 25, 3943–3957 CrossRef CAS.
- A. Kovalchuk, T. Abu-Husein, D. Fracasso, D. A. Egger, E. Zojer, M. Zharnikov, A. Terfort and R. C. Chiechi, Transition voltages respond to synthetic reorientation of embedded dipoles in self-assembled monolayers, Chem. Sci., 2016, 7, 781–787 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc00165k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |