Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure and aromaticity of carborane-fused carbo- and heterocycles†
Tek Long
Chan
 and
Zuowei
Xie
and
Zuowei
Xie
 *
*
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China. E-mail: zxie@cuhk.edu.hk
First published on 16th January 2018
Abstract
Conjugation between a 3-D icosahedral carborane and a fused 2-D π-ring system is ambiguous. To address this issue, we prepared several carborane-fused carbo- and heterocycles. Detailed studies on their molecular structures, NMR data, and NICS (nucleus-independent chemical shift) and ISE (isomerization stabilization energy) values as well as molecular orbital analyses clearly suggest the presence of (1) considerable aromatic character in the exo five-membered ring of carborane-fused carbo- and heterocycles and (2) considerable conjugation between a 3-D carborane and a fused 2-D π-ring system. These results will shed some light on the design of new carborane-based materials.
Introduction
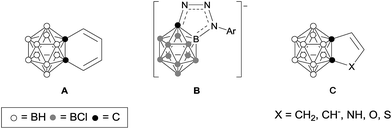
Icosahedral carboranes are carbon–boron molecular clusters, featuring a fully delocalized system of 26 skeletal electrons via 3c–2e bonding between tangential p orbitals and radial sp orbitals.1 Such clusters are aromatic molecules having 3-D aromaticity (σ-aromaticity),2 which are different from classical 2-D aromatic molecules such as benzenes (π-aromaticity).3 On the other hand, both classes of molecules share some common features such as thermal stability and ability to undergo electrophilic substitution reactions.4Conjugation between a 3-D carborane and an exo-X atom via cage CX vertex (X = atom with π-donor ability) has been evidenced by experimental and theoretical results, and such a unique σ–π conjugation is enhanced as the π-donor ability of X atom increases.5 However, conjugation between a 3-D carborane and a fused 2-D π-ring system is ambiguous. No σ–π conjugation is observed between the C2B10 cage and the diene moiety in benzocarborane (A in Fig. 1), in which the C–C double bonds are localized with the NICS(0) (NICS: nucleus-independent chemical shift) values of −0.7 to −3.4 ppm for exo-C6H4 six-membered ring in benzocarborane derivatives.6 In contrast, a considerable σ–π conjugation is reported for CB11 cage-fused heterocyclic anion [1,2-N3R-1-CB11Cl10]− (B in Fig. 1), leading to an aromatic exo-CBN3 five-membered ring with a NICS(0) value of −7.8 ppm.7
In view of wide applications of carboranes as a unique electronic sink and transmitter in optoelectronic materials,8 we initiated a research program to study the σ–π conjugation in o-carborane-fused five-membered carbo- and heterocycles, as well as the aromaticity of fused exo five-membered rings (C in Fig. 1). The results of this work would shed some light on the design of new carborane-fused π conjugated materials.
Result and discussion
Synthesis
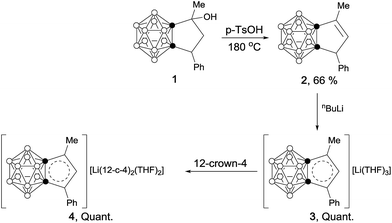
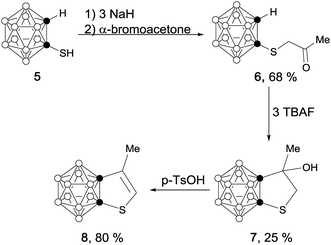
Scheme 1 outlines the synthetic route to carborane-fused cyclopentadienyl anions. Compound 1 was synthesized according to the method given in the literature.9 Solvent-free dehydration of 1 in the presence of 1 equiv. of p-TsOH at 180 °C gave 2 as colorless crystals in 66% yield. Treatment of 2 with 1 equiv. of n-BuLi in THF afforded the corresponding lithium salt 3 as a yellow powder in quantitative yield. Recrystallization of 3 from a THF solution containing 12-crown-4 ether produced 4 as yellow crystals in quantitative yield.Treatment of 1-thio-o-carborane10 (5) with an excess amount of NaH followed by reaction with α-bromoacetone gave 6 as a white solid in 68% yield. Cyclization of 6 in the presence of tetrabutylammonium fluoride (TBAF) generated compound 7 as a white solid in 25% yield. The carborane-fused thiophene (8) was synthesized as colorless crystals in 80% yield via solvent-free dehydration in the presence of 1 equiv. of p-TsOH at 180 °C for 4 hours (Scheme 2).
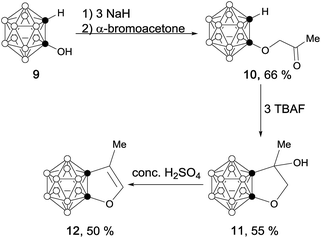
In a similar manner, carborane-fused furan (12) was prepared as a colorless liquid via concentrated H2SO4 mediated dehydration of 11 in 50% yield (Scheme 3).
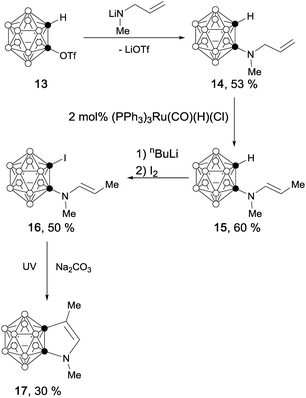
The reaction of o-carboranyl triflate (13) with lithium N-allylmethylamide afforded the corresponding amine 14 as a colorless liquid in 53% yield.11 Ruthenium-catalyzed olefin isomerization12 and cage carbon iodination gave compound 16 as a light yellow solid in 50% yield. UV irradiation of 16 produced carborane-fused pyrrole (17) as colorless crystals in 30% yield (Scheme 4).13
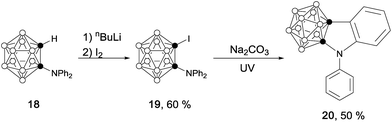
Similarly, iodination of 18,11 followed by UV irradiation of 19 generated a carborane-fused indole (20) as pale yellow crystals in 50% yield (Scheme 5).13
Structural characterization
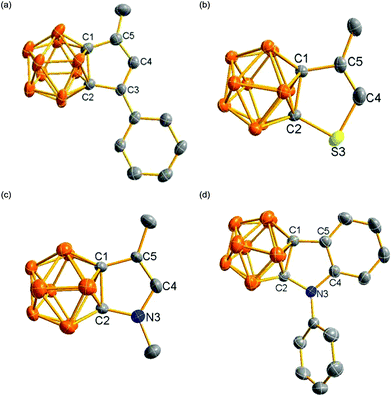
NMR spectroscopy serves as a useful tool to determine whether a compound has aromatic character.14 The fused five-membered ring proton was observed at 6.53 ppm for 3 and 6.10 ppm for 4, which was shifted downfield compared to that of 5.75 ppm in 2, suggesting that the fused five-membered ring in 3 and 4 has some aromatic character. On the other hand, the CH proton in carborane-fused heterocycles was observed at 6.27 ppm in 8, 6.58 ppm in 12 and 6.05 ppm in 17, which was compared to that of 6.87 ppm in 3-methylthiophene,15 7.16 ppm in 3-methylfuran15 and 6.35 ppm in 3-methyl-N-methylpyrrole,16 respectively. These measured proton chemical shifts were considerably downfield shifted in comparison to those observed in the corresponding dihydrothiophene,17 dihydrofuran,18 and dihydropyrrole,19 indicating that the fused five-membered rings in 8, 12 and 17 have some aromatic character.Single-crystal X-ray analyses confirmed the molecular structures of 2, 4, 8, 17 and 20. Their representative structures are shown in Fig. 2. The results clearly indicate that the fused five-membered rings in 4, 8, 17 and 20 are co-planar with the sum of the internal pentagonal angles being 540°.
As shown in Fig. 3, the bond distances of the fused five-membered ring in 4 are averaged in comparison with those observed in 2, suggesting the presence of some degree of delocalization within the five-membered ring. Except for the cage C(1)–C(2) distance, the measured distances and angles of the fused five-membered heterocycles in 8, 17 and 20 are comparable to those observed in thiophene,20 pyrrole21a and indole.21b
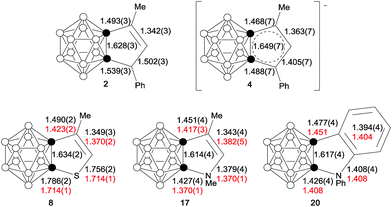 | ||
| Fig. 3 Selected bond distances (Å) of 2, 4, 8, 17 and 20. The distances in red are the experimental values (determined by the microwave spectroscopic method) of thiophene, pyrrole and indole. | ||
The UV-Vis spectra of 2, 4, 8, 12, 17 and 20 in THF were obtained, and they are shown in Fig. S7 and S8 in the ESI.† The UV-Vis spectrum of 4 (see Fig. S7 in the ESI†) displayed a new absorption band centered at 423 nm, which was assigned as π → π* absorption of the delocalized system of the exo five-membered ring.
The UV-Vis spectra of carborane-fused heterocycles (see Fig. S8 in the ESI†) showed absorptions between 287 and 290 nm attributable to n → σ* transition and between 306 to 330 nm corresponding to the π → π* transition of the delocalized exo five-membered ring system, which were red-shifted compared to those observed in heteroarenes (λmax = 205–218 nm)22 and benzene-fused heterocycles (λmax = 282–298 nm).23 These assignments were supported by time-dependent DFT (TD-DFT) calculations (see ESI†).
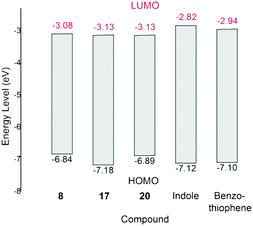
The cyclic voltammograms of 8, 17, 20, indole and benzothiophene obtained from solutions in THF are shown in Fig. S10 (ESI†). The absorption edge and reduction potentials of the aforementioned compounds are summarized in Table S1 in the ESI.† Based on these data, the energy levels of LUMO (the Lowest Unoccupied Molecular Orbital) and HOMO (the Highest Occupied Molecular Orbital) of these compounds were estimated and are shown in Fig. 4 (also see Table S1 in the ESI†). These results indicate that the electron-withdrawing nature of carboranyl moiety decreases the LUMO energy levels of 8, 17 and 20 compared to those of benzo-fused heterocycles. The DFT-calculated (at the B3LYP/6-311++G(d,p) level of theory) HOMO energy levels are in general agreement with the corresponding experimental values, whereas the DFT-calculated LUMO energy levels are higher than the corresponding experimental values (Table S2 in ESI†). Such differences are often observed in aromatic π systems.24
Computational studies
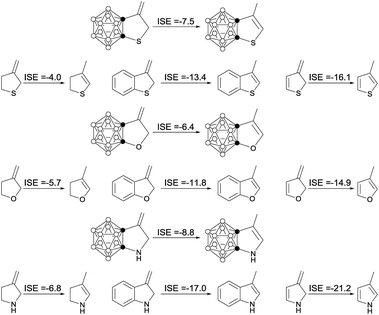
NICS values have been used extensively for the identification of aromatic properties of molecules.25 In this regard, NICS values of carborane-fused five-membered carbo- and heterocycles were calculated at the B3LYP/6-311++G(d,p) level of theory. For comparison, NICS values of cyclopentadiene, cyclopentadienide, thiophene, furan, pyrrole and their benzo derivatives were also calculated at the same level of theory. The results are compiled in Table 1. The data (−9.0 to −9.9 ppm) suggest that carborane-fused five-membered carbo- and heterocycles have considerable aromatic character. These results are consistent with those obtained from the aforementioned NMR data and structural parameters.Another parameter used to estimate aromatic stabilization energy is the isomerization stabilization energy (ISE).26 The calculated ISE data for carborane-fused heterocycles and the related systems are summarized in Table 2. The results clearly show that the ISE values of carborane-fused thiophene, furan and pyrrole are about half (−6.4 to −8.8 kcal mol−1) of those calculated for benzo-thiophene, -furan and -pyrrole that are typical aromatic molecules.
