Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nickel-catalyzed enantioselective allylic alkylation of lactones and lactams with unactivated allylic alcohols†
Aurapat
Ngamnithiporn
,
Carina I.
Jette
,
Shoshana
Bachman
,
Scott C.
Virgil
and
Brian M.
Stoltz
 *
*
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA. E-mail: stoltz@caltech.edu
First published on 24th January 2018
Abstract
The first nickel-catalyzed enantioselective allylic alkylation of lactone and lactam substrates to deliver products bearing an all-carbon quaternary stereocenter is reported. The reaction, which utilizes a commercially available chiral bisphosphine ligand, proceeds in good yield with a high level of enantioselectivity (up to 90% ee) on a range of unactivated allylic alcohols for both lactone and lactam nucleophiles. The utility of this method is further highlighted via a number of synthetically useful product transformations.
Introduction
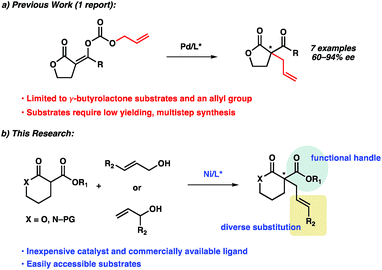
Since the seminal report in 1965 by Tsuji,1 transition metal-catalyzed allylic alkylation has emerged as one of the most powerful methods for the construction of stereocenters.2 In particular, with the use of prochiral nucleophiles that proceed through tetrasubstituted enolates, the transition metal-catalyzed enantioselective allylic alkylation has proven to be a formidable strategy for accessing chiral quaternary stereocenters in catalytic enantioselective fashion.3 Although this transformation has been studied for more than 50 years,4 the use of α-substituted lactones or lactams as prochiral nucleophiles remains significantly under-developed.5,6As part of our ongoing research program directed at the development of new strategies for constructing quaternary stereocenters,7 we were drawn to the α-acyl lactones and lactams, as we envisioned the α-acyl substituent would provide an additional functional handle for further synthetic manipulations. In addition, lactone products could also provide access to acyclic quaternary stereocenters via ring-opening reactions8 and reduction of the lactam products would enable direct access to functionalized piperidine rings, the most prevalent nitrogenous heterocycle in drug molecules.9 However, to the best of our knowledge, there has been only one report of a transition metal-catalyzed enantioselective allylic alkylation of monocyclic α-acyl lactone or lactam prochiral nucleophiles to furnish products bearing a quaternary stereocenter.5d
Recently, Cossy disclosed a palladium-catalyzed decarboxylative enantioselective allylic alkylation of enol carbonates derived from γ-butyrolactones (Scheme 1a). Various enol carbonates can be used to obtain diverse α-acyl quaternary butyrolactones in moderate to high levels of enantioselectivity. Nonetheless, the limited electrophile scope and challenging nucleophile synthesis limits the practicality of this transformation.
 | ||
| Scheme 1 Metal-catalyzed enantioselective allylic alkylations (AA) of α-acyl lactone and lactam prochiral nucleophiles. | ||
To address this limitation, we chose to investigate the enantioselective allylic alkylation of α-acyl lactones and lactams by using an inexpensive transition metal catalyst and easily accessible prochiral nucleophiles. Mashima's recent report on nickel-catalyzed enantioselective allylic alkylation of β-keto esters with allyl alcohol prompted us to probe nickel in our system.10,11 Furthermore, we anticipated that an intermolecular allylic alkylation would simplify the substrate synthesis and provide a more convergent approach to these α-quaternary products.12 Herein, we report the first example of nickel-catalyzed intermolecular enantioselective allylic alkylation using easily accessible α-acyl lactones and lactams as prochiral nucleophiles in conjunction with allylic alcohols as electrophilic coupling partners (Scheme 1b).
Results and discussion
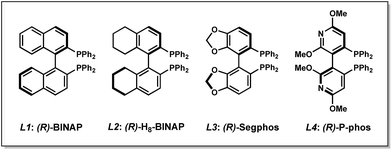
Our studies commenced with an investigation of the enantioselective allylic alkylation between α-ethoxycarbonyl lactone 1a and allyl alcohol (2a) using Ni(COD)2 and (R)-BINAP in diethyl ether at 0 °C. Although the α-quaternary lactone product 3aa was obtained in good yield, only moderate enantioselectivity was achieved.13 Seeking to improve the enantioselectivity, we elected to survey a wide variety of commercially available ligand scaffolds. Chiral bisphosphine ligands were discovered to exhibit superior enantioselectivity to other classes of ligands, including those commonly used in asymmetric allylic alkylations such as phosphinooxazolines (PHOX) or C2-asymmetric ligands pioneered by the Trost group.14 In the presence of Ni(COD)2 (10 mol%) and chiral bisphosphine ligands L1–L4 (12 mol%) in Et2O, the reaction proceeds with moderate levels of enantioselectivity (Table 1, entries 1–4). Using toluene or other ethereal solvents results in lower ee.15 The highest enantiomeric excess (ee) was achieved with (R)-P-phos (L4), which delivers α-quaternary lactone 3aa in 82% yield and 82% ee (entry 4). Decreasing the catalyst loading to 5 mol% requires exceedingly long reaction time (entry 5). An examination of different temperatures revealed that decreasing the temperature improves ee (entries 6–7), albeit with slightly diminished yields. Prolonged reaction time (48 h) at −10 °C affords product 3aa in 80% yield and 85% ee (entry 8). Importantly, a control experiment performed in the absence of the chiral ligand shows no background reaction (entry 9).| Entry | Ligand | Temp (°C) | % Yieldb | % eec |
|---|---|---|---|---|
| a Conditions: 1a (0.1 mmol), 2a (0.1 mmol), Ni(COD)2 (10 mol%), ligand (12 mol%) in Et2O (1.0 mL). b Yields determined by 1H NMR of crude reaction mixture using 1,3,5-trimethoxybenzene as a standard. c Determined by chiral SFC analysis of the isolated product. d Ni(COD)2 (5 mol%) and L4 (6 mol%) were used. e Reaction time = 48 h. | ||||
| 1 | L1 | 0 | 53 | 75 |
| 2 | L2 | 0 | 76 | 78 |
| 3 | L3 | 0 | 93 | 79 |
| 4 | L4 | 0 | 82 | 82 |
| 5d | L4 | 0 | 62 | 81 |
| 6 | L4 | 23 | 86 | 74 |
| 7 | L4 | −10 | 69 | 84 |
| 8e | L4 | −10 | 80 | 85 |
| 9 | — | 0 | 0 | — |
|
||||
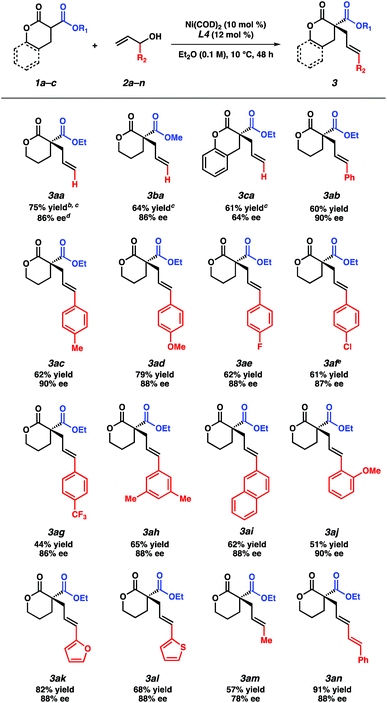
With the optimized reaction conditions in hand, we examined the scope of this asymmetric transformation (Table 2). The reaction of α-methoxycarbonyl lactone 1b, possessing a smaller alkyl group at the ester fragment, with allyl alcohol (2a) provides α-quaternary lactone 3ba in comparable yield and ee to the allylated product 3aa. Bicyclic lactone 1c could also be used to furnish product 3ca in slightly diminished yield and enantioselectivity. With respect to the electrophile scope, reactions between lactone 1a with various substituted allyl alcohols proceed with good ee (78–90% ee) at increased temperature (10 °C). Although a trend in enantioselectivity was not observed, we found that the electronic nature of the aryl substituent does affect the reactivity. Electrophiles containing electron rich aryl substituents provide the corresponding products in greater yields than their electron-deficient counterparts (3ac–3ag). Furthermore, we found that para- and meta-substituted aryl rings exhibit higher reactivity as compared to the ortho-substituted aryl ring (3ac, 3ah–aivs.3aj). Apart from the aryl-substituted electrophiles, we were pleased to find that heteroaryl substitution is also well-tolerated (3ak–3al). The reaction with an aliphatic electrophile affords product 3am in slightly diminished yield and ee. In addition, an alkenyl-substituted electrophile fares well under our reaction conditions, delivering product 3an in an excellent 91% yield and 88% ee.
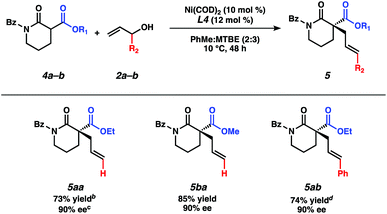
At this stage, we questioned whether we could leverage this transformation to include nitrogen-containing lactam nucleophiles. To our delight, under slightly modified reaction conditions using the same chiral bisphosphine ligand L4,16 α-ester lactams 4a–4b furnish products 5aa–5ba in good yields and with even higher enantioselectivity as compared to their lactone counterparts (Table 3). Examination of different protecting groups revealed that the benzoyl-protecting group is optimal.17 Reaction of α-ethoxycarbonyl benzoyl-protected lactam 4a with branched cinnamyl alcohol affords linear product 5ab in 74% yield and 90% ee.
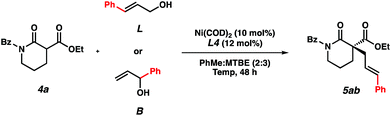
In order to gain mechanistic insights into this transformation, we compared the results from reactions using linear and branched cinnamyl alcohols (Table 4). Only the linear product was detected, indicating that a nickel π-allyl is likely an intermediate in the catalytic cycle.18 While additional studies are needed to establish the full reaction mechanism and stereocontrolling factors in the process, the ability of this catalyst combination to access a single product from two electrophilic coupling partners highlights its flexibility in potential synthetic applications.
| Entry | Elec. | Temp (°C) | % Conversionb | % Yieldb | % eec |
|---|---|---|---|---|---|
| a Reactions performed on 0.1 mmol scale. b Yields determined by 1H NMR of crude reaction mixture using benzyl ether as a standard. c Determined by chiral SFC analysis of the isolated product. | |||||
| 1 | L | 10 | 60 | 59 | 92 |
| 2 | B | 10 | 58 | 55 | 92 |
| 3 | L | 30 | >95 | 86 | 91 |
| 4 | B | 30 | 90 | 83 | 91 |
To demonstrate the synthetic utility of the α-quaternary products, we performed a number of product transformations on both α-quaternary lactone 3aa (Scheme 2) and lactam 5aa (Scheme 3). Selective reduction of the lactone functionality in 3aa provides diol 6 in 88% yield. Additionally, vinyl Grignard addition into lactone 3aa affords enone 7 in 67% yield with no erosion of enantioselectivity. These enantioenriched acyclic products 6 and 7 bearing a quaternary stereocenter are envisioned to be useful chiral building blocks as they contain multiple functional handles for further manipulations. For example, enantioenriched spirocycle 8 can be accessed via ring-closing metathesis followed by lactonization of enone 7.
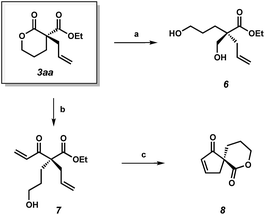 | ||
| Scheme 2 (a) NaBH4, CeCl3·7H2O, THF/MeOH, 0 °C, 88% yield; (b) vinyl-magnesium bromide, THF, −78 °C, 67% yield, 86% ee; (c) Grubbs' II (5 mol%), toluene, 40 °C; DBU, MeCN, 23 °C, 53% yield. | ||
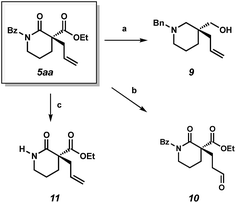 | ||
| Scheme 3 (a) LAH, ether, 65 °C, 80% yield; (b) CuCl·H2O (12 mol%), PdCl2(PhCN)2 (12 mol%), AgNO2 (6 mol%), t-BuOH, nitromethane under O2, 75% yield; (c) NaOEt, EtOH, 23 °C, 84% yield. | ||
We also performed experiments to probe the reactivity of our α-quaternary lactam products. Reduction of lactam 5aa with lithium aluminium hydride delivers chiral piperidine derivative 9, which is of potential value to medicinal chemists.9 Use of the aldehyde selective Wacker procedure19 affords aldehyde 10 in 75% yield. Lastly, cleavage of the benzoyl protecting group under basic conditions provides unprotected lactam 11 in 84% yield.
Conclusions
In summary, we have developed the first nickel-catalyzed enantioselective allylic alkylation of α-substituted lactones and lactams with free allylic alcohols. Utilizing a commercially available chiral bisphosphine ligand, α-quaternary lactones and lactams can be constructed in good yield (up to 91% yield) and with high enantiomeric excess (up to 90% ee). A broad range of functional groups are compatible with the reaction conditions. A number of product derivatizations showed the synthetic utility of this methodology for constructing small chiral building blocks with multiple functional handles. Future work to further elucidate the mechanism of this transformation is underway and will be reported in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The NIH-NIGMS (R01GM080269) and Caltech are thanked for support of our research program. A. N. thanks the Royal Thai Government Scholarship program. C. I. J. thanks the National Science Foundation for a predoctoral fellowship. Dr Michael Takase (Caltech) is acknowledged for assistance with X-ray analysis. We thank Dr Mona Shahgholi (Caltech) for mass spectrometry assistance.Notes and references
- J. Tsuji, H. Takahashi and M. Morikawa, Tetrahedron Lett., 1965, 6, 4387–4388 CrossRef
.
-
(a) B. M. Trost, Chem. Rev., 1996, 96, 395–422 CrossRef CAS PubMed
; (b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921–2943 CrossRef CAS PubMed
; (c) B. M. Trost, J. Org. Chem., 2004, 69, 5813–5837 CrossRef CAS PubMed
.
-
(a) Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Acc. Chem. Res., 2015, 48, 740–751 CrossRef CAS PubMed
; (b) V. Bhat, E. R. Welin, X. Guo and B. M. Stoltz, Chem. Rev., 2017, 117, 4528–4561 CrossRef CAS PubMed
; (c) J. C. Hethcox, S. E. Shockley and B. M. Stoltz, ACS Catal., 2016, 6, 6207–6213 CrossRef CAS PubMed
.
- For reviews, see:
(a) J. T. Mohr and B. M. Stoltz, Chem. Asian J., 2007, 2, 1476–1491 CrossRef CAS PubMed
; (b) S. Oliver and P. A. Evans, Synthesis, 2013, 45, 3179–3198 CrossRef CAS
.
- For α-quaternary lactones, see:
(a) X.-H. Li, S.-L. Wan, D. Chen, Q. R. Liu, C.-H. Ding, P. Fang and X.-L. Hou, Synthesis, 2016, 48, 1568–1572 CrossRef CAS
; (b) R. Akula and P. J. Guiry, Org. Lett., 2016, 18, 5472–5475 CrossRef CAS PubMed
; (c) J. James and P. J. Guiry, ACS Catal., 2017, 1397–1402 CrossRef CAS
; (d) M. N. Oliveira, J. Fournier, S. Arseniyadis and J. Cossy, Org. Lett., 2017, 19, 14–17 CrossRef PubMed
.
- For α-quaternary lactams, see:
(a) D. C. Behenna, Y. Liu, T. Yurino, J. Kim, D. E. White, S. C. Virgil and B. M. Stoltz, Nat. Chem., 2012, 4, 130–133 CrossRef CAS PubMed
; (b) B. M. Trost and M. U. Frederiksen, Angew. Chem., Int. Ed., 2005, 44, 308–310 ( Angew. Chem. , 2005 , 117 , 312–314 ) CrossRef CAS PubMed
.
- For selected examples, see:
(a) E. J. Alexy, S. C. Virgil, M. D. Bartberger and B. M. Stoltz, Org. Lett., 2017, 19, 5007–5009 CrossRef CAS PubMed
; (b) S. E. Shockley, J. C. Hethcox and B. M. Stoltz, Angew. Chem., Int. Ed., 2017, 56, 11545–11548 ( Angew. Chem. , 2017 , 129 , 11703–11706 ) CrossRef CAS PubMed
; (c) P. Starkov, J. T. Moore, D. C. Duquette, B. M. Stoltz and I. Marek, J. Am. Chem. Soc., 2017, 139, 9615–9620 CrossRef CAS PubMed
; (d) J. C. Hethcox, S. E. Shockley and B. M. Stoltz, Angew. Chem., Int. Ed., 2016, 55, 16092–16095 ( Angew. Chem. , 2016 , 128 , 16326–16329 ) CrossRef CAS PubMed
; (e) M. Hayashi, S. Bachman, S. Hashimoto, C. C. Eichman and B. M. Stoltz, J. Am. Chem. Soc., 2016, 138, 8997–9000 CrossRef CAS PubMed
; (f) D. C. Behenna, J. T. Mohr, N. H. Sherden, S. C. Marinescu, A. M. Harned, K. Tani, M. Seto, S. Ma, Z. Novák, M. R. Krout, R. M. McFadden, J. L. Roizen, J. A. Enquist, D. E. White, S. R. Levine, K. V. Petrova, A. Iwashita, S. C. Virgil and B. M. Stoltz, Chem.–Eur. J., 2011, 17, 14199–14223 CrossRef CAS PubMed
.
