Investigation of the role of writing-to-learn in promoting student understanding of light–matter interactions
Alena
Moon
,
Eleni
Zotos
,
Solaire
Finkenstaedt-Quinn
,
Anne Ruggles
Gere
and
Ginger
Shultz
 *
*
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109, USA. E-mail: gshultz@umich.edu
First published on 10th May 2018
Abstract
Fundamental quantum chemistry concepts—quantization of energy, electronic structure, and light–matter interaction—are essential for understanding chemistry and spectroscopy, an important tool for studying molecules. However, very few studies have investigated how students learn and understand these concepts or how their learning can be supported. Drawing on the capacity of writing to support learning of difficult concepts, we designed an intervention that targeted quantum concepts in the context of the use of spectroscopy for identifying chemical composition of the Orion Nebula. A quasi-experimental design with a pre-post assessment on a control and treatment group was used to identify the gains associated with completing the WTL activity. Results from a three-tiered assessment show that WTL students significantly improved in their explanations of the concept of spectroscopic transitions and their overall confidence in their understanding. Analysis of their writing, follow-up interviews, and feedback served to explain the changes observed on the pre-post assessment.
Introduction and rationale
Core quantum chemistry concepts like the nature of electromagnetic radiation, quantization of energy, electronic structure, and light–matter interactions serve as the foundation for spectroscopy, a ubiquitous tool for studying molecules. Though these concepts are essential to understanding the tools chemists use to probe molecules, we know very little about how students learn these concepts and what interventions might support learning of these concepts (Dangur et al., 2014; Aguiar and Correia, 2016; Körhasan and Wang, 2016). Much of the work on this topic area has resulted in laboratory activities that use spectroscopy (Armstrong et al., 2017; Mowry et al., 2017), development and evaluation of inquiry activities (Lucas and Rowley, 2011), and general elucidation of the topic (Tsaparlis, 2014, 2016). Given the importance of these concepts in the discipline of chemistry, further investigation of students’ understanding is needed.Writing-to-Learn (WTL) is a pedagogy that draws on the relationship between writing and learning to foster deep, conceptual learning in STEM (Connolly and Vilardi, 1989; Rivard, 1994; Reynolds et al., 2012). In contrast to traditional writing assignments in chemistry (e.g. laboratory reports or research poster), WTL activities prompt students to engage deeply with a specific concept or concepts through writing. WTL activities have been widely used and reported throughout STEM (Moore, 1993; Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017). Given the promise of writing activities for promoting conceptual learning about difficult concepts, like quantum chemistry concepts, we developed, implemented, and evaluated a Writing-to-Learn activity for introductory quantum mechanics students. Results from this study begin to fill a gap in the literature regarding students’ understanding of fundamental chemistry concepts and the efficacy of WTL. To this end, the study presented herein was guided by the following research questions:
(1) Do introductory physical chemistry students who completed a Writing-to-Learn activity show larger gains than a control group on a three-tiered assessment that measured understanding, confidence, and explanations for concepts targeted by the activity?
(2) How does analysis of written products—drafts, peer review, and revisions—explain any changes observed on the assessment?
(3) What were students’ perceptions of the Writing-to-Learn activity and how do students’ perceptions explain results from Research Questions 1 and 2?
A quasi-experimental design with a pre-post assessment for control and treatment groups was used to answer the posed research questions. Further analysis of students’ writing activity, follow-up interviews, and reflections serve to explain the observations.
Background
Student understanding of quantum concepts
Research shows that quantum concepts underlying spectroscopy are difficult for students (Johnston et al., 1998; Singh, 2001). These difficulties are at least partially enhanced by the highly mathematical nature of the topic (Dangur et al., 2014), which has prompted some to consider student difficulties with quantum mechanics as sourcing from student difficulty with mathematics (Caballero and Wilcox, 2015). In spite of this assumption, physical chemistry instruction has remained relatively constant utilizing a primarily mathematical treatment of quantum mechanics (Dangur et al., 2014). Meanwhile, research in physics and chemistry has demonstrated that doing mathematics is not the primary barrier to understanding of physical concepts (Stefani and Tsaparlis, 2009; Pepper et al., 2012; Smith et al., 2013; Caballero and Wilcox, 2015). Rather, students struggle to develop a conceptual understanding of mathematics that equips them to determine when an equation is appropriate and evaluate answers and models (Stefani and Tsaparlis, 2009; Pepper, et al., 2012; Smith et al., 2013; Caballero and Wilcox, 2015). Primarily mathematical treatment of quantum concepts in the classroom can result in students having limited understanding and ability to apply those concepts, whereas the incorporation of visual-conceptual tools promoted conceptual understanding (Dangur et al., 2014). This has prompted some chemists to argue for a shift towards qualitative, conceptual treatments of quantum mechanics at the introductory level (Kalkanis et al., 2003; Dangur et al., 2014), especially because quantum concepts are important for understanding many chemical concepts students will encounter (deSouza and Iyengar, 2013).The ability to apply these concepts is especially important for spectroscopy, though very few studies have investigated students’ understanding of basic spectroscopy (Körhasan and Wang, 2016). Many laboratory and instructional activities around spectroscopy have been published, but these are limited in effect without more fundamental investigations of how students learn the concepts underpinning spectroscopy and how this learning can be supported. Körhasan and Wang (2016) specifically investigated nine second-year physics students’ mental models of atomic spectra. Their results include four mental models ranging in scientific accuracy and sophistication: orbit model, no photon model, primitive scientific model of atomic spectra, and scientific model of atomic spectra. An orbit model treated electrons as residing in different orbits that give rise to spectral lines. The no photon model was similar to the orbit model, but recognized discrete energy levels and problematically equated them to spectral lines. The primitive scientific model of atomic spectra included bound electrons, discrete energy levels, and photon energy, but did not include electronic transitions between levels. Finally, the scientific model of atomic spectra included bound electrons, discrete energy levels, spectral lines, photon energy, and electronic transitions between quantum states. These models are helpful for beginning to understand how students make sense of key quantum mechanical concepts, but given the limited number of students interviewed, further investigation is warranted (Körhasan and Wang, 2016).