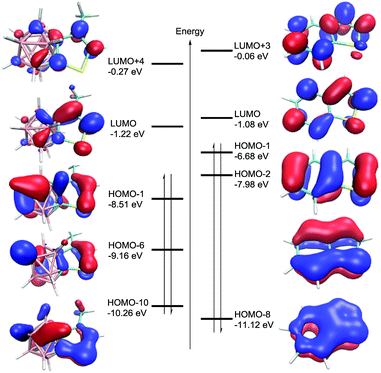
To understand the electronic structures of carborane-fused heterocycles, DFT calculations at the B3LYP/6-311++G(d,p) level of theory were performed for both 8 and benzothiophene. The optimized bond distances and angles are in very good agreement with the experimental values of 8 (see Table S5 in the ESI†). Their selected molecular orbitals (MOs) are shown in Fig. 5, revealing the significant mixing between the carborane cage and the fused exo ring. It is noteworthy that the five MOs (HOMO−1, HOMO−6, HOMO−10, LUMO+4 and LUMO) in 8 resemble those found in benzothiophene, corresponding to π and π* MOs. These results again suggest that the carborane moiety can utilize its cage carbon p orbitals to participate in the exo π bonding interactions, resulting in considerable conjugation between a 3-D cage molecule and a 2-D π system.
Conclusions
Several new carborane-fused cyclopentadienyl anion, furan, thiophene, pyrrole and indole have been prepared. NMR data, X-ray structural parameters, NICS and ISE values as well as molecular orbital analyses prove that the fused five-membered rings in the aforementioned carborane-fused cyclics have considerable aromatic character, and the conjugation between a 3-D cluster and a fused 2-D π system has been realized by the π donation of the exo heteroatom/carbanion. These results will shed some light on the design of new carborane-based π molecules for material applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Research Grants Council of the Hong Kong Special Administration Region (Project No. 14306114), NSFC/RGC Joint Research Scheme (Project No. N_CUHK442/14) and the incentive fund from the Faculty of Science, CUHK. T. L. C. acknowledges the Tertiary Education Services Office of Macau Special Administration for a postgraduate scholarship. We thank Dr Jiji Zhang for the valuable discussion on DFT calculations and Mr Ruofei Cheng for the measurements of cyclic voltammograms.Notes and references
- (a) R. N. Grimes, Carboranes, 3rd edn, Elsevier, Oxford, 2016 Search PubMed; (b) N. S. Hosmane, Boron Science: New Technologies and Applications, Taylor & Francis Books/CRC, Boca Raton, FL, 2011 Search PubMed; (c) Z. Qiu, S. Ren and Z. Xie, Acc. Chem. Res., 2011, 44, 299–309 CrossRef CAS PubMed; (d) J. Zhang and Z. Xie, Acc. Chem. Res., 2014, 47, 1623–1633 CrossRef CAS PubMed.
- For selected reviews, see: (a) R. B. King, Chem. Rev., 2001, 101, 1119–1152 CrossRef CAS PubMed; (b) Z. Chen and R. B. King, Chem. Rev., 2005, 105, 3613–3642 CrossRef CAS PubMed; (c) M. Zhao and B. M. Gimarc, Inorg. Chem., 1993, 32, 4700–4707 CrossRef CAS; (d) P. v. R. Schleyer and K. Najafian, Inorg. Chem., 1998, 37, 3454–3470 CrossRef CAS PubMed.
- For selected articles, see: (a) E. Hückel, Z. Phys., 1931, 70, 204–286 CrossRef; (b) E. Hückel, Z. Phys., 1931, 72, 310–337 CrossRef; (c) D. P. Craig and N. L. Paddock, Nature, 1958, 181, 1052–1053 CrossRef CAS; (d) A. T. Balaban, D. C. Oniciu and A. R. Katritzky, Chem. Rev., 2004, 104, 2777–2812 CrossRef CAS PubMed; (e) E. Matito, J. Poater, M. Solà and P. v. R. Schleyer, in Chemical Reactivity Theory: A Density Functional View, ed. P. K. Chattaraj, CRC Press, Boca Raton, FL, 2009, ch. 28, pp. 439–449 Search PubMed.
- (a) F. A. Carey and R. J. Sundberg, in Advanced Organic Chemistry Part B: Reactions and Synthesis, Springer, New York, 5th edn, 2007, ch. 11, pp. 1003–1062 Search PubMed; (b) D. Olid, R. Núñez, C. Viñas and F. Teixidor, Chem. Soc. Rev., 2013, 42, 3318–3336 RSC; (c) J. Poater, M. Solà, C. Viñas and F. Teixidor, Angew. Chem., Int. Ed., 2014, 53, 12191–12195 CrossRef CAS PubMed; (d) Z. Qiu, Tetrahedron Lett., 2015, 56, 963–971 CrossRef CAS; (e) Z. Xie, Sci. China: Chem., 2014, 57, 1061–1063 CrossRef CAS; (f) Y. Quan and Z. Xie, J. Am. Chem. Soc., 2014, 136, 15513–15516 CrossRef CAS PubMed; (g) W.-B. Yu, P.-F. Cui, W.-X. Gao and G.-X. Jin, Coord. Chem. Rev., 2017, 350, 300–319 CrossRef CAS; (h) B. J. Eleazer, M. D. Smith, A. A. Popov and D. V. Peryshkov, J. Am. Chem. Soc., 2016, 138, 10531–10538 CrossRef CAS PubMed; (i) B. J. Eleazer, M. D. Smith, A. A. Popov and D. V. Peryshkov, Chem. Sci., 2017, 8, 5399–5407 RSC; (j) R. M. Dziedzic, J. L. Martin, J. C. Axtell, L. M. A. Saleh, T.-C. Ong, Y.-F. Yang, M. S. Messina, A. L. Rheingold, K. N. Houk and A. M. Spokoyny, J. Am. Chem. Soc., 2017, 139, 7729–7732 CrossRef CAS PubMed; (k) E. A. Qian, A. I. Wixtrom, J. C. Axtell, A. Saebi, D. Jung, P. Rehak, Y. Han, E. H. Moully, D. Mosallaei, S. Chow, M. S. Messina, J.-Y. Wang, A. T. Royappa, A. L. Rheingold, H. D. Maynard, P. Král and A. M. Spokoyny, Nat. Chem., 2017, 9, 333–340 CrossRef CAS PubMed; (l) J. C. Axtell, K. O. Kirlikovali, D. Jung, R. M. Dziedzic, A. L. Rheingold and A. M. Spokoyny, Organometallics, 2017, 36, 1204–1210 CrossRef CAS; (m) J. Estrada and V. Lavallo, Angew. Chem., Int. Ed., 2017, 56, 9906–9909 CrossRef CAS PubMed; (n) J. F. Kleinsasser, S. P. Fisher, F. S. Tham and V. Lavallo, Eur. J. Inorg. Chem., 2017, 4417–4419 CrossRef CAS; (o) S. G. McArthur, R. Jay, L. Geng, J. Guo and V. Lavallo, Chem. Commun., 2017, 53, 4453–4456 RSC.
- (a) S.-h. Wu and M. Jones, Inorg. Chem., 1988, 27, 2005–2008 CrossRef CAS; (b) R. Coult, M. A. Fox, W. R. Gill and K. Wade, Polyhedron, 1992, 11, 2717–2721 CrossRef CAS; (c) L. A. Boyd, W. Clegg, R. C. B. Copley, M. G. Davidson, M. A. Fox, T. G. Hibbert, J. A. K. Howard, A. Mackinnon, R. J. Peace and K. Wade, Dalton Trans., 2004, 2786–2799 RSC; (d) M. A. Fox, C. Nervi, A. Crivello, A. S. Batsanov, J. A. K. Howard, K. Wade and P. J. Low, J. Solid State Electrochem., 2009, 13, 1483–1495 CrossRef CAS; (e) M. A. Fox, J. A. H. MacBride, R. J. Peace, W. Clegg, M. R. J. Elsegood and K. Wade, Polyhedron, 2009, 28, 789–795 CrossRef CAS; (f) J. M. Oliva, N. L. Allan, P. v. R. Schleyer, C. Viñas and F. Teixidor, J. Am. Chem. Soc., 2005, 127, 13538–13549 CrossRef CAS PubMed; (g) T. D. Getman, C. B. Knobler and M. F. Hawthorne, J. Am. Chem. Soc., 1990, 112, 4593–4594 CrossRef CAS; (h) K. Chui, H.-W. Li and Z. Xie, Organometallics, 2000, 19, 5447–5453 CrossRef CAS; (i) F. Zhang, J. Zhang, X. Fu and Z. Xie, Chem.–Asian J., 2013, 8, 1886–1891 CrossRef PubMed.
- Although the NICS values of parent benzocarborane are not reported, we use thiophene-fused benzocarboranes, o-carborane-based biphenyl and p-terphenyl derivatives as examples. (a) Y. Morisaki, M. Tominaga and Y. Chujo, Chem.–Eur. J., 2012, 18, 11251–11257 CrossRef CAS PubMed; (b) Y. Morisaki, M. Tominaga, T. Ochiai and Y. Chujo, Chem.–Asian J., 2014, 9, 1247–1251 CrossRef CAS PubMed.
- (a) J. H. Wright, C. E. Kefalidis, F. S. Tham, L. Maron and V. Lavallo, Inorg. Chem., 2013, 52, 6223–6229 CrossRef CAS PubMed; (b) A. L. Chan, J. Fajardo, J. H. Wright, M. Asay and V. Lavallo, Inorg. Chem., 2013, 52, 12308–12310 CrossRef CAS PubMed; (c) M. Asay, C. E. Kefalidis, J. Estrada, D. S. Weinberger, J. Wright, C. E. Moore, A. L. Rheingold, L. Maron and V. Lavallo, Angew. Chem., Int. Ed., 2013, 52, 11560–11563 CrossRef CAS PubMed.
- For selected examples, see: (a) K. R. Wee, W. S. Han, D. W. Cho, S. Kwon, C. Pac and S. O. Kang, Angew. Chem., Int. Ed., 2012, 51, 2677–2680 CrossRef CAS PubMed; (b) K. R. Wee, Y. J. Cho, S. Jeong, S. Kwon, J. D. Lee, I. H. Suh and S. O. Kang, J. Am. Chem. Soc., 2012, 134, 17982–17990 CrossRef CAS PubMed; (c) C. Shi, H. Sun, X. Tang, H. Lv, H. Yan, Q. Zhao, J. Wang and W. Huang, Angew. Chem., Int. Ed., 2013, 52, 13434–13438 CrossRef CAS PubMed; (d) H. Naito, Y. Morisaki and Y. Chujo, Angew. Chem., Int. Ed., 2015, 54, 5084–5087 CrossRef CAS PubMed; (e) N. Tsuboya, M. Lamrani, R. Hamasaki, M. Ito, M. Mitsuishi, T. Miyashita and Y. Yamamoto, J. Mater. Chem., 2002, 12, 2701–2705 RSC; (f) J. Guo, D. Liu, J. Zhang, J. Zhang, Q. Miao and Z. Xie, Chem. Commun., 2015, 51, 12004–12007 RSC; (g) S. Mukherjee and P. Thilagar, Chem. Commun., 2016, 52, 1070–1093 RSC; (h) R. Furue, T. Nishimoto, I. S. Park, J. Lee and T. Yasuda, Angew. Chem., Int. Ed., 2016, 55, 7171–7175 CrossRef CAS PubMed; (i) H. Naito, K. Nishino, Y. Morisaki, K. Tanaka and Y. Chujo, Angew. Chem., Int. Ed., 2017, 56, 254–259 CrossRef CAS PubMed.
- H. Nakamura, K. Aoyagi and Y. Yamamoto, J. Am. Chem. Soc., 1998, 120, 1167–1171 CrossRef CAS.
- A.-R. Popescu, A. D. Musteti, A. Ferrer-Ugalde, C. Viñas, R. Núñez and F. Teixidor, Chem.–Eur. J., 2012, 18, 3174–3184 CrossRef CAS PubMed.
- R. Cheng, J. Zhang, J. Zhang, Z. Qiu and Z. Xie, Angew. Chem., Int. Ed., 2016, 55, 1751–1754 CrossRef CAS PubMed.
- S. Xu, F. Haeffner, B. Li, L. N. Zakharov and S.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 6795–6799 CrossRef CAS PubMed.
- H. Ni, Z. Qiu and Z. Xie, Angew. Chem., Int. Ed., 2017, 56, 712–716 CrossRef CAS PubMed.
- T. M. Krygowski and M. K. Cyrański, Chem. Rev., 2001, 101, 1385–1420 CrossRef CAS PubMed.
- R. J. Abraham and M. J. Reid, J. Chem. Soc., Perkin Trans. 2, 2002, 1081–1091 RSC.
- H. McNab and M. E.-A. Murray, J. Chem. Soc., Perkin Trans. 1, 1988, 333–338 RSC.
- N. N. Sauer, R. Angelici, Y. C. J. Huang and W. S. J. Trahanovsky, J. Org. Chem., 1986, 51, 113–114 CrossRef CAS.
- S. H. Hong, D. P. Sanfers, C. W. Lee and R. H. Grubbs, J. Am. Chem. Soc., 2005, 127, 17160–17161 CrossRef CAS PubMed.
- G.-H. Hou, J.-H. Xie, P.-C. Yan and Q.-L. Zhou, J. Am. Chem. Soc., 2009, 131, 1366–1367 CrossRef CAS PubMed.
- B. Bak, D. Christensen, L. Hansen-Nygaard and J. Rastrup-Andersen, J. Mol. Spectrosc., 1961, 7, 58–63 CrossRef CAS.
- (a) L. Nygaard, J. T. Nielsen, J. Kirchheiner, G. Maltesen, J. Rastrup-Andersen and G. O. Sørensen, J. Mol. Struct., 1969, 3, 491–506 CrossRef CAS; (b) N. Sundaraganesan, H. Umamaheswari, B. D. Joshua, C. Meganathan and M. J. Ramalingam, J. Mol. Struct.: THEOCHEM, 2008, 850, 84–93 CrossRef CAS.
- A. R. Chaudhry, R. Ahmed, A. Irfan, A. Shaari and A. Al-Sehemi, Mater. Chem. Phys., 2013, 138, 468–478 CrossRef CAS.
- (a) A. Muranaka, S. Yasuike, C.-Y. Liu, J. Kurita, N. Kakusawa, T. Tsuchiya, M. Okuda, N. Kobayashi, Y. Matsumoto, K. Yoshida, D. Hashizume and M. Uchiyama, J. Phys. Chem. A, 2009, 113, 464–473 CrossRef CAS PubMed; (b) N. Singla, V. S. Bhadram, C. Narayana and P. Chowdhury, J. Phys. Chem. A, 2013, 117, 2738–2752 CrossRef CAS PubMed.
- (a) Z. Liang, Q. Tang, R. Mao, D. Liu, J. Xu and Q. Miao, Adv. Mater., 2011, 23, 5514–5518 CrossRef CAS PubMed; (b) X. Gu, B. Shan, Z. He and Q. Miao, ChemPlusChem, 2017, 82, 1034–1038 CrossRef CAS; (c) J.-L. Bredas, Mater. Horiz., 2014, 1, 17–19 RSC.
- (a) Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed; (b) H. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Org. Lett., 2006, 8, 863–866 CrossRef CAS PubMed; (c) P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- (a) J. Zhu, K. An and P. v. R. Schleyer, Org. Lett., 2013, 15, 2442–2445 CrossRef CAS PubMed; (b) C. Zhu, S. Li, M. Luo, X. Zhou, Y. Niu, M. Lin, J. Zhu, Z. Cao, X. Lu, T. Wen, Z. Xie, P. v. R. Schleyer and H. Xia, Nat. Chem., 2013, 5, 698–703 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1583053–1583058 for 2, 4, 8, 10, 17 and 20. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04722c |
| This journal is © The Royal Society of Chemistry 2018 |