Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cooperative catalytic methoxycarbonylation of alkenes: uncovering the role of palladium complexes with hemilabile ligands†
Kaiwu
Dong‡
 a,
Rui
Sang‡
a,
Zhihong
Wei
a,
Rui
Sang‡
a,
Zhihong
Wei
 a,
Jie
Liu
a,
Jie
Liu
 a,
Ricarda
Dühren
a,
Anke
Spannenberg
a,
Haijun
Jiao
a,
Ricarda
Dühren
a,
Anke
Spannenberg
a,
Haijun
Jiao
 a,
Helfried
Neumann
a,
Ralf
Jackstell
a,
Robert
Franke
bc and
Matthias
Beller
a,
Helfried
Neumann
a,
Ralf
Jackstell
a,
Robert
Franke
bc and
Matthias
Beller
 *a
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein Straße 29a, Rostock, 18059, Germany. E-mail: matthias.beller@catalysis.de
bEvonik Performance Materials GmbH, Paul-Baumann-Str. 1, 45772 Marl, Germany
cLehrstuhl für Theoretische Chemie, Ruhr-Universität Bochum, 44780 Bochum, Germany
First published on 7th February 2018
Abstract
Mechanistic studies of the catalyst [Pd2(dba)3/1,1′-bis(tert-butyl(pyridin-2-yl)phosphanyl)ferrocene, L2] for olefin alkoxycarbonylation reactions are described. X-ray crystallography reveals the coordination of the pyridyl nitrogen atom in L2 to the palladium center of the catalytic intermediates. DFT calculations on the elementary steps of the industrially relevant carbonylation of ethylene (the Lucite α-process) indicate that the protonated pyridyl moiety is formed immediately, which facilitates the formation of the active palladium hydride complex. The insertion of ethylene and CO into this intermediate leads to the corresponding palladium acyl species, which is kinetically reversible. Notably, this key species is stabilized by the hemilabile coordination of the pyridyl nitrogen atom in L2. The rate-determining alcoholysis of the acyl palladium complex is substantially facilitated by metal–ligand cooperation. Specifically, the deprotonation of the alcohol by the built-in base of the ligand allows a facile intramolecular nucleophilic attack on the acyl palladium species concertedly. Kinetic measurements support this mechanistic proposal and show that the rate of the carbonylation step is zero-order dependent on ethylene and CO. Comparing CH3OD and CH3OH as nucleophiles suggests the involvement of (de)protonation in the rate-determining step.
1. Introduction
Transition metal complexes play a crucial role in homogeneous catalysis both for basic organic synthesis and on a large scale in the chemical industry.1 In general, elementary catalytic steps occur at a given metal center and the stabilizing ligands remain untouched during the reaction. By tuning the electronic and steric properties of the ligands, the activity and/or selectivity at the metal center can be adjusted. In contrast to this classic scenario, metal–ligand cooperative catalysis requires the ligand to take part in the activation and/or recognition of the substrates or intermediates.2 For a given catalytic cycle, this strategy is a powerful tool to accelerate the rate-limiting step by lowering the energy of the key transition state. This multifunctional catalysis, which mimicks natural enzymes, opens up new avenues to synergistically activate small molecules and develop new and potentially ‘greener’ catalytic processes.3Hence, in the past two decades, several types of ligand have been developed for this purpose. Typically, substituents such as amino, carboxylate and hydroxyl groups are introduced at a specific position of the ligand.4 Most of these systems have been favorably applied for catalytic (de)hydrogenation reactions.4a–f,5
In order to expand the use of this compelling concept, we became interested in its application for carbonylation reactions. In fact, the hydroformylation and alkoxycarbonylation of alkenes constitute the most common methodologies in industry to synthesize aliphatic oxygenated compounds,6 which are found widely in our daily life. As a representative example, the palladium/L1-catalyzed methoxycarbonylation of ethylene7 followed by condensation with paraformaldehyde is used to produce methyl methacrylate, which is an important monomer in the polymer industry.
Interestingly, Drent et al. showed the superiority of diphenylphosphinopyridine (Ph2P(2-Py)) in the carbonylation of propyne with CO.8 Here, the 2-pyridyl moiety in this ligand is suggested to promote the nucleophilic attack of an alcohol on the key palladium acyl species, which is often rate-limiting, via metal–ligand cooperativity.9 Inspired by this seminal work, very recently we developed highly efficient palladium catalysts for more significant olefin alkoxycarbonylations.10 Compared to ligand L1, which is currently applied in industry, ligand L2 shows very high activity even at room temperature (Scheme 1). The yield of the reaction with L2 is nearly quantitative within 3 hours, while that with L1 is only less than 10% under the same reaction conditions.10b The key feature of L2 is the combination of amphoteric and sterically hindered moieties on the P atoms. Although the built-in base is thought to be responsible for the enhancement of the activity, until now its exact role has been unclear. Obviously, a better understanding of this substantial effect could be of enormous importance for the rational design of advanced industrial catalysts.
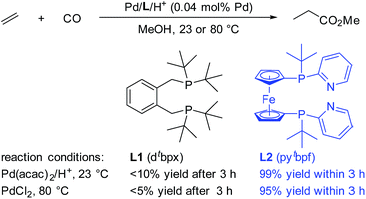 | ||
| Scheme 1 Comparison between L1 and L2 in the Pd-catalyzed ethylene methoxycarbonylation. L2 was used as a mixture of meso- and rac-isomers. | ||
Thus, a detailed investigation into ethylene methoxycarbonylation catalyzed by a palladium/L2 complex is presented here for the first time. Stoichiometric model reactions, X-ray crystallographic and ESI-mass spectroscopic studies as well as density functional theory computations reveal the importance of metal–ligand cooperativity and provide the basis for understanding the outstanding efficiency of this catalyst, which paves the way for rational catalyst development in the future.
2. Results and discussion
2.1. Kinetic analysis
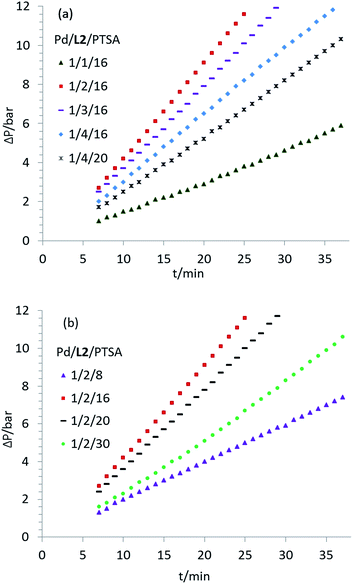
To gain insight into the mechanism of the Pd/L2–catalyzed methoxycarbonylation, we performed some kinetic experiments under the previously optimized reaction conditions using ethylene as a model substrate due to its industrial relevance. The initial rate of this process was estimated by an online gas drop of ethylene and CO (see ESI† for experimental details). However, it is noted that our reaction was carried out in a closed autoclave under neither isobaric nor isothermal conditions. During the reaction, the total pressure was reduced considerably over time (up to 20 bar) due to the consumption of ethylene and CO, and this can change the partial pressure and the solubility of the gas molecules in solvent. At the same time, the temperature increased up to 7 °C (using 0.0420 mmol of Pd source) over time due to its exothermic properties, and this again can influence the properties of the gas molecules. In addition, it is worth mentioning that ethylene gas does not follow the ideal gas rule,11 and this makes such a pressure-dependent reaction more complicated than expected on the basis of the ideal gas models. All of these factors can influence our kinetic model as well as the corresponding kinetic parameters. Thus, our results can only be qualitative rather than quantitative.Variation of the Pd(0)- and (II)-precursors revealed an induction time of almost 3 h using Pd(acac)2. Advantageously, the reaction proceeded immediately in the presence of [Pd2(dba)3·CHCl3] (ESI, Fig. S1†), which suggests that the catalytic cycle is triggered by a low valent Pd(0) species. When the ratio of Pd(0)/L2 changes from 1/1 to 1/2, formation of the active complex is promoted and the initial reaction rate increases substantially, which also indicates the possibility of reversible coordination of the ligand to the metal center (Fig. 1a). However, a further increase in the amount of L2 inhibited the reaction, probably due to the blocking of free coordination sites. To disprove the notion that the reduced activity is an effect of the acid consumption by the built-in base in L2, control experiments with additional amounts of PTSA (p-toluenesulfonic acid) were performed (Fig. 1a). The reaction rate increased significantly when the concentration of PTSA increased within a certain range (Pd/L2/PTSA from 1/2/8 to 1/2/16, Fig. 1b). Considerably negative effects were observed both at lower and higher concentrations of PTSA.
A nearly zero-order dependence on the concentration of ethylene and CO was observed (ESI, Fig. S4 and S5†), demonstrating their facile incorporation in the corresponding palladium hydride and palladium alkyl complexes. Even at room temperature these elementary steps are not rate-limiting. On the other hand, the reaction has a 1.05-order dependence on the concentration of the palladium catalyst (Fig. S6†), indicating that the active Pd/L2/PTSA complex is involved in the rate-limiting step.
Notably, ethylene carbonylation using Pd/L2/PTSA proceeded well, even at 0 °C, albeit 24 h was needed to reach full conversion. Gratifyingly, at 100 °C the catalyst loading can be decreased to as low as 0.9 ppm, and the desired product was afforded with very high activity and chemoselectivity (TON: >1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; TOF: 46
000; TOF: 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; selectivity: 99%, see ESI† for details). To estimate the kinetic parameters, we plotted the Arrhenius and Eyring equations in the temperature range of 296 to 333 K (Fig. S7†). The estimated activation energy (Ea = 44.5 kJ mol−1) and activation enthalpy (ΔH‡ = 41.9 kJ mol−1) are comparable.
000 h−1; selectivity: 99%, see ESI† for details). To estimate the kinetic parameters, we plotted the Arrhenius and Eyring equations in the temperature range of 296 to 333 K (Fig. S7†). The estimated activation energy (Ea = 44.5 kJ mol−1) and activation enthalpy (ΔH‡ = 41.9 kJ mol−1) are comparable.
Under otherwise identical conditions, we carried out ethylene methoxycarbonylation in CH3OD and found only the mono-deuterated β-isomer product, in contrast with the previously reported labelling results using L1 at a higher temperature.12 To further check the H/D exchange products, we slightly modified our procedure and started the reaction at first using CD3OD and ethylene without CO for 3 h, and then further with CO. Finally we found the multi-deuterated α- and β-isomer products (Fig. S12†). This difference might be associated with the ethylene concentration in solution as well as the respective reaction rates.
2.2. Characterization of the catalytic intermediates
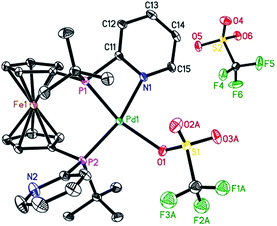
To characterize the assumed active palladium hydride species,13 [Pd(L2)(dba)] was prepared according to the procedure reported for the [Pd(L1)(dba)] complexes.7 Next, the slow addition of trifluoromethanesulfonic acid (TfOH) into the solution of Pd(L2)(dba) in methanol at room temperature should provide the desired active species. Unfortunately, no suitable crystals for X-ray crystallography were obtained. However, in the presence of benzoquinone, the corresponding Pd(II) complex with L2 was formed in Et2O. Notably, this is the first detailed characterization of an L2/Pd(II) complex. Single crystals of [Pd(L2)(OTf)]OTf were obtained in CH2Cl2/Et2O at 0 °C. As shown in Fig. 2, the complex has a distorted square-planar geometry. The palladium atom is coordinated to the two phosphorus centers and one nitrogen atom from one pyridyl ring of L2, as well as one oxygen atom from [OTf]−1. The bond length of N–Pd is exactly 2.116(3) Å, while those of P–Pd are 2.2363(8) and 2.2695(8) Å, respectively. Bite angles of 70.07(7)° for N1–Pd1–P1 and 97.20(3)° for P1–Pd1–P2 were observed. The crystal structure of [Pd(L2)(OTf)]OTf shows that L2 could also act as a bidentate or a tridentate ligand under different conditions. Actually, L2 as a bidentate ligand has been observed in the previously reported pre-catalyst Pd(L2)(NMM) (NMM, N-methylmaleimide).10bUsing this novel palladium complex and a catalytic amount of PTSA, the methoxycarbonylation of ethylene gave the desired ester under otherwise identical conditions. In the presence of an additional amount of L2, similar activity compared to the in situ generated catalyst was observed (Fig. S11†).
Next, we were interested in the structural assignment of intermediates in the catalytic cycle by means of in situ spectroscopy. While 31P and 1H NMR investigations proved unsuccessful, to our delight, electrospray ionization mass spectroscopy (ESI-MS) analysis allowed for the detection of palladium complexes in the crude solution from our standard reaction. NMR control experiments showed the preferential protonation of the pyridyl nitrogen atom compared to the phosphorous atom in L2. As shown in Fig. S12,† ESI-MS signals at m/z = 622.8 and 792.8, with their characteristic isotope distribution, matched with the calculated patterns for [Pd(L2)(H)]+ and [Pd(L2)(OTs)]+. However, these results cannot distinguish the location of the proton in the protonated form [Pd(L2)(H)]+, and DFT computations prefer the protonation of the pyridyl nitrogen atom over the Pd center by 11.3 kJ mol−1, indicating a possible equilibrium between the protonation of the pyridyl nitrogen atom and the Pd center. Besides, no double protonation has been found by ESI-MS. In addition, oxidation of the ligand was observed, and the corresponding palladium complexes were also detected (m/z = 517.0, 533.0 and 549.0, as well as 656.8, 666.8 and 870.8).14 In fact, a comparison with the complex of L1 was carried out under similar conditions, and the protonation of L1 as well as the cation complex [Pd(L1)(H)]+ were also detected by ESI-MS.
2.3. DFT analysis
To understand the significant difference in activity of the currently used industrial ligand, L1, and our ligand, L2, we carried out detailed density functional theory computations. In our calculations, we used the real-sized ligands, catalysts (L1Pd and L2Pd) and substrates (ethylene, CO and CH3OH) in the gas phase and with the incorporation of methanol solvation, as well as in methanol solution including van der Waals dispersion correction. All of these computational details and results are given in the ESI.† Since there are plentiful mechanistic investigations into the methoxycarbonylation reaction,7a,13,15,16 we followed the well-accepted catalytic cycle starting from the cationic [LPdH]+ complex. The first step is olefin coordination and insertion into Pd–H with the formation of the alkyl complex, the second step is CO coordination and insertion with the formation of the acyl complex, and the last step is methanolysis resulting in the formation of the ester and the regeneration of the active [LPdH]+ catalyst.On the basis of the computed effective and apparent barriers, we found that L1Pd (Table S2†) is less active than L2Pd (Table S3†) in the gas phase and in methanol solution, as well as in methanol solution including van der Waals dispersion correction. Inspired by Mecking’s work,13,15b we computed the methanolysis step assisted by a three-methanol cluster [Section S4.2(a)†] using L1 as the ligand. However, no expected energy lowering and stabilizing effects were found, and the single methanol route has a lower effective barrier than the three-methanol assisted route in the gas phase (155.1 vs. 160.6 kJ mol−1) and in methanol solution (177.2 vs. 191.7 kJ mol−1). The reason for this artificial effect in Mecking’s work comes from the constrained planar geometry of the three-methanol cluster, which represents a higher order saddle point (three imaginary frequencies), and is 25.1 kJ mol−1 higher in energy than the non-planar energy minimum structure calculated in our work. Since the potential energy surfaces in the gas phase and in methanol solution are very similar, and including van der Waals dispersion correction gives a negative apparent free energy barrier (Scheme S3†), only the results including solvation are used for discussion and comparison.
We found that L2Pd is more active than L1Pd in both the gas phase and in methanol solution on the basis of the computed effective (between the lowest and highest points) and apparent (between the reference and highest points) barriers. Therefore, we present here only the results in methanol solution to show their differences in stability and activity in the catalytic cycle (Fig. 3).
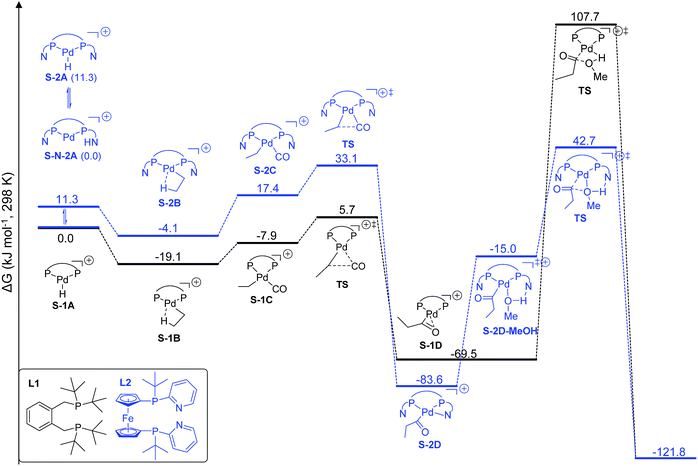 | ||
| Fig. 3 Potential free energy surface using [L1Pd–H]+ and [L2Pd–H]+ in ethylene methoxycarbonylation (S denotes solvation). | ||
Starting from the active catalyst, [L1Pd–H]+, the first step of ethylene insertion into Pd–H is barrierless and the formation of the ethyl complex [L1Pd–CH2CH3]+ is exergonic by 19.1 kJ mol−1. In the second step, CO coordination to the ethyl intermediate is endergonic by 11.2 kJ mol−1 and the effective barrier of CO insertion is 24.8 kJ mol−1, and the formation of the acyl complex [L1Pd–COCH2CH3]+ is strongly exergonic by 50.4 kJ mol−1. The final step of methanolysis has an effective barrier of 177.2 kJ mol−1 and is exergonic by 52.3 kJ mol−1; the transition state represents the highest point on the potential energy surface and is therefore rate-determining.
For the L2Pd catalyst, it is found that the nitrogen atom of the pyridyl ring has a higher proton affinity than the palladium atom by 11.3 kJ mol−1, and both protonated forms can have a dynamic equilibrium (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Starting from the [L2Pd–H]+ complex, the formation of the ethyl complex [L2Pd–CH2CH3]+ is barrierless and exergonic by 4.1 kJ mol−1, revealing the reversibility of this step. In the second step, CO coordination to the ethyl complex is endergonic by 21.5 kJ mol−1 and the effective barrier of CO carbonylation is 37.2 kJ mol−1, and the formation of the acyl complex is exergonic by 79.5 kJ mol−1. Finally, the effective barrier of methanolysis is 126.3 kJ mol−1, which is much lower than that using L1 by 50.9 kJ mol−1. This is in qualitative agreement with our experimentally observed activity trend between L1 and L2 at room temperature (Scheme 1).
1). Starting from the [L2Pd–H]+ complex, the formation of the ethyl complex [L2Pd–CH2CH3]+ is barrierless and exergonic by 4.1 kJ mol−1, revealing the reversibility of this step. In the second step, CO coordination to the ethyl complex is endergonic by 21.5 kJ mol−1 and the effective barrier of CO carbonylation is 37.2 kJ mol−1, and the formation of the acyl complex is exergonic by 79.5 kJ mol−1. Finally, the effective barrier of methanolysis is 126.3 kJ mol−1, which is much lower than that using L1 by 50.9 kJ mol−1. This is in qualitative agreement with our experimentally observed activity trend between L1 and L2 at room temperature (Scheme 1).
Taking the active catalyst, CO and methanol as a reference, the apparent free energy barrier from using L2 (42.7 kJ mol−1) is lower than that (107.7 kJ mol−1) from using L1 by 65.0 kJ mol−1; such a low barrier for L2 and the remarkable difference between L1 and L2 rationalize clearly the observed activity of the L2 complex. Since the largest difference between L1Pd and L2Pd is found in the methanolysis step, we analyzed the transition state structures. Detailed structural analysis of the transition state shows that pyridyl N-assisted H–O bond dissociation and O–C bond formation occur when using L2; there is no such additional stabilization of the transition state when using L1 (Fig. 4).
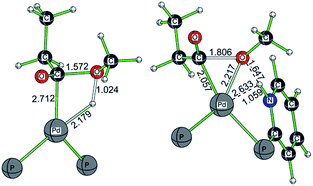 | ||
| Fig. 4 Optimized transition state structures for methanolysis using L1 and L2 (only the central part of the structure is shown; other parts are omitted for clarity). | ||
For the transition state with L1, methanolysis proceeds through a concerted pathway via the formation of the O–C bond (1.572 Å) and Pd–H bond (2.179 Å), as well as the breaking of the O–H bond (1.024 Å) and Pd–C bond (2.712 Å). For the transition state with L2, however, the proton from methanol is readily transferred to the N atom of the pyridyl ring (N–H: 1.059 Å), and the transferred proton also interacts with the oxygen atom of the CH3O group via hydrogen bonding (O–H: 1.647 Å). At the same time, both methoxy and acyl groups are coordinated to the Pd center (C–Pd: 2.057 Å; O–Pd: 2.217 Å), and the critical C–O distance from the nucleophile attack of the negatively charged CH3O group to the acyl group is 1.806 Å. This kind of pyridyl N-assisted methanolysis has been reported by Bühl et al., in their work on the methoxycarbonylation of alkynes catalyzed by a Pd complex bearing a chelating 2-pyridyl-diphenylphosphine ligand,8,9 where the pyridyl N atom can stabilize the transition state via N–H interactions.
Comparing the energies of the intermediates and the transition states of the different complexes, it is evident that only the acyl complex and the transition state of the N-assisted methanolysis are lower in energy for ligand L2. In particular, the latter step is substantially different to that for L1Pd due to the base-assisted activation of the nucleophile (methanol).
In addition, it should be noted that ligand L2 might prevent the formation of palladium black by the hemi-labile coordination of the pyridine nitrogen atom.
2.4. Catalytic cycle
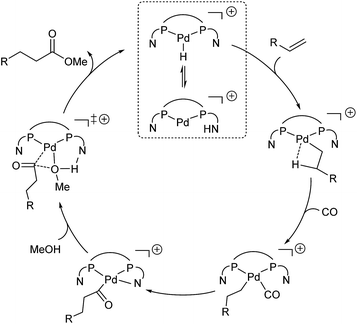
On the basis of the experiments vide supra and the computed potential energy surface, we propose the following catalytic cycle for the L2Pd-catalyzed methoxycarbonylation of alkenes (Fig. 5). The first step is the protonation of the complex, and the proton is in equilibrium between the N atom of the pyridyl ring on the phosphorus ligand and the Pd center. Subsequently, the barrierless and exergonic insertion of the alkene into the Pd–H bond proceeds to give the corresponding alkyl complex. The third step is CO coordination and insertion with the formation of the acyl species. Finally, N-assisted methanolysis takes place, which is energetically favorable for L2Pd.2.5. Extension of alkene methoxycarbonylation
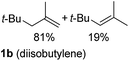
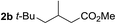
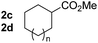
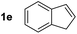
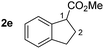
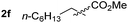
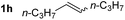
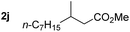
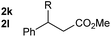
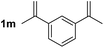
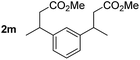
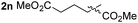
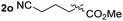
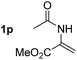
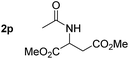
So far, palladium-catalyzed alkoxycarbonylations using L2 have only been reported for ethylene, propene and butenes. To demonstrate the superiority of this ligand with a built-in base, the methoxycarbonylation of various alkenes, including sterically hindered and functionalized substrates, was investigated. As shown in Table 1, aside from tetramethylethylene 1a, the sterically hindered alkene 1b (an industrial mixture of C8 alkenes, known as “diisobutene”, from the dimerization of isobutene) was converted into a single ester, 2b, in quantitative yield. Similarly, cyclic substrates including cyclohexene, cyclooctene and indene proved to be suitable substrates, giving the corresponding esters 2c–e in high yields. The excellent TON of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the methoxycarbonylation of 1c demonstrated the outstanding efficiency of the catalyst, albeit in lower yield. When terminal and internal olefins were employed using ligand L2, the corresponding products 2f–i were afforded in high yields, although the regioselectivity was somewhat lower compared to previous data using L1.7 This is explained by the accelerated reaction of the nucleophile with the internal acyl complex. 1,1-disubstituted double bonds were carbonylated selectively into the desired esters 2j–m in very high yields. Interestingly, diester 2m is a promising monomer for polymerization. Gratifyingly, our catalyst also allowed the alkoxycarbonylation of functionalized olefins 1n and 1o with ester and cyano groups, which afforded the ester with high yields and selectivity. Even the carbonylation of the less reactive push–pull substituted unsaturated bond of 1p proceeded well, and product 2p was obtained in 70% yield as a single isomer.
000 for the methoxycarbonylation of 1c demonstrated the outstanding efficiency of the catalyst, albeit in lower yield. When terminal and internal olefins were employed using ligand L2, the corresponding products 2f–i were afforded in high yields, although the regioselectivity was somewhat lower compared to previous data using L1.7 This is explained by the accelerated reaction of the nucleophile with the internal acyl complex. 1,1-disubstituted double bonds were carbonylated selectively into the desired esters 2j–m in very high yields. Interestingly, diester 2m is a promising monomer for polymerization. Gratifyingly, our catalyst also allowed the alkoxycarbonylation of functionalized olefins 1n and 1o with ester and cyano groups, which afforded the ester with high yields and selectivity. Even the carbonylation of the less reactive push–pull substituted unsaturated bond of 1p proceeded well, and product 2p was obtained in 70% yield as a single isomer.
| Alkene | Ester | Yield/%, n/iso |
|---|---|---|
a Reaction conditions: alkene 1 (2.0 mmol) and Pd(acac)2/L2/PTSA (0.2/0.8/3.2 mol%) in MeOH (2 mL) under CO (40 bar) at 120 °C for 20 h. Isolated yields are shown.
b Yields were detected by GC analysis using isooctane as the internal standard.
c
1b (40 mmol) and Pd(acac)2/L2/PTSA (0.005/0.1/0.4 mol%) in MeOH (20 mL). TON of 9600.
d
1c (80 mmol) and Pd(acac)2/L2/PTSA (0.001/0.5/0.2 mol%) in MeOH (20 mL) for 72 h. TON of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000.
|
||
|
|
99b, 99/1 |
|
|
99, 99/1 |
| 48c, 99/1 | ||
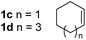
|
|
99b (55b,d) |
| 98 | ||
|
|
70 |
| 1/2 = 74/26 | ||
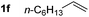
|
|
99b, 75/25 |
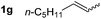
|
99b, 72/28 | |
|
99b, 71/29 | |
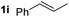
|

|
92, 79/21 |
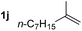
|
|
99, 99/1 |
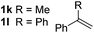
|
|
98, 99/1 |
| 98, 99/1 | ||
|
|
99, 99/1 |
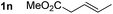
|
|
99b, 77/23 |
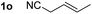
|
|
98, 80/20 |
|
|
70, 99/1 |
3. Conclusions
The mechanism of the palladium-catalyzed alkoxycarbonylation of olefins using the state-of-the-art ligand L2 has been elucidated for the first time. More specifically, the multifunctional roles of the 2-pyridyl moiety in L2 are shown. On one hand, this integrated base acts as a proton shuttle for the formation of the palladium hydride and the rate-determining N-assisted methanolysis. On the other hand, the nitrogen atom is able to improve the durability of the catalyst via hemilabile coordination to the palladium center in the catalytic cycle. Experimental and DFT computational studies support the metal–ligand cooperativity in these alkene carbonylation reactions. In this respect, we believe this work will stimulate the more rational design of advanced catalysts for carbonylations and other reactions involving (de)protonation steps.Crystal structures have been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition number CCDC 1554504 ([Pd(L2)(OTf)]OTf).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by Evonik Performance Materials GmbH and the State of Mecklenburg-Vorpommern as well as the Leibniz Association (Leibniz Competition, SAW-2016-LIKAT-1). We thank the analytical team for their kind support.Notes and references
- J. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2009 Search PubMed.
- (a) R. Peters, Cooperative Catalysis: Designing Efficient Catalysts for Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2015 Search PubMed; (b) P. Kalck, Homo- and Heterobimetallic Complexes in Catalysis: Cooperative Catalysis, Springer International Publishing, 2016 Search PubMed; (c) B. Zhao, Z. Han and K. Ding, Angew. Chem., Int. Ed., 2013, 52, 4744–4788 CrossRef CAS PubMed; (d) T. Zell and D. Milstein, Acc. Chem. Res., 2015, 48, 1979–1994 CrossRef CAS PubMed; (e) D. G. A. Verhoeven and M.-E. Moret, Dalton Trans., 2016, 45, 15762–15778 RSC.
- (a) D. G. H. Hetterscheid, J. I. van der Vlugt, B. de Bruin and J. N. H. Reek, Angew. Chem., Int. Ed., 2009, 48, 8178–8181 CrossRef CAS PubMed; (b) X. Wang, P. Guo, Z. Han, X. Wang, Z. Wang and K. Ding, J. Am. Chem. Soc., 2014, 136, 405–411 CrossRef CAS PubMed; (c) A. Nerush, M. Vogt, U. Gellrich, G. Leitus, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2016, 138, 6985–6997 CrossRef CAS PubMed; (d) D. A. DiRocco, Y. Ji, E. C. Sherer, A. Klapars, M. Reibarkh, J. Dropinski, R. Mathew, P. Maligres, A. M. Hyde, J. Limanto, A. Brunskill, R. T. Ruck, L.-C. Campeau and I. W. Davies, Science, 2017, 356, 426–430 CrossRef CAS PubMed; (e) Y.-C. Lin, E. Hatzakis, S. M. McCarthy, K. D. Reichl, T.-Y. Lai, H. P. Yennawar and A. T. Radosevich, J. Am. Chem. Soc., 2017, 139, 6008–6016 CrossRef CAS PubMed; (f) B. M. Lindley, Q. J. Bruch, P. S. White, F. Hasanayn and A. J. M. Miller, J. Am. Chem. Soc., 2017, 139, 5305–5308 CrossRef CAS PubMed.
- (a) R. Noyori and T. Ohkuma, Angew. Chem., Int. Ed., 2001, 40, 40–73 CrossRef CAS; (b) L. A. Saudan, C. M. Saudan, C. Debieux and P. Wyss, Angew. Chem., Int. Ed., 2007, 46, 7473–7476 CrossRef CAS PubMed; (c) M. Rakowski DuBois and D. L. DuBois, Chem. Soc. Rev., 2009, 38, 62–72 RSC; (d) B. L. Conley, M. K. Pennington-Boggio, E. Boz and T. J. Williams, Chem. Rev., 2010, 110, 2294–2312 CrossRef CAS PubMed; (e) C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588–602 CrossRef CAS PubMed; (f) J. R. Khusnutdinova and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12236–12273 CrossRef CAS PubMed; (g) D.-S. Kim, W.-J. Park and C.-H. Jun, Chem. Rev., 2017, 117, 8977–9015 CrossRef CAS PubMed.
- (a) K.-N. T. Tseng, J. W. Kampf and N. K. Szymczak, J. Am. Chem. Soc., 2016, 138, 10378–10381 CrossRef CAS PubMed; (b) R. Xu, S. Chakraborty, S. M. Bellows, H. Yuan, T. R. Cundari and W. D. Jones, ACS Catal., 2016, 6, 2127–2135 CrossRef CAS.
- G. Kiss, Chem. Rev., 2001, 101, 3435–3456 CrossRef CAS PubMed.
- (a) W. Clegg, M. R. J. Elsegood, G. R. Eastham, R. P. Tooze, X. Lan Wang and K. Whiston, Chem. Commun., 1999, 1877–1878 RSC; (b) C. J. Rodriguez, D. F. Foster, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2004, 1720–1721 RSC.
- E. Drent, P. Arnoldy and P. H. M. Budzelaar, J. Organomet. Chem., 1993, 455, 247–253 CrossRef CAS.
- (a) L. Crawford, D. J. Cole-Hamilton, E. Drent and M. Bühl, Chem.–Eur. J., 2014, 20, 13923–13926 CrossRef CAS PubMed; (b) L. Crawford, D. J. Cole-Hamilton and M. Bühl, Organometallics, 2015, 34, 438–449 CrossRef CAS.
- (a) K. Dong, X. Fang, S. Gülak, R. Franke, A. Spannenberg, H. Neumann, R. Jackstell and M. Beller, Nat. Commun., 2017, 8, 14117–14123 CrossRef PubMed; (b) K. Dong, R. Sang, X. Fang, R. Franke, A. Spannenberg, H. Neumann, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 5267–5271 CrossRef CAS PubMed; (c) K. Dong, R. Sang, J. Liu, R. Razzaq, R. Franke, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 6203–6207 CrossRef CAS PubMed.
- (a) J. H. Dymond and E. B. Smith, The Virial Coefficients of Pure Gases and Mixtures, Clarendon Press, Oxford, 1980 Search PubMed; (b) D. Heller, Chem. Ing. Tech., 1992, 64, 725–726 CrossRef CAS.
- G. R. Eastham, R. P. Tooze, M. Kilner, D. F. Foster and D. J. Cole-Hamilton, J. Chem. Soc., Dalton Trans., 2002, 1613–1617 RSC.
- P. Roesle, C. J. Dürr, H. M. Möller, L. Cavallo, L. Caporaso and S. Mecking, J. Am. Chem. Soc., 2012, 134, 17696–17703 CrossRef CAS PubMed.
- The oxidation of ligand L2 was suggested to occur during the characterization of the reaction mixture by ESI-MS under an air atmosphere.
- (a) A. Scrivanti, V. Beghetto, E. Campagna, M. Zanato and U. Matteoli, Organometallics, 1998, 17, 630–635 CrossRef CAS; (b) P. Roesle, L. Caporaso, M. Schnitte, V. Goldbach, L. Cavallo and S. Mecking, J. Am. Chem. Soc., 2014, 136, 16871–16881 CrossRef CAS PubMed.
- (a) G. R. Eastham, B. T. Heaton, J. A. Iggo, R. P. Tooze, R. Whyman and S. Zacchini, Chem. Commun., 2000, 609–610 RSC; (b) W. Clegg, G. R. Eastham, M. R. J. Elsegood, B. T. Heaton, J. A. Iggo, R. P. Tooze, R. Whyman and S. Zacchini, J. Chem. Soc., Dalton Trans., 2002, 3300–3308 RSC; (c) P. W. N. M. van Leeuwen, M. A. Zuideveld, B. H. G. Swennenhuis, Z. Freixa, P. C. J. Kamer, K. Goubitz, J. Fraanje, M. Lutz and A. L. Spek, J. Am. Chem. Soc., 2003, 125, 5523–5539 CrossRef CAS PubMed; (d) J. J. R. Frew, K. Damian, H. Van Rensburg, A. M. Z. Slawin, R. P. Tooze and M. L. Clarke, Chem.–Eur. J., 2009, 15, 10504–10513 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc02964k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |