Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Te-containing carbon dots for fluorescence imaging of superoxide anion in mice during acute strenuous exercise or emotional changes†
Wei
Zhang
*,
Ruixia
Wang
,
Wei
Liu
,
Xin
Wang
,
Ping
Li
*,
Wen
Zhang
,
Hui
Wang
and
Bo
Tang
 *
*
College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Institute of Biomedical Sciences, Shandong Normal University, Jinan 250014, P. R. China. E-mail: tangb@sdnu.edu.cn
First published on 8th November 2017
Abstract
Acute strenuous exercise and emotional changes are closely related to important aspects of human health. The superoxide anion (O2˙−), as one of the primary reactive oxygen species (ROS), is intimately associated with major diseases. However, there is no relevant in vivo research for directly indicating the link between O2˙− level and acute physical exercise or emotional changes. Hence, we constructed three fluorescent probes for the detection of O2˙−, including a Te-containing molecular probe and Se- and Te-containing CDs, and evaluated their properties such as selectivity, sensitivity, instantaneity and dynamic response to O2˙−. Through performance comparisons, we found that the Te-containing CDs exhibited reversibility, instantaneity and the highest sensitivity (LOD ∼ 8.0 pM), under guarantees of specific recognition of O2˙−, which ensure they are suitable for tracing native level changes in O2˙− within living systems. The probe was applied for monitoring the levels of O2˙− in mice under the state of intense exercise, irritability and mild depression, which led to the levels of O2˙− significantly increasing compared to the normal condition. Furthermore, we used the Te-containing CDs for real-time and dynamic imaging of O2˙− fluxes in the brain of mild depression mice and witnessed a positive correlation between O2˙− levels and depression. This work provides a new strategy for studying the relationship between acute exercise or emotional changes and diseases at the level of ROS.
Introduction
Strenuous physical exercise and acute emotional changes are closely related to human health.1–6 The superoxide anion (O2˙−), as one of the primary ROS and an important signal molecule, is associated with major diseases.7–9 So are the levels of ROS, especially the first produced O2˙−, related to the state of acute exercise or emotional alterations? To explore the relationship between O2˙− and the above-mentioned states, the fluorescence imaging method is an ideal approach because of the advantages of being nondestructive and the ability to afford high spatial-temporal resolution.10,11 Given the special properties of O2˙− including inordinate low levels and mutual transformation between ROS in living systems, the fluorescent probes should possess an ultra-high sensitive, reversible and instantaneous response to O2˙−. Currently, with the aim to monitor O2˙− levels in cells and in vivo, many analytical methods have been developed.12,13 However, limited by sensitivity and reversibility, existing imaging results of O2˙− in cells and in vivo are basically achieved by external stimuli, which cannot realize real-time analysis of native O2˙− fluctuation in biological processes.14–17 In our previous work, we developed a two-photon fluorescent probe (TCA) for dynamic and reversible imaging of O2˙−.16 Nevertheless, due to the detection limit of TCA being at the nanomolar level, the O2˙− level was measured under external stimuli. In order to break through the limitation of the sensitivity of existing probes and to achieve detection of the true endogenous O2˙− level in vivo, we constructed a polymer nanoprobe (PCLA-O2˙−) based on chemiluminescence resonance energy transfer, which demonstrates imaging of O2˙− at the picomolar level in mice.17 However, because of the deficiency of reversibility, the PCLA-O2˙− was not able to dynamically image O2˙−. Therefore, in order to directly uncover the linkage between the O2˙− level and acute exercise or emotional changes, ideal fluorescence imaging probes for real-time visualization of O2˙− levels in vivo are still scarce.Currently, CDs have attracted extensive interest owing to their good biocompatibility, excellent two-photon properties, optical stability and slow diffusion. CD-based nanosensors have been used for sensing pH, metal ions, H2S, and so forth.18–23 To construct CD-based nanosensors, the procedure of engineering CD surfaces with diverse functions is generally complicated. Therefore, improved methods for building a CD-based nanosensor are still highly demanded. In previous studies, Te and Se have been successfully confirmed as active sites to mimetic glutathione peroxidase.24 These properties of Te and Se inspired researchers to design a series of Te- and Se-containing probes that can be applied for dynamic and reversible imaging of active small molecules such as ROS and mercaptan in cells.25–33 Considering that the recognition ability of Se- and Te-based active sites is mainly focused on ROS, in order to realize the dynamic fluorescence imaging of native O2˙− fluctuation during intensive exercise or acute emotional changes, the introduction of Se- and Te-based active sites into CD-based nanosensors may offer a useful perspective on O2˙− recognition.