Writing-to-Learn in undergraduate chemistry
Carefully designed classroom tasks, or formative assessments, are needed to support meaningful learning of key concepts, like those highlighted above (Laverty et al., 2016). Tasks that can support this learning should provide students with an authentic context and the opportunity to integrate concepts. WTL tasks have been shown to support this type of learning by helping students “make connections, think deeply, and facilitate conceptual change” (Keys, 1999). Across grade levels and discipline, and specifically in STEM, WTL activities have effectively promoted learning (Rivard, 1994; Bangert-Drowns et al., 2004; Klein and Boscolo, 2016). Grounded in the premise that writing facilitates conceptual learning, the writing activities themselves vary widely with differing lengths, objectives, and scaffolding (Keys, 1999). Multiple local studies involving unique writing-based interventions have demonstrated the capacity of writing to support learning (Shibley et al., 2001; Whelan and Zare, 2003; Margerum et al., 2007; Lillig, 2008; Reilly and Strickland, 2010; Shultz and Gere, 2015; Finkenstaedt-Quinn et al., 2017).Shultz and Gere (2015) developed a WTL activity including an initial draft, peer review, and revision, for a general chemistry course that targeted the concept of Lewis structures and the nature of science. Analysis of both drafts showed that students improved in their summary of important themes, discussion of pre-Lewis theories, and comparison to conventional theory. On a post survey of the nature of science, students demonstrated a more sophisticated conception of the nature of science, recognizing its non-absolute nature and the role of creativity in developing theories and explanations (Shultz and Gere, 2015). One study designed WTL activities for physical chemistry, which targeted the role of ethics in physical chemistry (Reilly and Strickland, 2010). These activities consisted of a case study and a topic-based essay. Results from a pre- and post-assessment show that students changed their ideas on ethical considerations in science (Reilly and Strickland, 2010). Moore (1993) specifically investigated how differences in scaffolding of writing impacted course exam grades. In this study, students were separated into four groups: (1) no writing assignments, (2) writing assignments with no writing instruction or feedback, (3) writing assignments with writing instruction but no feedback, and (4) writing assignments with no writing instruction but feedback and the opportunity to revise according to that feedback. Results showed that group four performed significantly higher on exams and reported more frequently that writing helped them learn and that they would use it as a tool in the future. These results lend to the key role that feedback and revision play in the success of writing activities for learning (Moore, 1993). These studies demonstrate some value of WTL activities, but further study of the relationship between engaging in a writing activity and the learning that takes place is needed (Reynolds et al., 2012). The study presented herein contributes to an understanding of that relationship.
Writing as sociocultural activity
Writing is theorized as a sociocultural activity through which students internalize knowledge (Vygotsky, 1978; Prior, 2006). Learning occurs when students internalize the social activity in which they participate. Writing as a social activity involves efforts to understand, communicate, and co-construct knowledge through writing, which in turn impacts students’ lived experiences. That is, this social activity both influences and is influenced by students’ emotions; particularly, it offers the “gift of confidence” (Mahn and John-Steiner, 2008). As a result of the fusion of thinking and affect, a collaborative zone of proximal development is established. Mahn and John-Steiner (2008) describe the relationship between collaborative activity (writing) and emotion.“In producing shared texts, collaborators expand their partner's early drafts; they strive to give shape to their communicative intent by combining precision—or word meaning—with the fluidity of the sense of words. They live, temporarily, in each other's heads. They also draw on their mutuality as well as their differences in knowledge, working styles and temperament.”
The Writing-to-Learn activity investigated in this study engaged students in a process of drafting, peer review, and revision in response to a writing prompt. This activity requires students to individually interact with social variables embedded in the prompt: identity, audience, genre, and problem stakeholders. Students negotiate the meaning of the target concept in their writing as they consider these variables. Further, the students undergo peer review and revision. The process of peer review is explicitly a social activity whereby students engage in negotiating meaning with each other. In this activity, it is expected that students draw insight from the drafts that they read and the feedback that they receive from their peers that then impact their revisions. This theorized relationship guided us to expect the social activity of writing to promote confidence and, consequently, learning.
Methods
Participants and settings
This study was conducted in an undergraduate course titled Introduction to Physical Chemistry at a large Midwestern research university. This course served as an introduction to quantum chemistry, spectroscopy, chemical thermodynamics, and chemical kinetics. Topics in this course were covered in greater depth than in a general chemistry course, but less than in a full physical chemistry course sequence. The participants were primarily majors in three disciplines: chemistry, biochemistry, and chemical engineering. Chemical engineering majors (referred to as Group 1) took a 1 credit hour course only covering quantum chemistry and spectroscopy, while chemistry and biochemistry majors (referred to as Group 2) took the whole course for 3 credit hours. The course was divided into two sections, Section A with 100 students, and Section B with 68 students. Each section had students from Group 1 and 2. Section A completed a whole homework set composed of ten traditional physical chemistry problems, and Section B completed the Writing-to-Learn assignment and half of a homework set. All data collection and analysis accounted for ethical considerations, as determined by our ethical review board.Intervention description and design
The writing prompt targeted light–matter interactions and spectroscopy. Students were instructed to write a synopsis of an astronomy professor's research for the university community to be included in the university research newsletter. This astronomy professor has used the HIFI (Heterodyne Instrument for the Far Infrared) instrument to determine the chemical composition of star-forming regions in the Orion nebula (Orion-KL). Students were instructed to summarize this professor's research by explaining the quantum mechanical nature of light and matter, how light and matter interact, and how these interactions can be used to determine chemical identity. The complete writing prompt is included in Appendix 1, Fig. 5.Participants wrote a first draft in response to a prompt, underwent peer review, and revised their own papers based on peer feedback. The prompt provided students with the following rhetorical scaffolds: problem context, identity, audience, genre, and problem stakeholders. Each of these pieces were intended to frame how students made meaning out of the content as they considered the rhetorical variables to decided how and what to write. Additionally, the original prompt gave students specific concepts to include in their writing. During peer review and revision, those concepts were captured in a rubric that the students used to evaluate each other and themselves. In this way, the same concepts were emphasized throughout the WTL activity.
Data collection
An external three-tiered assessment was given to all students at the beginning of the semester and again after the intervention. The three tiers included a multiple-choice question, a confidence rating, and a short answer explanation (Sreenivasulu and Subramaniam, 2013). For each question, respondents rated how confident they were in their response (1 to 5, 5 being very confident) and explained the reasoning for their chosen multiple-choice answer. Multiple-choice questions were selected from existing assessments found in the literature (Bardar et al., 2007; Dick-Perez et al., 2016). There were five multiple-choice questions that targeted the process of light absorption (Q1), the process of light emission (Q2, Q3), the relationship between electromagnetic radiation frequency and wavelength (Q4), and differentiating between spectroscopic transitions (Q5). In total, 46 WTL and 63 non-WTL pre- and post-tests were collected.Data collected from the activity includes all written work (draft, peer review given and received, and revision). The activity took place over a two-week period. Students were given one week to complete their draft, half a week to complete peer review, and half a week to complete revisions. Peer review was facilitated electronically through a tool embedded in the course management system (CMS). Because of CMS restrictions, peer review was structured so that Group 1 students reviewed each other and Group 2 students reviewed each other. Two additional qualitative data sources were collected: follow-up interviews and open-ended feedback. Upon submission of the final draft, an open-response survey (N = 43) was administered. In this survey, students were asked what they liked about the activity, what was challenging to write about, and if they were willing to participate in an interview about the writing activity. Three of the 43 students who had completed the writing activity were interviewed. The interview protocol was intended to confirm that the writing prompt was clear and understood as designed, as well as prompt students to reflect on how they learned by completing the writing activity.
Data analysis
To complete an ordinal logistic regression for the explanation tier, short answers were scored for quality. For each question, explanations were scored using a 3-point rubric. Table 1 below shows the general rubric for scoring each question. This rubric was tailored to each specific question.
| Score | Description |
|---|---|
| 0 | Contains one of the following: No response. Irrelevant. Contains obvious alternative conception. Missing all correct components. |
| 1 | Missing one correct component. Related, but ambiguous. |
| 2 | Correctly includes all relevant components. |
To ensure reliable scoring of students’ explanations, two of the authors developed the scoring criteria together while coding. Once a complete rubric was developed, the first author explained the scoring rubric to a group of four chemistry education researchers who independently scored a 10% sample of the data set. For each question, Krippendorff's alpha was used to calculate inter-rater reliability to ensure that students’ explanations were accurately and consistently scored. For the first four questions, acceptable values for Krippendorff's alpha were obtained (Q1: 0.875; Q2: 0.865; Q3: 0.918; Q4: 0.799; Q5: 0.443), allowing us to conduct further statistical analysis on the scores (Krippendorff, 2004). The final question (Q5) scoring reliability was particularly low because the answers to this question were so poor, nearly all of them warranted a zero. Examples of these answers were “This is a guess” and “I’m not familiar with these definitions.” For this reason, the lack of variability in responses contributed to a low agreement, as determined by the Krippendorff's alpha coefficient (Krippendorff, 2004). In light of this, we considered the score acceptable for moving forward with analysis.
Both the interviews and open-ended feedback elicited student comments on the prompt and the implementation as well as perceptions of their own learning. For the purpose of this study, our analysis focused only on the latter. Given the findings from the first two research questions, we particularly wanted to understand how students perceived of the writing activity, especially peer review and revision, impacting their own learning. We coded for features of the prompt, peer review, and revision that were either helpful or challenging. The first and second authors independently read and coded comments as helpful and challenging features. These authors then met and discussed codes until consensus was reached regarding students’ perceptions.
Results
Research Question 1: Do introductory physical chemistry students who completed a Writing-to-Learn activity show larger gains than a control group on a three-tiered assessment that measured understanding, confidence, and explanations for concepts targeted by the activity?Multiple-choice tier
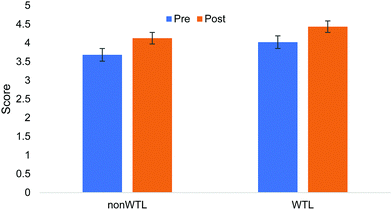
The linear regression model considered the effect of three factors—pre-score, section (WTL or non-WTL), and major (engineering or not)—for explaining variance of the dependent variable, total post multiple choice. Section membership was found to have no effect on the total post-score (maximum of 5 possible, indicating each question was answered correctly), as determined by a linear regression. Analysis revealed that when controlling for pre-score and major, students in intervention were more likely to perform higher on the post-test than their control counterparts, but not significantly (B: −0.253; S.E.: 0.186; Sig.: 0.179; 95% C.I.: −0.622–0.117). Fig. 1 shows that students in the intervention group had higher pre- and post-total scores than the control group, with comparable changes from pre-test to post-test. Question by question analysis using binary logistic regression revealed no significant differences between the intervention and treatment groups.Confidence tier
For overall confidence, controlling for whether they were in engineering or not and pre-score, WTL students had higher post-confidence than their non-WTL peers, as shown in Table 2. Fig. 2 represents the difference observed between WTL and non-WTL students in overall confidence. Question by question analysis revealed no significant differences between WTL and non-WTL students on post-confidence score. Given the roughly equivalent overall pre-scores, we believe that the increase in score for WTL students over their non-WTL counterparts is indeed a result of completing the activity.| Dependent variable | Factors | B (S.E.) | Sig. | 95% C.I. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Total confidence | Pre | 0.627 (0.088) | 0.000 | 0.452 | 0.802 |
| Section (non-WTL) | −1.279 (0.647) | 0.051 | −2.561 | 0.003 | |
| Major (E) | −0.861 (0.639) | 0.181 | −2.129 | 0.407 | |
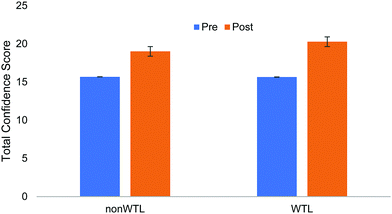 | ||
| Fig. 2 Total pre- and post-scores confidence tier of assessment (25 total, indicating maximum confidence on all 5 questions). | ||
Explanation tier
A linear regression with overall post-explanation as the dependent variable (10 pts total, 0–2 for each of 5 questions) revealed no significant effect of section membership (B: −0.611; S.E.: 0.522; Sig.: 0.245; 95% CI lower bound: −1.646; upper bound: 0.424). However, a question by question analysis using ordinal logistic regression did reveal a significant effect by section membership for Q5, which targeted spectroscopic transitions, which is shown in Appendix 2, Table 3.For each question, the pre-score was a significant predictor of increased odds of scoring higher on the post-explanation. For example, given a one-point increase in Q2 explanation pre-score, there is a 0.84 increase in the odds of the explanation post-score being higher, while holding all other variables constant. This is not surprising as students with stronger pre-performances will likely have strong post-performance. However, for Q5, the section membership significantly predicted the probability of scoring high on the explanation post-score. This assessment question targeted spectroscopic transitions, which was a key concept targeted by the writing assignment. Interestingly, the low pre-score indicates low prior knowledge of this topic relative to the other assessed topics, shown in Fig. 3. This is consistent with the fact that students likely have previously seen the concepts targeted by the first four questions (i.e., absorption, emission, electromagnetic radiation). Shown in Appendix 2, Table 3, given the same pre-score and major, the odds of scoring highly on this question are 2.83 times greater for WTL students relative to non-WTL students. For this specific question, WTL students are more likely to write higher-scoring post-explanations than their non-WTL peers. This is particularly interesting given the expectation that writing promotes deep conceptual learning and provides opportunities to construct explanations. That is, the writing activity required students to explain difficult concepts to a less scientifically literate audience, which might explain why WTL students outperformed their non-WTL counterparts on explaining the concept targeted by question 5 (differentiating between spectroscopic transitions) for which they had the least prior knowledge.
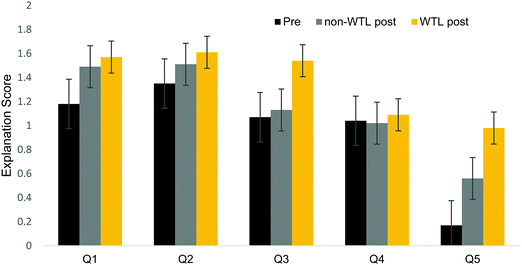 | ||
| Fig. 3 Question by question scores of explanation tier where each explanation was scored on a 3 point scale with a maximum score of 2. | ||
Research Question 2: How does analysis of written products—drafts, peer review, and revisions—explain any changes observed on the assessment?
To answer the second research question, the students’ writing activity was analyzed as a whole. Analysis of students’ writing—first draft, peer review, and revisions—revealed that the bulk of students’ revisions related to the concept targeted by question 5: rotational, vibrational, and electronic transitions. Student revisions, ranging in length from one phrase to multiple sentences, demonstrated an understanding of and a differentiation between rotational, vibrational, and electronic spectroscopic transitions. Initial drafts included a general discussion of electrons transitioning between energy levels with little to no discussion of the different types of transitions. Two themes arose in the revisions of this concept: (1) pairing each transition with the appropriate electromagnetic radiation and (2) discussing the molecular motions that correspond to each transition. The example below illustrates characteristic revisions students made as a result of the peer review and revision process.
In Elaina's draft, she wrote:
The electron does not gradually move from one level to another, it jumps up or down levels in measureable amounts. When an atom absorbs enough energy it can jump up to a higher level, or conversely, the electron can move down a level and emit a certain amount of energy. Think of it like climbing a ladder, you can only go up or down specific amounts and nothing in between. This idea of quantization revolutionized our understanding of physics and the way world we live in. Different atoms and molecules have unique energy levels, levels that we can measure and record by applying electromagnetic radiation.
In Elaina's revision, she wrote:
Energy within an atom is not continuous but rather is quantized, only available to be absorbed or emitted in discrete amounts (energy levels). This concept can be imagined as someone climbing a ladder, the person can only go up or down specific amounts, but not any distance between the rungs. Electromagnetic radiation comes a wide variety of forms depending on its wavelength, or energy: (visible, infrared, microwave, ultra-violet, radio wave, x-ray, etc.). These different forms of radiation excite molecules in different ways. Radiation in the microwave region causes molecules to rotate and thus changes their rotational state. Visible and UV radiation cause electronic transitions which are the movement of electrons to higher energy levels (higher rungs on the ladder). Radiation within the IR region cause molecules to vibrate in different ways, depending on the wavelength of light and the molecule. We can use these interactions between matter and electromagnetic radiation to produce spectra which in turn gives us information about the matter involved.
In her draft, Elaina adequately explained quantization of energy and made a broad statement that electromagnetic radiation can be used to measure energy levels of electrons. This statement was clarified in Elaina's revision. She explained that there are different types of electromagnetic radiation, named three forms of light, and provided a clear connection between the forms of light and the associated spectroscopic transition. Mark made similar kinds of revisions, which can be found in Appendix 3, Table 4. In his draft, Mark vaguely described the process of absorption. In the revision, Mark included additional information to explain what happens when an atom or molecule absorbs energy. Mark identified electronic, vibrational, and rotational transitions, described the associated molecular motions, and provided connections to the associated forms of electromagnetic radiation.
Elaina's and Mark's work were exemplars of many of the remaining participants. Of the 47 students that made revisions to their draft, 41 made revisions regarding spectroscopic transitions—the topic of question 5 of the three-tiered assessment. Like Elaina and Mark, these revisions included multiple-sentence sophisticated explanations of spectroscopic transitions. Treating the writing as a social network allowed us to view the writing activity as a whole, thereby revealing how many students reviewed each other and made revisions on this topic.
To capture all of the writing as a social activity, sociograms were created to represent which authors reviewed each other and how they revised their own writing. Because peer review in the WTL intervention was split into groups where Group 1 review each other and Group 2 reviewed each other, a sociogram was created for each group. In each of the following figures, the nodes represent each participant. The arrows are representative of peer review—each arrow originates at the participant giving peer review and ends at the participant that received that feedback.
In Fig. 4a, Group 1 is arranged to show authors and reviews. In Fig. 4b, pink nodes indicate authors in Group 1 that made scientifically normative revisions regarding spectroscopic transitions. The size of the node corresponds to the magnitude of revisions: minor, one sentence, or multiple sentences. Shown in Fig. 4b, of the authors that made revisions, all but one made multiple-sentence revisions, similar to the examples shown in the previous section (Elaina and Mark). This is particularly interesting given the literature showing that revision is often difficult for students, especially substantial revisions to the content (Graham et al., 1995). The pink arrows indicate reviews that included specific feedback regarding spectroscopic transitions. A similar pattern was found for Group 2, which is illustrated in Appendix 4, Fig. 6a and b.
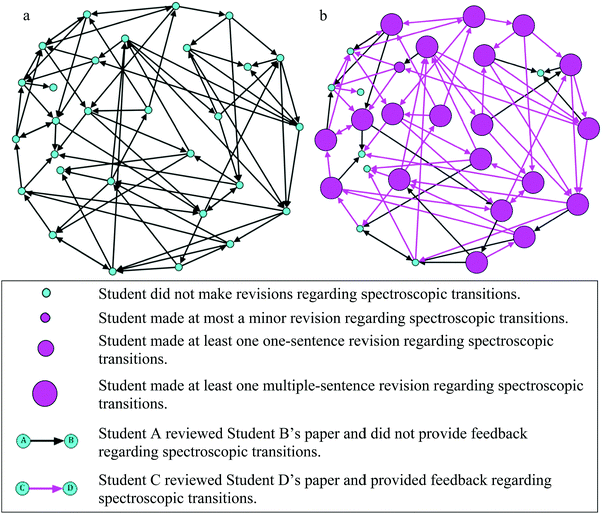 | ||
| Fig. 4 (a) Sociogram for Group 1 representing authors’ first drafts and peer review, (b) sociogram for Group 1 representing the magnitude of revisions and peer review. | ||
The bulk of social writing activity centered around spectroscopic transitions (71% peer reviewed and 82% of the authors revised). This concept was reviewed and revised relatively more frequently than the other concepts targeted by the assessment (absorption: 29% peer review, 46% revision; emission: 8% peer review, 14% revision; electromagnetic radiation: 62% review, 46% revisions). The difference in the amount of review and revision for each concept is particularly revealing of how WTL supports larger gains on an assessment for an unfamiliar concept (e.g., spectroscopic transitions) relative to familiar ones (e.g., absorption). Our second research question aimed at understanding the relationship, if any, between students’ engagement in the writing activity and the outcomes observed by the assessment. These results suggest that there is a relationship between performance on the assessment and how students participated in the writing activity. That is, for the concepts with which they engaged more actively (more frequent and extensive review and revision), students performed better on the assessment than non-WTL students relative to other concepts.
Research Question 3: What were students’ perceptions of the Writing-to-Learn activity, and how do students’ perceptions explain results from Research Questions 1 and 2?
Additional data sources—three follow-up reflective interviews and 43 feedback responses—equipped us to understand how students perceived of the WTL activity as well as further explain the results reported above. Students’ perceptions of helpful and challenging features of the writing activity reveal the ways that writing served to support their understanding more than a traditional problem set. Of the 43 feedback responses, 17 explicitly stated that the activity helped them develop a “deeper understanding” of the material. This perception can be explained by the ways in which the writing required them to interact with the material. In interviews and feedback, students voiced that a difficult component of the WTL activity was getting ideas “to flow.” In an effort to do this, we argue that students were synthesizing ideas. While discussing reviewing others, Diana voiced the challenge of making the concepts flow together.
Okay, the first two were formatted and written very strangely. And they think they were struggling a lot with what I was struggling with, of how to get the concepts to really flow. So the one person did—which I realized when I was reading mine that I did something similar—it was like a line-by-line summary of everything we covered in the class. And then not really looping it back into the actual research prompt. The one person's [essay] was all sentence fragments, so that made it difficult.—Diana
Further, of the 43 responses to the request for feedback on the prompt, 15 students voiced a similar challenge. This student specifically frames the challenge as developing a “full understanding” of a difficult concept.
I found it most challenging to develop a full understanding of how electromagnetic radiation shows us the distant particles of a nebula but also taking such difficult concepts and explaining them in ways that cater to the general [university] population.
These quotes illustrate how students perceived this activity as prompting them to orchestrate multiple concepts in order to develop a “full understanding.” Some students described slightly different variations of similar cognitive processes they engaged in while writing. One student explained that overcoming the challenge of organizing his essay effectively helped him organize his own thinking about the material. Another student claimed that the writing assignment forced them to “conceptualize the bigger picture.” It is expected that this kind of thinking—reorganizing or seeing the bigger picture—was able to support understanding of difficult and unfamiliar concepts.
In this case, spectroscopy was a difficult and unfamiliar concept. Additionally, we observed that students wrote and reviewed each other more about this concept relative to the others. This was reflected in the interviews and feedback with many suggesting spectroscopy was the most difficult to write about or that their thinking changed most for this concept.
Um spectroscopy is probably the hardest thing to write about…Well it definitely helped me, like writing it out actually helped me understand it a lot more than maybe just reading about it. So, I think as I had to explain it to other people I sort of had to explain to myself and that worked well for me.—Madison
When prompted to reflect on how her thinking had changed throughout the writing process, Madison says “definitely the IR spectroscopy and actually how he used it a little bit…once I understood IR more, I understood how he used it a bit more.” In the feedback responses, 11 of 43 (more than for any other concept) discussed spectroscopy as a particularly difficult concept to write about.
I think the most challenging thing to write about was the vibrational motion of a molecule. It's somewhat difficult to try and explain something like the motion of a molecule and how it interacts with electromagnetic radiation, with all of the specific rules. Putting it into a synopsis form becomes difficult without it's just a bunch of chemistry-related jargon that some might not understand.
This WTL activity required students to interact with this concept in a unique way. Writing about spectroscopy is a way for students to develop their conceptual understanding. Finally, we observed some themes in the role of peer review and revision in students’ perception of their understanding. This was interesting in light of the finding that students in the WTL section were more likely than non-WTL students to have higher post-overall confidence scores. Confidence in understanding came up in the interviews and feedback. Particularly, students voiced that the peer review prompted them to be certain about their understanding in order to give feedback to their peers. Diana explains this when describing how she approached peer review and how it impacted her understanding of the content.
Yeah, just because when I was giving the review critiques, I wanted to make sure that I was absolutely certain of what I was saying, you know? So I actually did, I went back and reviewed some of the concepts, just to make sure that I was absolutely certain. It made me doubt some of the things I’d written. But, yeah.—Diana
It is evident that in an effort to become certain, Diana built confidence in her own understanding. Similar comments were made in the feedback. One student says that the writing assignment showed them areas they were “unsure about.” Both writing to the initial prompt and undergoing peer review helped students develop not only in their understanding, but their confidence in their understanding. One way that the writing activity accomplished this was by promoting metacognition and exposing to students concepts they might not understand.
Limitations
Findings from this work contribute meaningfully to our understanding of the relationship between writing and learning, specifically in a quantum context. Writing is a complex and dynamic activity that has the potential to support multiple meaningful types of learning (Klein and Boscolo, 2016). The assessment used in this study targeted a very specific type of learning—understanding, confidence, and ability to explain a certain set of concepts as measured primarily by multiple choice questions. Though we considered students’ confidence and explanations, their responses were bound by a few limited multiple-choice items. It is possible that different assessments that target broader learning outcomes may reveal more gains associated with writing. Additionally, not all concepts are equally supported by writing. It is possible that spectroscopic transitions required synthesis that was supported well by writing. For this reason, it is necessary to implement similar types of prompts in other contexts to tease out how context may act as a moderating variable in writing to learn. Finally, this intervention was implemented in one class. It is necessary to repeat the intervention in other similar courses to ensure that the writing activity is indeed giving rise to outcomes observed in this study.Discussion and implications
Results from this study reveal that writing as a visual-conceptual tool promotes a conceptual understanding of concepts of light–matter interactions (Kalkanis et al., 2003; Dangur et al., 2014). In this particular case, the writing assignment supported gains in WTL students’ confidence in their understanding and ability to explain a key concept of spectroscopic transitions. These kinds of outcomes are precisely those that were absent in highly mathematical treatments of quantum instruction (Stefani and Tsaparlis, 2009; Dangur et al., 2014). The writing task in this study explicitly prompted students to write about these concepts in the context of authentic research. That is, the task required students to apply and synthesize concepts of absorption, emission, light, and spectroscopy. The concept for which they had the lowest prior knowledge also required synthesis of concepts they had been previously exposed to. Writing showed to support their ability to synthesize those concepts, which is key to developing a conceptual understanding of quantum concepts (Johnston et al., 1998). This was further supported by qualitative results indicating that the students used this activity to synthesize their ideas.Additionally, these results add to knowledge about how writing supports learning (Bangert-Drowns et al., 2004; Klein and Boscolo, 2016), especially considering a sociocultural theory of writing (Prior, 2006). This study explicitly related participation in social activity to an increase in confidence and conceptual development. In Vygotskian terms, intermental activity—between participants—promoted intramental activity—within mind of participants (Vygotsky, 1978). This was evidenced by the bulk of the social component (i.e., peer review and revisions) concerning the concept for which conceptual gains were observed. Further, Mahn and John-Steiner (2008) argued that confidence is a unique student outcome of social activities that involve the collaborative production of text, for example. As a result of the activity in this study, the WTL students showed larger gains in confidence in their understanding than their non-WTL counterparts. We argue that this outcome is uniquely tied to the practice of peer review. That is, when students have to critically review their peers’ ideas and defend their own, they develop confidence in their understanding (Mahn and John-Steiner, 2008). Engaging in explicit reflection, in this case facilitated by peer review and revision, is key to building confidence in understanding. These results provide important empirical evidence that elucidates how the social components of writing support learning (Klein and Boscolo, 2016).
Given the difficulty of the topic of spectroscopy and the relative absence of reported interventions for developing students’ understanding of quantum concepts, these results offer a promising approach for instructors to target this concept. In particular, this WTL activity provided students with an opportunity to synthesize their knowledge that a traditional problem set did not afford. It is expected that this opportunity for synthesis in the form of WTL can be extended to other difficult concepts in chemistry. WTL activities could be particularly well suited for supporting learning of threshold concepts (Park and Light, 2009; Loertscher et al., 2014; Körhasan and Wang, 2016), particularly for their capacity to facilitate synthesis of ideas and uncover implicit schemas (Talanquer, 2015). To design and implement WTL activities effectively, there are critical components that must be included in an activity (Stains and Vickrey, 2017). Our results suggest that the critical components of Writing-to-Learn are structural—they concern the way that the writing prompt is designed and implemented with students. The writing prompt should include a context that prompts students to engage with a difficult concept. An audience should be selected so that a consideration of audience actually informs their writing choices (e.g. a non-science audience for spectroscopy requires students to explain fundamental concepts). Our results further demonstrate the important role that peer review and revision served in supporting students’ understanding and confidence in that understanding. To support effective peer review and revision in this study, a rubric that targeted specific concepts was referenced throughout the activity. This type of clear rubric serves to direct student attention to specific concepts, thereby ensuring that students are considering the concepts that are being targeted by the writing activity.
Conflicts of interest
There are no conflicts to declare.Appendix 1
Fig. 5.Appendix 2
Table 3.| Question | Variable | Exp_B (S.E.) | Sig. | 95% C.I. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| *p < 0.01.a Section (non) and Major (E) indicate that these measures were determined with respect to the WTL group and non engineers, which were both set to zero in the model. | |||||
| Q1 | Pre | 2.02 (0.264) | 0.008* | −1.219 | −0.184 |
| Section (non) | 1.41 (0.429) | 0.418 | −1.188 | 0.494 | |
| Major (E) | 2.18 (0.433) | 0.071 | −1.631 | 0.068 | |
| Q2 | Pre | 2.32 (0.249) | 0.001* | 0.353 | 1.330 |
| Section (non) | 1.03 (0.443) | 0.940 | −0.901 | 0.834 | |
| Major (E) | 1.12 (0.433) | 0.783 | −0.729 | 0.967 | |
| Q3 | Pre | 2.95 (0.249) | 0.000* | 0.594 | 1.569 |
| Section (non) | 2.32 (0.440) | 0.055 | −1.706 | 0.019 | |
| Major (E) | 2.22 (0.422) | 0.057 | −1.629 | 0.025 | |
| Q4 | Pre | 3.75 (0.306) | 0.000* | 0.723 | 1.921 |
| Section (non) | 1.47 (0.377) | 0.305 | −1.127 | 0.353 | |
| Major (E) | 1.42 (0.370) | 0.370 | −1.077 | 0.373 | |
| Q5 | Pre | 3.89 (0.473) | 0.004* | 0.431 | 2.287 |
| Section (non) | 2.83 (0.388) | 0.007* | −1.801 | −0.281 | |
| Major (E) | 1.37 (0.385) | 0.414 | −1.070 | 0.440 | |
Appendix 3
Table 4.| Draft | […] the energy to move an electron must be added or removed in discrete values, or quanta. Only electromagnetic waves with energy equal to the change in energy of an electron can be used to move an electron between energy levels. Atoms and molecules with energy gaps that do not match the energy of the waves will not absorb waves with other energy values. The energy of a wave is directly proportional and indirectly proportional to wavelength. This property of quantum mechanics allows for the production of spectra that show the wavelengths of light that are and are not absorbed by a specific atom or molecule. |
| Revision | […] the energy to move an electron must be added or removed in discrete values, or quanta. Only electromagnetic waves with energy equal to the change in energy of an electron can be used to move an electron between energy levels. Atoms and molecules with energy gaps that do not match the energy of the waves will not absorb waves with other energy values. The energy of a wave is directly proportional and indirectly proportional to wavelength. This property of quantum mechanics allows for the production of spectra that show the wavelengths of light that are and are not absorbed by a specific atom or molecule. There are three main types of electronic energy changes that can occur in a molecule: vibrational, rotational, and electronic. Rotational energy transitions involve a molecule rotating around a fixed point along the molecule and electromagnetic waves in the microwave range can excite rotational changes. Electronic changes occur when electrons transition between energy levels and these transitions occur at energies that match UV and visible light electromagnetic radiation. The transitions that are important for far infrared spectroscopy are vibrational transitions. These involve vibrations such as stretching and bending that alter the dipole (or charge distribution) within a molecule and have energy changes that are in the infrared range. |
Appendix 4
Fig. 6.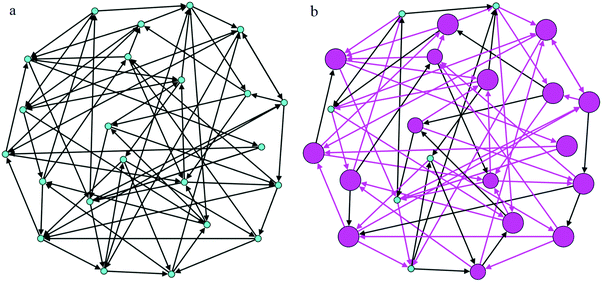 | ||
| Fig. 6 (a) Sociogram for Group 2 representing authors’ first drafts and peer review, (b) sociogram for Group 2 representing the magnitude of revisions and peer review. | ||
References
- Aguiar J. G. and Correia P. R. M., (2016), Using concept maps as instructional materials to foster the understanding of the atomic model and matter–energy interaction, Chem. Educ. Res. Pract., 17(4), 756–765.
- Armstrong C., Burnham J. A. J. and Warminski E. E., (2017), Combining Sustainable Synthesis of a Versatile Ruthenium Dihydride Complex with Structure Determination Using Group Theory and Spectroscopy, J. Chem. Educ., 94(7), 928–931.
- Bangert-Drowns R. L., Hurley M. M. and Wilkinson B., (2004), The effects of school-based writing-to-learn interventions on academic achievement: a meta-analysis, Rev. Educ. Res., 74(1), 29–58.
- Bardar E. M., Prather E. E., Brecher K. and Slater T. F., (2007), Development and validation of the light and spectroscopy concept inventory, Astron. Educ. Rev., 5(2), 103–113.
- Bastian M., Heymann S. and Jacomy M., (2009), Gephi: an open source software for exploring and manipulating networks, International AAAI Conference on Weblogs and Social Media.
- Caballero M. D. and Wilcox B. R., (2015), Unpacking students’ use of mathematics in upper-division physics: where do we go from here? Eur. J. Phys., 36(6), 65004.
- Connolly P. and Vilardi T., (1989), Writing To Learn Mathematics and Science, Teachers College Press, 1234 Amsterdam Ave., New York, NY 10027.
- Dangur V., Avargil S., Peskin U. and Dori Y. J., (2014), Learning quantum chemistry via a visual-conceptual approach: students’ bidirectional textual and visual understanding, Chem. Educ. Res. Pract., 15(3), 297–310.
- deSouza R. T. and Iyengar S. S., (2013), Using quantum mechanics to facilitate the introduction of a broad range of chemical concepts to first-year undergraduate students, J. Chem. Educ., 90(6), 717–725.
- Dick-Perez M., Luxford C. J., Windus T. L. and Holme T., (2016), A quantum chemistry concept inventory for physical chemistry classes, J. Chem. Educ., 93(4), 605–612.
- Finkenstaedt-Quinn S. A., Halim A. S., Chambers T. G., Moon A., Goldman R. S., Gere A. R. and Shultz G. V., (2017), Investigation of the Influence of a Writing-to-Learn Assignment on Student Understanding of Polymer Properties, J. Chem. Educ., 94(11), 1610–1617.
- Graham S., MacArthur C. and Schwartz S., (1995), Effects of goal setting and procedural facilitation on the revising behavior and writing performance of students with writing and learning problems, J. Educ. Psychol., 87(2), 230–240.
- Johnston I. D., Crawford K. and Fletcher P. R., (1998), Student difficulties in learning quantum mechanics, Int. J. Sci. Educ., 20(4), 427–446.
- Kalkanis G., Hadzidaki P. and Stavrou D., (2003), An Instructional Model for a Radical Conceptual Change Towards Quantum Mechanics Concepts, Sci. Educ., 87(2), 257–280.
- Keys C. W., (1999), Revitalizing instruction in scientific genres: connecting knowledge production with writing to learn in science, Sci. Educ., 83(2), 115–130.
- Klein P. D. and Boscolo P., (2016), Trends in research on writing as a learning activity, J. Writ. Res., 7(3), 311–350.
- Körhasan N. D. and Wang L., (2016), Students’ mental models of atomic spectra, Chem. Educ. Res. Pract., 17(4), 743–755.
- Krippendorff K., (2004), Reliability in content analysis, Hum. Commun. Res., 30(3), 411–433.
- Laverty J. T., Underwood S. M., Matz R. L., Posey L. A., Carmel J. H., Caballero M. D., Fata-Hartley C. L., Ebert-May D., Jardeleza S. E. and Cooper M. M., (2016), Characterizing college science assessments: the three-dimentsional learning assessment protocol, PLoS One, 11(9), e0162333.
- Lillig J. W., (2008), Writing across the semester: a non-standard term paper that encourages critical data analysis in the upper-division chemistry classroom, J. Chem. Educ., 85(10), 1392.
- Loertscher J., Green D., Lewis J. E., Lin S. and Minderhout V., (2014), Identification of threshold concepts for biochemistry, CBE Life Sci. Educ., 13(3), 516–528.
- Lucas T. and Rowley N. M., (2011), Enquiry-based learning: experiences of first year chemistry students learning spectroscopy, Chem. Educ. Res. Pract., 12(4), 478–486.
- Mahn H. and John-Steiner V., (2008), The Gift of Confidence: A Vygotskian View of Emotions, in Learning for Life in the 21st Century, Blackwell Publishing, pp. 46–58.
- Margerum L. D., Gulsrud M., Manlapez R., Rebong R. and Love A., (2007), Application of calibrated peer review (CPR) writing assignments to enhance experiments with an environmental chemistry focus, J. Chem. Educ., 84(2), 292.
- Moore R., (1993), Does writing about science improve learning about science? J. Coll. Sci. Teach., 22(4), 212.
- Mowry C., Milofsky R., Collins W. and Pimentel A. S., (2017), Laser-induced breakdown spectroscopy for qualitative analysis of metals in simulated Martian soils, J. Chem. Educ., 94(10), 1507–1511.
- Park E. J. and Light G., (2009), Identifying Atomic Structure as a Threshold Concept: Student mental models and troublesomeness, Int. J. Sci. Educ., 31(2), 233–258.
- Pepper R. E., Chasteen S. V., Pollock S. J. and Perkins K. K., (2012), Observations on student difficulties with mathematics in upper-division electricity and magnetism, Phys. Rev. Spec. Top.-Phys. Educ. Res., 8(1), 010–111.
- Prior P., (2006), A sociocultural theory of writing, in Handbook of writing research, Guilford Press, pp. 54–66.
- Reilly J. T. and Strickland M., (2010), A writing and ethics component for a quantum mechanics, physical chemistry course, J. Coll. Sci. Teach., 39(4), 35.
- Reynolds J. A., Thaiss C., Katkin W. and Thompson R. J., (2012), Writing-to-learn in undergraduate Science Education: a community-based, conceptually driven approach, CBE-Life Sci. Educ., 11(1), 17–25.
- Rivard L. O. P., (1994), A review of writing to learn in science: implications for practice and research, J. Res. Sci. Teach., 31(9), 969–983.
- Shibley Jr I. A., Milakofsky L. K. and Nicotera C. L., (2001), Incorporating a substantial writing assignment into organic chemistry: library research, peer review, and assessment, J. Chem. Educ., 78(1), 50.
- Shultz G. V. and Gere A. R., (2015), Writing-to-Learn the Nature of Science in the Context of the Lewis Dot Structure Model, J. Chem. Educ., 92(8), 1325–1329.
- Singh C., (2001), Student understanding of quantum mechanics, Am. J. Phys., 69(8), 885–895.
- Smith T. I., Thompson J. R. and Mountcastle D. B., (2013), Student understanding of Taylor series expansions in statistical mechanics, Phys. Rev. Spec. Top.-Phys. Educ. Res., 9(2), 020110.
- Sreenivasulu B. and Subramaniam R., (2013), University students’ understanding of chemical thermodynamics, Int. J. Sci. Educ., 35(4), 601–635.
- Stains M. and Vickrey T., (2017), Fidelity of implementation: an overlooked yet critical construct to establish effectiveness of evidence-based instructional practices, CBE-Life Sci. Educ., 16(1), rm1.
- Stefani C. and Tsaparlis G., (2009), Students' levels of explanations, models, and misconceptions in basic quantum chemistry: a phenomenographic study, J. Res. Sci. Teach., 46(5), 520–536.
- Talanquer V., (2015), Threshold concepts in chemistry: the critical role of implicit schemas, J. Chem. Educ., 92(1), 3–9.
- Theobald R. and Freeman S., (2014), Is it the intervention or the students? Using linear regression to control for student characteristics in undergraduate STEM education research, CBE-Life Sci. Educ., 13(1), 41–48.
- Tsaparlis G., (2014), Linking the macro with the submicro levels of chemistry: demonstrations and experiments that can contribute to active/meaningful/conceptual learning, in Learning with understanding in the chemistry classroom, Springer Science and Business Media, Springer, Dordrecht, pp. 41–61.
- Tsaparlis G., (2016), The logical and psychological structure of physical chemistry and its relevance to graduate students' opinions about the difficulties of the major areas of the subject, Chem. Educ. Res. Pract., 17(2), 320–336.
- Vygotsky L. S., (1978), Mind in society: the development of higher psychological processes, Cambridge, MA: Harvard University Press.
- Whelan R. J. and Zare R. N., (2003), Teaching effective communication in a writing-intensive analytical chemistry course, J. Chem. Educ., 80(8), 904.
| This journal is © The Royal Society of Chemistry 2018 |