-
(a) W. Liu, D. D. Xu, O. Repič and T. J. Blacklock, Tetrahedron Lett., 2001, 42, 2439–2441 CrossRef CAS
; (b) L. Delhaye, A. Merschaert, K. Diker and I. N. Houpis, Synthesis, 2006, 9, 1437–1442 Search PubMed
.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed
.
- Y. Kita, R. D. Kavthe, H. Oda and K. Mashima, Angew. Chem., Int. Ed., 2016, 55, 1098–1101 (
Angew. Chem.
, 2016
, 128
, 1110–1113
) CrossRef CAS PubMed
.
- For recent success in Ni-catalyzed allylic alkylations from the past 15 years, see:
(a) Y. Bernhard, B. Thomson, V. Ferey and M. Sauthier, Angew. Chem., Int. Ed., 2017, 56, 7460–7464 (
Angew. Chem.
, 2017
, 129
, 7568–7572
) CrossRef CAS PubMed
; (b) S.-C. Sha, H. Jiang, J. Mao, A. Bellomo, S. A. Jeong and P. J. Walsh, Angew. Chem., Int. Ed., 2016, 55, 1070–1074 ( Angew. Chem. , 2017 , 128 , 1082–1086 ) CrossRef CAS PubMed
; (c) J. Wang, P. Wang, L. Wang, D. Li, K. Wang, Y. Wang, H. Zhu, D. Yang and R. Wang, Org. Lett., 2017, 19, 4826–4829 CrossRef CAS PubMed
; (d) S. Son and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 2756–2757 CrossRef CAS PubMed
; (e) J. D. Shields, D. T. Ahneman, T. J. A. Graham and A. G. Doyle, Org. Lett., 2014, 16, 142–145 CrossRef CAS PubMed
; (f) H. D. Srinivas, Q. Zhou and M. P. Watson, Org. Lett., 2014, 16, 3596–3599 CrossRef CAS PubMed
.
- These α-quaternary products could alternatively be accessed via phase-transfer catalysis, see:
(a) M. W. Ha, H. Lee, H. Y. Yi, Y. Park, S. Kim, S. Hong, M. Lee, M.-h. Kim, T.-s. Kim and H.-g. Park, Adv. Synth. Catal., 2013, 355, 637–642 CrossRef CAS
; (b) Y. Park, Y. J. Lee, S. Hong, M.-h. Kim, M. Lee, T.-s. Kim, J. K. Lee, S.-s. Jew and H.-g. Park, Adv. Synth. Catal., 2011, 353, 3313–3318 CrossRef CAS
.
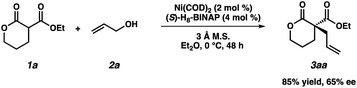
- The quaternary lactone product 3aa was isolated in 86% yield and with 65% ee under Mashiama's optimized conditions:
 .
. - Additional 36 ligands were examined, see the ESI† for details.
- See the ESI† for the investigation of different solvents.
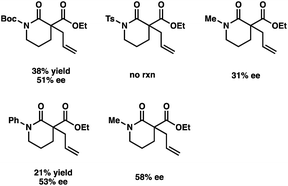
- Additional optimization of reaction parameters for lactam nucleophile is required due to the insolubility of substrate in Et2O. See the ESI† for results from the optimization.
- Results from other protected-acyl lactams under the slightly modified conditions:
 .
. - Previous reports also proposed a nickel π-allyl intermediate in the catalytic cycle, see ref. 10, 11a and b.
- K. E. Kim, J. Li, R. H. Grubbs and B. M. Stoltz, J. Am. Chem. Soc., 2016, 138, 13179–13182 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H NMR, 13C NMR, and IR spectra, SFC traces of racemic and chiral compounds. CCDC 1815141. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc05216b |
| This journal is © The Royal Society of Chemistry 2018 |