Based on the above strategies, we developed three O2˙− fluorescent probes (FO–PTe, Te-CDs and Se-CDs) (Scheme 1 and Fig. S1†). Among them, the Te-containing molecular probe (FO–PTe) with 9-fluorenone as a fluorophore was covalently linked with two Te-containing moieties, which could achieve dynamic and reversible detection of O2˙− through the redox properties of the Te-center. Two other kinds of Se- and Te-containing CD were prepared from Te- and Se-containing molecular probes (FO–PTe and FO-PSe) as the carbon source, respectively. The observed results demonstrated that all three probes had good selectivity for O2˙−. More importantly, the Te-CDs and Se-CDs exhibited excellent reversibility and an instantaneous response. Their reversibility was attributed to the redox of the Te- or Se-center by further characterization. In particular, the detection limit of Te-CDs reached 8.0 pM. These probes were applied in live cells and tumor tissues to image O2˙−. The results indicated that the Te-CDs exhibited the highest sensitivity to track the endogenous O2˙− level without external stimulation. Through two-photon fluorescence imaging, we found that the O2˙− level significantly increased in mice during acute strenuous exercise and emotional changes. Finally, we explored the O2˙− levels in the brains of the depressive mice using Te-CDs, which instantaneously and dynamically indicated the changes in O2˙− concentration.
Results and discussion
Reaction mechanism, characterization and spectral properties of the probes
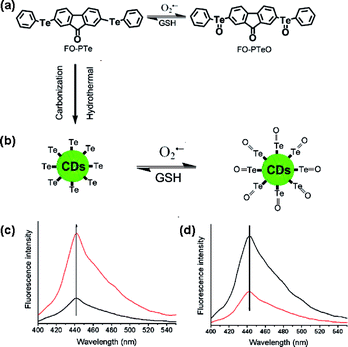
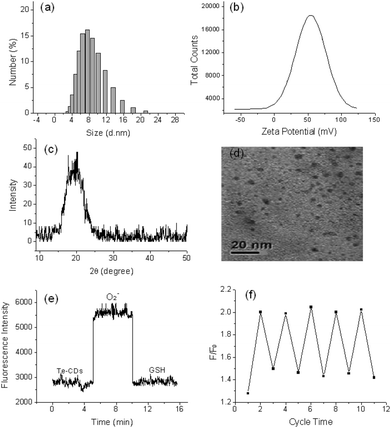
Three fluorescent probes were constructed and the structure, reaction mechanism and spectral properties are shown in Scheme 1 and Fig. S1.† The Te-based active site with 9-fluorenone as the fluorophore was employed to fabricate the reversible organic molecular fluorescent probe FO-PTe. After reaction with O2˙−, the fluorescence intensity of FO-PTe enhanced, and the resultant product was FO-PTeO. When FO-PTeO underwent a reaction with glutathione (GSH), its fluorescence intensity became quenched. Thus, the reversible and dynamic response of O2˙− might be realized through the redox of the Te-based active site in FO-PTe. Using FO-PTe as the carbon source, the functional Te-CDs were prepared by a single hydrothermal reaction step. The fluorescence intensity of the Te-CDs (λex, 380 nm; λem, 440 nm) increased after reaction with O2˙− (Scheme 1c), and the obtained product is TeO-CD. After reaction with GSH, the fluorescence of TeO-CDs decreased and the product is Te-CD (Scheme 1d). Through redox cycling between Te and Te![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Scheme 1b), the probe has the ability to dynamically sense O2˙−. In order to further study the reaction mechanism and the analytical properties of the Te-CDs, we prepared Se-CDs using the molecular probe FO-PSe as a carbon source.34–36 The fluorescence response of Se-CDs (λex, 440 nm and λem, 550 nm) with O2˙−/GSH was also investigated (Fig. S1c and d†). According to further characterization, we found that the structure of the directly obtained CDs was SeO-CD using the molecular probe FO-PSe as the carbon source, and then Se-CDs were produced after treatment with GSH. These results suggested that the reversible response to O2˙− might also be realized by redox cycling of the Se-based active site. In addition, the corresponding structure and the recognition mechanism of the two CDs were well characterized by TEM, DSL, XRD, Z-potential, XPS and Te and Se NMR (Fig. 1a–d, S2–S7†).
O (Scheme 1b), the probe has the ability to dynamically sense O2˙−. In order to further study the reaction mechanism and the analytical properties of the Te-CDs, we prepared Se-CDs using the molecular probe FO-PSe as a carbon source.34–36 The fluorescence response of Se-CDs (λex, 440 nm and λem, 550 nm) with O2˙−/GSH was also investigated (Fig. S1c and d†). According to further characterization, we found that the structure of the directly obtained CDs was SeO-CD using the molecular probe FO-PSe as the carbon source, and then Se-CDs were produced after treatment with GSH. These results suggested that the reversible response to O2˙− might also be realized by redox cycling of the Se-based active site. In addition, the corresponding structure and the recognition mechanism of the two CDs were well characterized by TEM, DSL, XRD, Z-potential, XPS and Te and Se NMR (Fig. 1a–d, S2–S7†).
Reversibility of the probes
To determine whether the probes have reversible recognition ability, O2˙−/GSH was used to further test their fluorescence response over time. The fluorescence intensity of the Te-CDs significantly enhanced after the reaction with O2˙− (Fig. 1e). In the meantime, the fluorescence intensity decreased rapidly when reacted with GSH, which indicated the good reversibility and instantaneity of the probe. Then the reversibility of the probe was further characterized through multiple redox cycling experiments with O2˙−/GSH (Fig. 1f). The same experiment was performed to examine the reversibility of the molecular probes FO-PTe and Se-CDs with O2˙−/GSH. The experimental results exhibited the fast reaction speed and good reversibility of FO-PTe and Se-CDs (Fig. S8 and S9†). Therefore, these results demonstrate that the redox cycling of the Se- or Te-based active site makes the probes dynamically and reversibly trace O2˙−.Selectivity of the probes
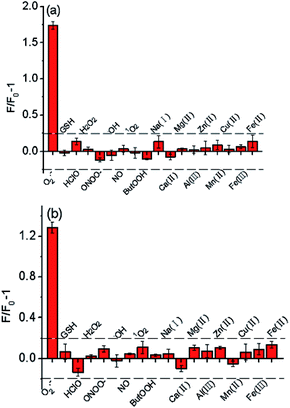
To verify the selectivity of the probes, we investigated their fluorescence response to a number of biologically relevant small molecule and metal ion solutions. The fluorescence intensity of Te-CDs enhanced obviously after addition of O2˙− (Fig. 2a), while there was little change in the fluorescence intensity in the presence of other reactive species, suggesting the great recognition ability of Te-CDs toward O2˙−. Next we confirmed the good selectivity of the molecular probe FO-PTe (Fig. 2b). As for the Se-containing CDs, we found that the fluorescence of the newly prepared CDs was significantly quenched after reaction with reducing species such as GSH and vitamin C (Vc), and the fluorescence was largely unchanged when other species were added (Fig. S10a†). After the newly prepared Se-containing CDs were treated with a reducing agent, the product fluoresced strongly when O2˙− was added and there was low fluorescence in the presence of some other biological substances (Fig. S10b†). This confirmed that the directly prepared Se-containing CDs were SeO-CDs. Meanwhile, the structure was Se-CD after treatment with the reducing agent GSH. Altogether, these results provide strong evidence that Se or Te as active sites give the probes outstanding selectivity for O2˙−.Sensitivity of the probes
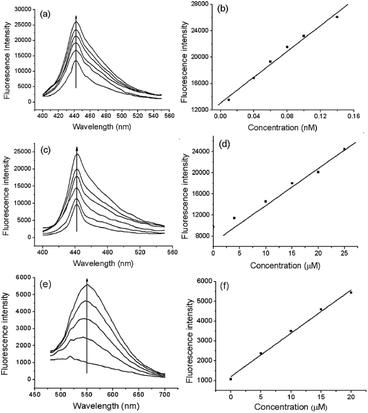
We compared the sensitivity of the probes. Fig. 3a shows that the fluorescence intensity of Te-CDs gradually enhanced as O2˙− concentrations increased, and the linear relationship in the concentration range of 0.01–0.14 nM had a correlation coefficient of 0.9934. Notably, the detection limit reached 8.0 pM (n = 11 and S/N = 3), which is a record sensitivity. Fig. 3c shows the fluorescence response of the molecular probe FO-PTe with different concentrations of O2˙−, and the linear relationship in the concentration range of 4.0–25.0 μM with a correlation coefficient and detection limit of 0.9912 and 0.15 μM, respectively (n = 11 and S/N = 3). Next, the response of Se-CDs with O2˙− was also investigated (Fig. 3e). The probe showed a linear relationship in the concentration range of 5.0–20.0 μM, and the correlation coefficient and detection limit were 0.9989 and 0.12 μM, respectively (n = 11 and S/N = 3). By comparison, Te-CDs exhibited ultra-high sensitivity, which is suitable for the imaging of O2˙− at native levels in cells and in vivo because the O2˙− level is about 10−10 M in biological systems.1 The reasons for the difference in detection limit may be as follows: (1) comparing the Te-CDs and the molecular probe FO-PTe, there is an obviously different luminescence mechanism between the CDs and the molecular probes, and a much larger two-photon absorption cross section of Te-CDs (9707 GM, ESI†) heightening the sensitivity and (2) comparing the Se-CDs and Te-CDs, the difference of the Se- and Te-based CDs lies in the difference between Se and Te. According to the radius formula of quantum dots, the theoretical radius of Te-CDs is smaller than that of Se-CDs.34 Considering that the atomic radius of Te is larger than that of Se, and the specific surface area of a quantum dot increases sharply with the decrease in size, we suppose that the effective ratio of the Te-based active site in Te-CDs is higher than that of the Se-based active site in Se-CDs. The above reasons lead to the Te-CDs having the best sensitivity among these probes.Imaging analysis of O2˙− in live cells
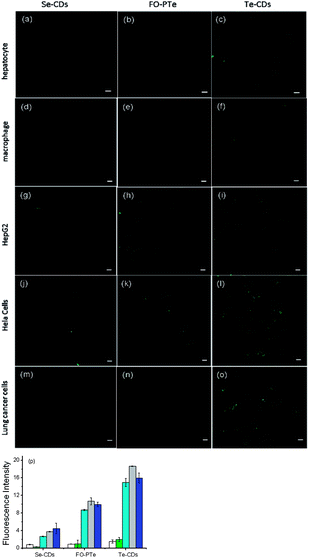
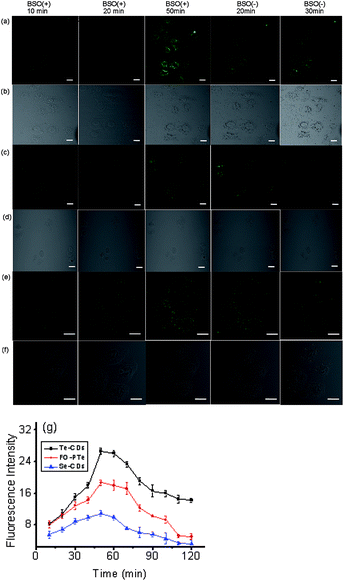
We next investigated how the three probes responded to O2˙− inside different cells (hepatocyte, HepG2 cells, macrophages, Hela cells and lung cancer cells, Fig. 4).37,38 In two-photon fluorescence images, clearer fluorescence was observed within tumor cells than normal cells, indicating higher O2˙− concentrations than in normal cells. In particular, the Te-CDs (Fig. 4p) showed the brightest fluorescence, demonstrating a supersensitive imaging capability for O2˙−. These results manifest that the Te-CD probe possesses obvious advantages for tracking native O2˙− in cells without external stimulation.To further test the performance of the probes for imaging O2˙−, three probes were used to monitor the changes in O2˙− levels in hepatocytes during L-buthionine sulfoximine (BSO) induced apoptosis (Fig. 5). We observed that the fluorescence within these cells gradually elevated as time went on, which should be attributable to the rapid increase of O2˙− during the process of apoptosis. After removal of BSO, intracellular fluorescence brightness was obviously weakened. These results suggested that these probes could measure real-time dynamic changes in O2˙− concentrations due to their fluorescence response reversibility. Through comparing fluorescence images of the three probes, the Te-CDs also present the most distinct fluorescence, thereby proving to be equipped with fascinating characteristics for supersensitive and dynamic imaging. In addition, after addition of Tiron (superoxide scavenger), the fluorescence image brightness obviously weakened (Fig. S17 and S18†), which provides evidence that the changes in fluorescence were due to changes in O2˙−.
Imaging analysis of O2˙− in tumor tissue of mice
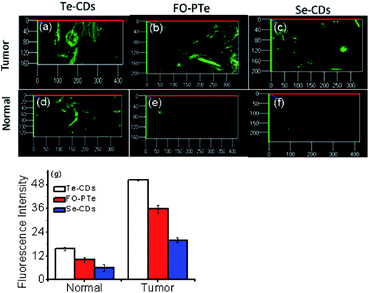
In order to examine the feasibility of the probes in living animals, they were applied to the normal abdomen tissue and breast tumor tissue of mice (Fig. 6). Apparently, much brighter fluorescence appeared in the tumor tissue than the normal tissue. This signified a pronouncedly higher O2˙− level in the mouse tumor tissue. These imaging results suggested that the three probes were able to track O2˙− in small animals, combined with deep tissue penetration and reduced background fluorescence of two-photon microscopy. Significantly, the Te-CDs exhibited the best imaging quality with O2˙−, especially in the normal tissue. Moreover, the imaging depth reached more than 800 μm (Fig. S19†). This phenomenon is due to the very high sensitivity, passive targeting and slow diffusion rate of the Te-CDs. From these results, we believe that the Te-CDs have enough capability to implement the imaging of O2˙−in vivo without an external stimulus.O2˙− fluctuations in mice under intense exercise conditions and emotional changes
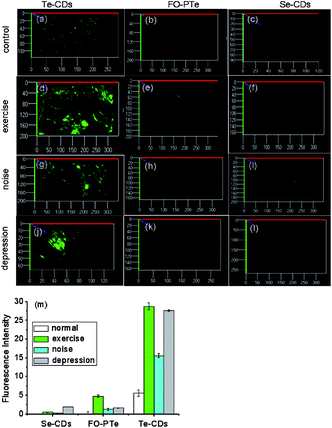
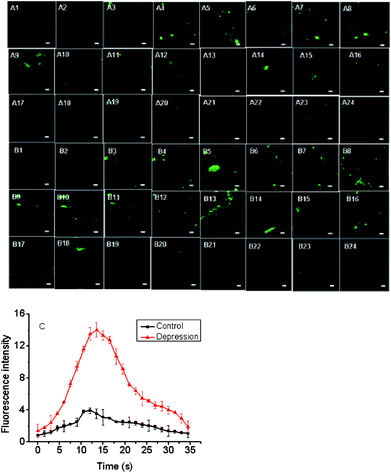
To explore how acute strenuous exercise and emotional changes influence O2˙− levels in small animals, we constructed three mouse models including: intense exercise of 1.5 h treadmill running, induced irritability by noise interference and mild depression after forced swimming.39,40 The three probes (FO–PTe, Te-CDs and Se-CDs) were then employed to observe O2˙− concentrations in the abdomen of these mice (Fig. 7). Notably, the abdomen of the normal mice injected with Te-CDs displayed perceptible fluorescence (Fig. 7a), which was due to native O2˙− levels in the control group. Furthermore, stronger fluorescence was observed for the mice under acute exercise, irritability and depressive states (Fig. 7d, g, j and m), revealing the distinct rise in O2˙− concentrations. This means that the O2˙− increase may lead to oxidative damage to organisms. Moreover, the superoxide scavenger (Tiron) was used to support the observed fluorescence that can be assigned to the presence and reactivity of O2˙− towards the probe. The fluorescence image brightness obviously weakened (Fig. S22†), suggesting that the changes in fluorescence were from the changes in O2˙−.To further confirm the changes of O2˙− in the mild depression model, the Te-CD probe was applied in the brain for real-time imaging. We found that the fluorescence brightness increased gradually and then decreased gradually over time (Fig. 8), reflecting dynamic changes of the O2˙− levels in the brain of the depressed mouse (Fig. 8c). We speculated that the changes of O2˙− during imaging were mainly due to “superoxide flashes”, which involve the transient openings of mitochondrial permeability transition pore stimulating superoxide production by the electron transport chain.15 Compared with the control group, the concentration of O2˙− in the brain of the depressed mice increased significantly. These results suggest a close relationship between ROS and depression.
Conclusions
For research on health or disease, the relationship between the O2˙− level and acute exercise and emotional changes was revealed using three probes, including Te-CDs, FO-PTe and Se-CDs, which were developed for monitoring intrinsic levels of O2˙− based on the Se or Te active sites. These probes can instantaneously and dynamically respond to O2˙− with specific recognition. Interestingly, Te-CDs displayed the highest and a record sensitivity with the detection limit at a picomolar level. Application of the three probes in different cells and tissues by two-photon fluorescence imaging confirmed that the Te-CDs were the most suitable for tracking O2˙− fluctuation without external stimulation. Mouse abdomen imaging using Te-CD, uncovered that the level of O2˙− increased sharply under intense exercise, irritability and mild depression. Furthermore, dynamic imaging in the brain of the mild depression model revealed O2˙− at a higher level. This work provides a new strategy and ideal imaging tool to unravel the influence of acute exercise and emotional change on health from the ROS level.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21390411, 21535004 and 21305080). The Primary Research Plan of Shandong Province (2017GSF18196). Natural Science Foundation of Shandong Province (ZR2015JL008).Notes and references
- M. P. Murphy, A. Holmgren, N. G. Larsson, B. Halliwell, C. J. Chang, B. Kalyanaraman, S. G. Rhee, P. J. Thornalley, L. Partridge, D. Gems, T. Nyström, V. Belousov, P. T. Schumacker and C. C. Winterbourn, Cell Metab., 2011, 13, 361–366 CrossRef CAS PubMed.
- D. R. Green, L. Galluzzi and G. Kroemer, Science, 2011, 333, 1109–1112 CrossRef CAS PubMed.
- F. He, J. Li, Z. Liu, C. C. Chuang, W. Yang and L. Zuo, Front. Physiol., 2016, 7, 486 Search PubMed.
- M. Viikki, S. Anttila, O. Kampman, A. Illi, M. Huuhka, E. Setälä-Soikkeli, N. Mononen, T. Lehtimäki and E. Leinonen, Neurosci. Lett., 2010, 477, 105–108 CrossRef CAS PubMed.
- J. M. Encinas, A. Vaahtokari and G. Enikolopov, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8233–8238 CrossRef CAS PubMed.
- M. Bilici, H. Efe, M. A. Koroglu, H. A. Uydu, M. Bekaroglu and O. Deger, J. Affective Disord., 2001, 64, 43–51 CrossRef CAS PubMed.
- M. Schieber and N. S. Chandel, Curr. Biol., 2014, 24, R453–R462 CrossRef CAS PubMed.
- M. Valkoa, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef PubMed.
- F. Esposito, R. Ammendola, R. Faraonio, T. Russo and F. Cimino, Neurochem. Res., 2004, 29, 617–628 CrossRef CAS PubMed.
- D. J. Stephens and V. J. Allan, Science, 2003, 300, 82–86 CrossRef CAS PubMed.
- J. H. Lee, C. S. Lim, Y. S. Tian, J. H. Han and B. R. Cho, J. Am. Chem. Soc., 2010, 132, 1216–1217 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS PubMed.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- W. Wang, H. Fang, L. Groom, A. Cheng, W. Zhang, J. Liu, X. Wang, K. Li, P. Han, M. Zheng, J. Yin, W. Wang, M. P. Mattson, J. P. Y. Kao, E. G. Lakatta, S. S. Sheu, K. Ouyang, J. Chen, R. T. Dirksen and H. Cheng, Cell, 2008, 134, 279–290 CrossRef CAS PubMed.
- W. Zhang, P. Li, F. Yang, X. Hu, C. Sun, W. Zhang and D. Chen, J. Am. Chem. Soc., 2013, 135, 14956–14959 CrossRef CAS PubMed.
- P. Li, L. Liu, H. Xiao, W. Zhang, L. Wang and B. Tang, J. Am. Chem. Soc., 2016, 138, 2893–2896 CrossRef CAS PubMed.
- P. C. Hsu and H. T. Chang, Chem. Commun., 2012, 48, 3984–3986 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- J. Ge, Q. Jia, W. Liu, L. Guo, Q. Liu, M. Lan, H. Zhang, X. Meng and P. Wang, Adv. Mater., 2015, 27, 4169–4177 CrossRef CAS PubMed.
- L. Pan, S. Sun, A. Zhang, K. Jiang, L. Zhang, C. Dong, Q. Huang, A. Wu and H. Lin, Adv. Mater., 2015, 27, 7782–7787 CrossRef CAS PubMed.
- C. Ding, A. Zhu and Y. Tian, Acc. Chem. Res., 2014, 47, 20–30 CrossRef CAS PubMed.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- S. T. Manjare, Y. Kim and D. G. Churchill, Acc. Chem. Res., 2014, 47, 2985–2998 CrossRef CAS PubMed.
- F. Yu, P. Li, B. Wang and K. Han, J. Am. Chem. Soc., 2013, 135, 7674–7680 CrossRef CAS PubMed.
- F. Yu, P. Li, G. Li, G. Zhao, T. Chu and K. Han, J. Am. Chem. Soc., 2011, 133, 11030–11033 CrossRef CAS PubMed.
- B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666–11667 CrossRef CAS PubMed.
- K. Xu, M. Qiang, W. Gao, R. Su, N. Li, Y. Gao, Y. Xie, F. Kong and B. Tang, Chem. Sci., 2013, 4, 1079–1086 RSC.
- S. Wang, N. Li, W. Pan and B. Tang, TrAC, Trends Anal. Chem., 2012, 39, 3–37 CrossRef CAS.
- W. Liu, X. Li, Y. S. Wong, W. Zheng, Y. Zhang, W. Cao and T. Chen, ACS Nano, 2012, 6, 6578–6591 CrossRef CAS PubMed.
- S. T. Manjare, S. Kim, W. D. Heo and D. G. Churchill, Org. Lett., 2014, 16, 410–412 CrossRef CAS PubMed.
- K. Xu, Y. Zhang, B. Tang, J. Laskin, P. J. Roach and H. Chen, Anal. Chem., 2010, 82, 6926–6932 CrossRef CAS PubMed.
- M. R. Detty, P. B. Merkel, R. Hilf, S. L. Gibson and S. K. Powers, J. Med. Chem., 1990, 33, 1108–1116 CrossRef CAS PubMed.
- B. Han, J. Yuan and E. Wang, Anal. Chem., 2009, 81, 5569–5573 CrossRef CAS PubMed.
- W. Zhang, W. Liu, P. Li, J. Kang, J. Wang, H. Wang and B. Tang, Chem. Commun., 2015, 51, 10150–10153 RSC.
- V. Georgakilas, J. A. Perman, J. Tucek and R. Zboril, Chem. Rev., 2015, 115, 4744–4822 CrossRef CAS PubMed.
- R. Liu, L. Zhang, X. Lan, L. Li, T. T. Zhang, J. H. Sun and G. H. Du, Neuroscience, 2011, 10, 408–419 CrossRef PubMed.
- S. Thiberge, S. Blazquez, P. Baldacci, O. Renaud, S. Shorte, R. Ménard and R. Amino, Nat.Protoc., 2007, 2, 1811–1818 CrossRef CAS PubMed.
- R. D. Porsolt, L. M. Pichon and M. L. Jalfre, Nature, 1977, 266, 730–732 CrossRef CAS PubMed.
- H. Takeda, M. Tsuji, M. Inazu, T. Egashira and T. Matsumiya, Eur. J. Pharmacol., 2002, 449, 261–267 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc03878j |
| This journal is © The Royal Society of Chemistry 2018 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: