The development, validation, and interpretation of a content coding map for analyzing chemistry lessons in Chinese secondary schools
Changlong
Zheng
 *,
Langsen
Li
*,
Langsen
Li
 ,
Peng
He
,
Peng
He
 and
Mengying
Jia
and
Mengying
Jia
Institute of Chemical Education, Northeast Normal University, Changchun, Jilin 130024, China. E-mail: Zhengcl@nenu.edu.cn
First published on 27th October 2018
Abstract
Although the content of science lessons has been analyzed from different perspectives by developing a set of codes (e.g., K. J. Roth, S. L. Druker, H. E. Garnier, M. Lemmens, C. Chen, T. Kawanaka, and R. Gallimore, (2006), Teaching science in five countries: results from the TIMSS 1999 video study, Washington, DC: National Center for Education Statistics), none of the existing coding systems have investigated it from a subject-specific and dynamic perspective. Aiming to fill this gap, this study develops a content coding map (CCM) to classify the content of chemistry lessons (CCL) into 12 types based on their roles and values. The CCM was constructed based on semi-structured interviews and revised by applying the initial CCM to six videotaped lessons. Furthermore, the coding was reviewed by an expert committee to confirm the content validity and evaluated by 86 in-service teachers using a questionnaire with responses measured on a five-point Likert scale to test for face validity. These 12 types of CCL were organized into five related groups in the CCM: core knowledge and practices (CKP), connections among CKP (C-CKP), expansion of CKP (E-CKP), scaffolding for CKP (S-CKP), and meaningless content in relation to CKP (MC-CKP). Each group is illustrated and discussed using specific types of CCL. The CCM, which provides a new way to explore chemistry classrooms, can be used as an analytic tool for chemistry educators to investigate the CCL and can serve as a guide for chemistry teachers when designing lessons.
Introduction
Effective classroom teaching is a complex task that is influenced by various factors such as teaching behavior (e.g., van der Lans et al., 2018) and lesson content (e.g., Clough et al., 2009). Not only do teaching behavior and lesson content impact the effectiveness of classroom teaching, but also the degree to which teaching behavior is matched to lesson content is a crucial element in effective teaching (Zheng et al., 2014). Decisions regarding what content to teach and what type of behavior to adopt are interrelated and should be made in light of teaching objectives (Olson et al., 2016). For example, although curriculum frameworks and standards documents advocate scientific inquiry as an instructional approach (e.g., NGSS Lead States, 2013; Chinese Ministry of Education, 2017), inquiry-based teaching is not suitable for all types of content, and consideration needs to be given to time constraints and the availability of resources (Anderson, 2002; Crawford, 2007; Ramnarain and Schuster, 2014). Despite the fact that the degree to which teaching behavior is matched to lesson content is a vital issue, few previous studies have paid specific attention to this connection. To understand the relationship between these two elements, first it is necessary to clarify our understanding of each of them. Some existing studies have developed coding schemes and categorized rubrics to identify various types of teaching behavior (e.g., Ramnarain and Schuster, 2014; Schuster et al., 2018). However, we could only find a few coding schemes for classifying lesson content (Roth et al., 2006). Previous coding schemes in science education have only paid attention to an integrated science curriculum without considering the features of specific disciplines such as chemistry. It is important to classify the content of chemistry lessons, but an appropriate coding scheme for such a classification is not currently available. Thus, a coding scheme with an acceptable level of validity and reliability is needed. To address this gap, this study aims to develop a content coding map (CCM) to classify the content of chemistry lessons (CCL) into various types based on their roles and values. Broader knowledge of the CCL can serve as a guide for chemistry teachers to select and organize the content of lessons and has significant implications for education scholars undertaking research into lesson analysis.Previous studies
Numerous studies have developed coding schemes or rubrics to analyze lessons and have attracted considerable attention around the world. Many of the results in relation to lesson analysis were presented in the Learner's Perspective Study (LPS) (Clarke et al., 2006) and the Third International Mathematics and Science Study (TIMSS) (Stigler et al., 1999; Hiebert et al., 2003), which investigated lesson features in the international teaching community. However, the LPS only focused on mathematics classrooms, whereas the TIMSS included mathematics and science teaching. Because this study concentrates on chemistry lessons, the TIMSS is especially relevant.The TIMSS used two video-based studies in 1995 and 1999 to investigate and describe teaching practices. These supplemented the TIMSS 1995 and 1999 student assessments. The TIMSS 1999 Video Study (Hiebert et al., 2003; Roth et al., 2006) focused on mathematics and science lessons in an attempt to discover national patterns and international variations using a large sample of teachers in various countries. A conceptual framework was developed with the aim of capturing the various features of science lessons (see Fig. 1). The TIMSS conceptual framework regarded the lesson as the center of the investigation and emphasized the coordination of Schwab's four commonplaces of teaching: the learner, the teacher, the milieu, and the subject matter (Schwab, 1969, 1971, 1973). As shown in Fig. 1, a lesson is a complex system that includes interactions among the students’ actions, the teacher's actions, and the science content. In addition, these aspects of a lesson system are shaped by the larger culture (Roth et al., 2006).
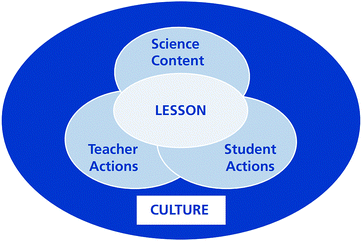 | ||
| Fig. 1 Conceptual framework of the TIMSS 1999 Video Study (Roth et al., 2006). | ||
In light of the framework, the TIMSS 1999 Video Study developed a set of codes to analyze various features of the students’ actions, the teacher's actions, and the science content. In particular, the content of science lessons was divided into six areas, each with its own code: (a) content disciplines and topics; (b) types of science knowledge; (c) source of the science content; (d) content coherence; (e) challenge presented by the content; and (f) multiple sets and types of evidence. More specifically, the content discipline categories included earth science, life science, physics, chemistry, and other areas, whereas content topics were coded using the TIMSS Guidebook to Examine School Curricula (McNeely, 1997). Science knowledge was divided into six types based on the coding scheme: canonical knowledge, knowledge of real-life issues, procedural and experimental knowledge, classroom safety knowledge, nature of science knowledge, and metacognitive knowledge. There were four sources of science content: the teacher, textbook or workbook, worksheet, and other sources. Content coherence was divided into three categories: doing activities with no conceptual links, learning content with weak or no conceptual links, and learning content with strong conceptual links. The challenge presented by the content was classified into three levels by the coding team: challenging content, basic and challenging content, and basic content. Multiple sets and types of evidence were captured by three indicators: more than one set of first-hand data, more than one phenomenon, and more than one visual representation (Roth et al., 2006).
Although the TIMSS 1999 Video Study classified the selected content of science lessons, the subject specified by the selected content of the science lessons was not mentioned in their set of codes. Furthermore, Roth et al. (2006) investigated the content of lessons from multiple static perspectives. Thus, developing an appropriate coding scheme using a dynamic perspective, specifically within the chemistry discipline, will enable us to generate new insights into analyzing chemistry lessons. In light of the TIMSS conceptual framework and the classification of science content, this study aims to develop a discipline-specific CCM to analyze the CCL.
Previous studies in the domain of chemistry education have developed various rubrics, coding schemes, or protocols to investigate classrooms, mainly focusing on two aspects: students’ actions and teachers’ actions. For example, regarding students’ actions, Crujeiras-Pérez and Jiménez-Aleixandre (2017) developed a rubric to analyze students’ planning investigations in the chemistry laboratory, Kulatunga et al. (2013) constructed a coding scheme to investigate the features of student group argumentation in a general chemistry course, and Seng and Hill (2014) established a coding scheme to identify several categories of peer feedback during chemistry investigative task discussion. In terms of teachers’ actions, Nehring et al. (2017) constructed two model-based coding schemes to investigate the complexity of teachers’ questions during chemistry instruction, while some studies (e.g., Herrington et al., 2011; Yezierski and Herrington, 2011) used the Reformed Teaching Observation Protocol to evaluate chemistry teachers’ instructional quality. Furthermore, several studies regarded the teacher and students as a unified system and developed coding schemes to investigate chemistry classrooms including both the students’ actions and the teacher's actions. For instance, Fay et al. (2007) constructed a rubric to distinguish among levels of inquiry in the undergraduate chemistry laboratory, which provided a standard for examining the laboratory curriculum, while Philipp et al. (2014) developed a protocol to evaluate the use of representations in secondary school chemistry lessons. Nevertheless, none of the existing coding schemes focuses on the lesson content, which is identified as an important element of lesson analysis in the TIMSS conceptual framework. Therefore, developing a CCM to classify the CCL should help to fill this gap in the literature.
The current study
Content of chemistry lessons
Although an integrated science curriculum has been promoted by emphasizing the unity of science (Lee, 1992; Cervetti et al., 2012), many countries (e.g., China and Israel) have insisted on teaching chemistry independently based on its discipline-specific features (Tümay, 2016) and developed chemistry curriculum standards such as the National Chemistry Curriculum Standard for Junior High School (NCCSJHS) (Chinese Ministry of Education, 2017). These national standards determine the core knowledge and practices (CKP), which set out what students should know and be able to do, and thus determine the most important content in lessons. However, “the vague nature of the required standard” (Olson et al., 2016, p. 6) has led teachers to reconsider their content selection and organization with respect to their unique teaching situation (DeBoer, 2011). In particular, it has been determined that some degree of scaffolding should be used in lessons to support students’ better understanding of CKP. One type of scaffolding, the developmental history of chemical concepts (e.g., the processes and results of classical experiments), is a commonly used CCL to support students in understanding the key concepts in chemistry (Hashweh, 1996; Ngai et al., 2014; do Amaral et al., 2018). For example, to teach the concept of atomic structure, a chemistry teacher might present the developmental history of atomic structure models, such as Dalton's atomic theory, the Thomson atomic model, the Rutherford atomic model, and the Bohr atomic model. In addition, the CCL in this study include other types of content. For instance, “explaining abstract concepts in chemistry” (Benny and Blonder, 2018, p. 122) is useful in enabling students to understand the foundations of chemical knowledge, while the use of socio-scientific issues or real-life experiences related to the CKP in chemistry as a stepping stone is appropriate for supporting students in learning these CKP (Sadler, 2004; Belova and Eilks, 2015). Experimental facts and phenomena (i.e., empirical data) are kinds of commonly used CCL when constructing chemical theories or deducing chemical laws (Harshman and Yezierski, 2015). Thus, there are various types of CCL, and classifying them provides a novel insight into chemistry lessons.Objectives of this study
The rubrics or coding schemes that have previously been applied in relation to chemistry education have not paid sufficient attention to content. Moreover, previous studies regarding the classification of content have concentrated on the integrated science curriculum instead of the specific discipline of chemistry, and have conducted their analyses from a static perspective (e.g., types of science knowledge, source of science content, and content coherence) (Roth et al., 2006). Based on the TIMSS conceptual framework, this study adopts a novel approach by developing a CCM from a dynamic perspective to classify the CCL into various types based on their roles and values.CCL in the context of Chinese secondary schools
For a better understanding of the aims of this study, it is necessary to introduce the context of secondary school chemistry classrooms in China, where the chemistry discipline is taught independently and a national chemistry standard has been formulated. The NCCSJHS (Chinese Ministry of Education, 2017) has determined that the chemistry curriculum in China includes a total of 11 topics which are generally taught in 10th and 11th grades. In most schools, students are organized into fixed classes with permanent classmates and teachers, and the number of students per class usually ranges from 40 to 60. Students usually attend four chemistry lessons each week, and each lesson lasts for 40 minutes.Each lesson in Chinese secondary schools contains a variety of content. In this regard, chemistry lessons can be divided into several “primitive units” that present fundamental content for further analysis. A primitive unit is a single concept related to either core knowledge or practices in which the teacher defines, states, describes, demonstrates, illustrates, explains, provides examples, or reviews a concept or skill. The following transcript provides an example of a primitive unit.
[Teacher] OK. Based on the conduction experiment, we know that some chemical compounds ionize and generate free-moving ions. Can somebody tell me whether the ionization is a chemical reaction?
[Teacher] What is the definition of a chemical reaction? Think about it in terms of the chemical bond.
[Student 1] Old bond breaking and new bond forming.
[Teacher] Then is the process of ionization a chemical reaction?
[Students] No.
[Teacher] Not a chemical reaction. The process of ionization only breaks the old bond without forming a new bond.
This primitive unit can be described as a discussion between a teacher and his/her students on the definition of a chemical reaction to confirm that ionization is not a chemical reaction. The present study strictly focuses on the content of each primitive unit which is regarded as the CCL. The CCL in the above episode can be labeled as “understanding ionization by reviewing the definition of a chemical reaction.” This study divides chemistry lessons into several primitive units, the content of each corresponding to one CCL.
Development and validation of the content coding map
Construction of the initial content coding map
To construct the initial content coding map, we conducted face-to-face interviews with four expert teachers and two chemistry educators. Each of the four expert teachers had at least 20 years of teaching experience and held a master's degree, while the two chemistry educators had a doctorate in chemistry education and held tenured university positions. Because of their expertise in both the theory and practice of teaching and learning chemistry, we explored their views on the classification of the CCL.Iterative preliminary interviews were conducted to develop the following interview questions:
(1) What kinds of CCL do teachers present in teaching secondary school chemistry?
(2) What are the roles and values of these CCL?
(3) How do you classify these CCL in terms of their roles and values?
Given these questions, all interviewees were asked to watch a videotaped chemistry lesson, after which they answered the questions in the context of the content-specific lesson. Their views on the roles and values of the CCL were labeled as an indicator pool for the initial CCM. All interviews were video-recorded and transcribed verbatim, and permission was obtained from all interviewees for the interviews to be used in this study. The development of this initial CCM was undertaken iteratively by analyzing the results of the interviews. The initial CCM, with 16 types of CCL, is presented in the first column in Table 1.
| Types of CCL (initial version) | Types of CCL (final version) | Treatments |
|---|---|---|
| Note: to differentiate between the initial and final versions, we have attached asterisks to the initial items (e.g., CKP-1*). CKP = core knowledge and practices; C-CKP = connections of CKP; S-CKP = scaffolding for CKP; E-CKP = expansion of CKP; MC-CKP = meaningless content in relation to CKP. | ||
| CKP-1* Chemistry core knowledge as the main properties of typical chemical substances | CKP-1 Chemistry core knowledge as the main properties of typical chemical substances | — |
| CKP-2* Chemistry core knowledge as concepts, theories, and laws | CKP-2 Chemistry core knowledge as concepts, theories, and laws | — |
| CKP-3a* Chemistry core practices as laboratory skills | CKP-3 Chemistry core practices as basic skills | Revised (combine laboratory skills, chemical symbol representation skills, and stoichiometry as the basic skills of chemistry) |
| CKP-3b* Chemistry core practices as chemical symbol representation skills | ||
| CKP-3c* Chemistry core practices as stoichiometry | ||
| CKP-4* Chemistry core practices as scientific inquiry | CKP-4 Chemistry core practices as scientific inquiry | — |
| C-CKP-1a* Explanations of chemical-related phenomena using chemistry core knowledge and practices | C-CKP-1 Applications of chemistry core knowledge and practices | Revised (combine explanations of phenomena and problem-solving in the applications of core knowledge and practices) |
| C-CKP-1b* Chemical-related problem-solving using chemistry core knowledge and practices | ||
| C-CKP-2* Clarifying the connections among chemistry core knowledge and practices | C-CKP-2 Systematic networks between chemistry core knowledge and practices | Revised (modify expression to be more concise) |
| S-CKP-1a* Chemical-related evidence scaffolding for students learning chemistry core knowledge and practices | S-CKP-1 Directly supporting students’ learning of chemistry core knowledge and practices | Revised (combine chemistry evidence and theoretical reasoning as direct support for students’ learning, corresponding to indirect support in S-CKP-2) |
| S-CKP-1b* Chemistry theoretical reasoning scaffolding for students learning chemistry core knowledge and practices | ||
| S-CKP-2* Stepping stones for students learning chemistry core knowledge and practices | S-CKP-2 Indirectly supporting students’ learning of chemistry core knowledge and practices | Revised (modify the expression to correspond to S-CKP-1 and provide more clarity) |
| E-CKP-1a* Explanations to help students’ understanding of chemistry core knowledge and practices | E-CKP-1 Promoting students’ understanding of chemistry core knowledge and practices | Revised (combine teachers’ explanations and illustrations to promote students’ learning) |
| E-CKP-1b* Illustrations to assist students’ understanding of chemistry core knowledge and practices | ||
| E-CKP-2* Extensions of chemistry core knowledge and practices | E-CKP-2 Reasonable extensions of chemistry core knowledge and practices | Revised (extension was classified into reasonable and unreasonable parts) |
| MC-CKP-1* Inappropriate content for learning chemistry core knowledge and practices | MC-CKP-1 Inappropriate content for learning chemistry core knowledge and practices | — |
| — | MC-CKP-2 Unreasonable extensions of chemistry core knowledge and practices | Added (derived from E-CKP-2*) |
Revision of the initial content coding map
The next stage was to confirm the appropriateness of the initial CCM in relation to topics taught in chemistry lessons. Therefore, six videotaped chemistry lessons were selected covering the main topics outlined in the content standards of the NCCSJHS (Chinese Ministry of Education, 2017). These lessons were collected from a university–government–school (UGS) initiative in northeast China. The six lessons were broken down into 146 CCLs, and the initial CCM was used to analyze these lessons and determine whether the indicators were able to accurately code the CCL. The types and percentages of CCL in the six lessons are shown in Table 2.| Types of CCL | Lesson | Total | Percentage (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Sodium | Aluminum hydroxide | Ionization | Reaction rate | Primary battery | Proteins | |||
| Note: CKP = core knowledge and practices; C-CKP = connections of CKP; S-CKP = scaffolding for CKP; E-CKP = expansion of CKP; MC-CKP = meaningless content in relation to CKP. | ||||||||
| CKP-1* Chemistry core knowledge as the main properties of typical chemical substances | 6 | 3 | 4 | 4 | 3 | 3 | 23 | 15.75 |
| CKP-2* Chemistry core knowledge as concepts, theories, and laws | 1 | 0 | 3 | 1 | 1 | 2 | 8 | 5.48 |
| CKP-3a* Chemistry core practices as laboratory skills | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 2.05 |
| CKP-3b* Chemistry core practices as chemical symbol representation skills | 0 | 1 | 1 | 1 | 1 | 0 | 4 | 2.74 |
| CKP-3c* Chemistry core practices as stoichiometry | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1.37 |
| CKP-4* Chemistry core practices as scientific inquiry | 1 | 1 | 2 | 1 | 2 | 0 | 7 | 4.79 |
| C-CKP-1a* Explanations of chemical-related phenomena using chemistry core knowledge and practices | 0 | 0 | 2 | 1 | 2 | 3 | 8 | 5.48 |
| C-CKP-1b* Chemical-related problem-solving using chemistry core knowledge and practices | 0 | 3 | 0 | 1 | 0 | 0 | 4 | 2.74 |
| C-CKP-2* Clarifying the connections among chemistry core knowledge and practices | 5 | 2 | 3 | 1 | 1 | 2 | 14 | 9.59 |
| S-CKP-1a* Chemical-related evidence scaffolding for students learning chemistry core knowledge and practices | 2 | 0 | 1 | 1 | 2 | 0 | 6 | 4.11 |
| S-CKP-1b* Chemistry theoretical reasoning scaffolding for students learning chemistry core knowledge and practices | 1 | 0 | 0 | 0 | 1 | 2 | 4 | 2.74 |
| S-CKP-2* Stepping stones for students learning chemistry core knowledge and practices | 6 | 3 | 3 | 2 | 3 | 3 | 20 | 13.70 |
| E-CKP-1a* Explanations to help students’ understanding of chemistry core knowledge and practices | 2 | 4 | 5 | 4 | 5 | 3 | 23 | 15.75 |
| E-CKP-1b* Illustrations to assist students’ understanding of chemistry core knowledge and practices | 0 | 1 | 2 | 0 | 2 | 1 | 6 | 4.11 |
| E-CKP-2* Extensions of chemistry core knowledge and practices | 0 | 1 | 2 | 0 | 1 | 2 | 6 | 4.11 |
| MC-CKP-1* Inappropriate content for learning chemistry core knowledge and practices | 0 | 2 | 0 | 2 | 1 | 3 | 8 | 5.48 |
| Total | 25 | 21 | 29 | 21 | 26 | 24 | 146 | 100.00 |
From Table 2, it can be seen that the percentages of some types of CCL, such as CKP-3a*, CKP-3b*, and CKP-3c*, were less than 5%, which means that those types may not emerge frequently in secondary school chemistry lessons. Therefore, two rules were applied to refine the initial CCM: the percentages of all types of CCL should be higher than 5%, and some types of CCL with low percentages can be combined based on their similar roles and values. For example, we combined CKP-3a*, CKP-3b*, and CKP-3c* into a single type of CCL, CKP-3, which had a percentage of 6.16%. The details of the treatments and changes can be seen in Table 1. In particular, we further divided E-CKP-2* into E-CKP-2 and MC-CKP-2 because not all extensions of chemistry CKP are reasonable. After reviewing all six lessons, the statements and number of indicators were revised to reflect the context of the chemistry lessons.
Validation and format of the final content coding map
An expert panel was established to validate the content of the revised CCM, a method that has been adopted in previous studies (e.g., Abd-El-Khalick et al., 2015; Çiçek and Ilhan, 2017). The panel included three expert teachers (all of whom were involved in the initial stage) and three chemistry educators (two of whom were involved in the initial stage). All panel members were informed of the reason for the development of the CCM and were asked to review the revised CCM based on the results of the analysis of the six videotaped lessons. Their opinions regarding the indicators in the revised version were used to further refine the final CCM.To guarantee the face validity of the final CCM, a questionnaire was distributed to 86 in-service chemistry teachers who were undertaking a national professional development program, and who were willing to provide their opinions voluntarily. Responses were measured on a five-point Likert-type scale where 5 = “Strongly agree” and 1 = “Strongly disagree”. Teachers from 27 provinces in China were selected by their local education bureau as outstanding representatives of their profession. Details regarding the demographics of these teachers are presented in Table 3.
| Frequency | Percent (%) | |
|---|---|---|
| Gender | ||
| Female | 41 | 47.7 |
| Male | 45 | 52.3 |
| Total | 86 | 100.0 |
| Teaching years | ||
| 1–5 | 4 | 4.7 |
| 6–10 | 10 | 11.6 |
| 11–15 | 20 | 23.3 |
| 16–20 | 17 | 19.8 |
| 21–25 | 22 | 25.6 |
| 26–30 | 11 | 12.8 |
| 31–35 | 2 | 2.3 |
| Total | 86 | 100.0 |
| Region | ||
| North China | 10 | 11.6 |
| Northeast China | 6 | 7.0 |
| East China | 24 | 27.9 |
| South Central China | 21 | 24.4 |
| Northwest China | 10 | 11.6 |
| Southwest China | 15 | 17.4 |
| Total | 86 | 100.0 |
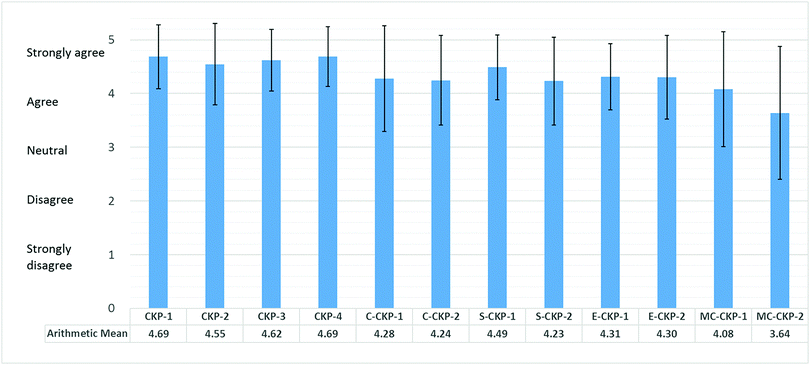
Responses of “Strongly agree” (5) and “Agree” (4) indicated that the respondents agreed with the category for CCL, responses of “Strongly disagree” (1) and “Disagree” (2) indicated that they did not agree with the category for CCL, and a response of “Neutral” (3) meant that they were unsure. Scores above 3 were taken to signify their agreement with the rationality of the coding items (Arends-Tóth et al., 2006). The mean score and standard deviation were calculated for each item and are presented in Fig. 2. It can be seen that the mean scores for 11 of the 12 items were higher than “Agree” (4), and even the lowest mean score (for item E2) was above the midpoint. These responses provide strong evidence supporting the final CCM. Thus, the final CCM items (see the second column in Table 1) were determined.
Interpretation of the content coding map
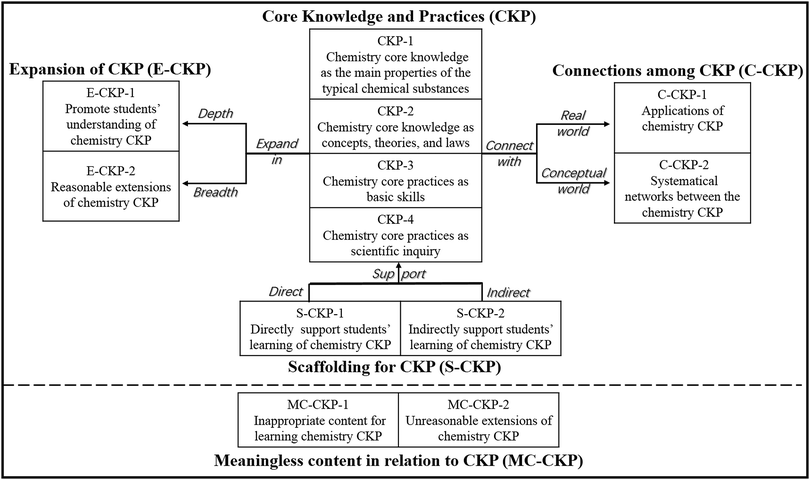
This study was the first attempt to classify the CCL into 12 types. To enable a more systematic approach, these 12 types were divided into five groups based on their roles and values: core knowledge and practices (CKP), connections among CKP (C-CKP), scaffolding for CKP (S-CKP), expansion of CKP (E-CKP), and meaningless content in relation to CKP (MC-CKP). The relationships between these groups are shown in Fig. 3. The aim of chemistry teaching is to help students to understand chemistry CKP. To ensure that students grasp chemistry CKP, teachers always provide some forms of scaffolding for students to support their learning (i.e., S-CKP) and expand the depth (i.e., E-CKP-1) and breadth (i.e., E-CKP-2) of the CKP to improve learning effectiveness (Sawyer, 2005; Yuriev et al., 2017). Furthermore, connecting those CKP to both the real world (i.e., C-CKP-1) and the conceptual world (i.e., C-CKP-2) is a vital aspect of lessons (Broman and Parchmann, 2014; Talanquer and Pollard, 2010). In addition to this valuable content, chemistry classrooms occasionally include irrelevant content (i.e., MC-CKP), which has no positive effect on students’ understanding of CKP. This content is separated by a dashed line in Fig. 3. In this section, each group of specific types of CCL was interpreted in more detail using examples from the video-recorded lessons that were collected in the process of revising the initial CCM.Core knowledge and practices
“The focus of the lessons is the core knowledge and practices, which are determined by the curriculum standards” (expert teacher, interview). Many countries have developed standards documents (e.g., NGSS Lead States, 2013; Chinese Ministry of Education, 2017) to determine content and to guide instruction and assessment (Liu et al., 2009). The context for this study is the chemistry standard published in China. The NCCSJHS (Chinese Ministry of Education, 2017) formulated 11 topics, each of which comprised three parts: content requirements, achievement expectations, and teaching suggestions. Content requirements define what is important for students to know and do (Schmidt et al., 2005), while achievement expectations stipulate the specific level of achievement that students should reach with respect to the content requirements. Content requirements mainly involve factual knowledge, conceptual knowledge, chemistry skills, and scientific inquiry (Chinese Ministry of Education, 2017).Factual knowledge, which is labeled CKP-1, refers to the main properties (e.g., redox properties and acid–base properties) of typical chemical substances (e.g., sodium and aluminum hydroxide). Conceptual knowledge, which is labeled CKP-2, includes chemical concepts (e.g., electrolysis and ionization), theories (e.g., atomic theory and transition-state theory), and laws (e.g., the ideal gas law). Factual and conceptual knowledge can be combined to form chemistry core knowledge, which summarizes what is important for students to know, including the facts, concepts, theories, and laws of chemistry.
In addition to this knowledge, the chemistry skills, which are labeled as CKP-3, that were mentioned by the NCCSJHS (Chinese Ministry of Education, 2017) include laboratory skills (Taber, 2016), chemical symbol representation skills (Wang et al., 2017), and stoichiometry (Gulacar et al., 2013), for instance, configuring solutions, writing ionic equations, and calculating reaction rates. Moreover, the NCCSJHS emphasized the importance of scientific inquiry, which is labeled as CKP-4, in the list of content requirements. To be specific, under the topic of Chemistry and experimental inquiry, the NCCSJHS stated that students should “know the key elements of the process of scientific inquiry such as asking questions, proposing a hypothesis…constructing conclusions, and evaluating findings” (p. 12). Thus, scientific inquiry should be regarded as a vital component of chemistry lessons (Herrington et al., 2011; Vhurumuku, 2011). For example, a teacher guided students in exploring the factors influencing the reaction rate by designing and analyzing a controlled trial. Considering that chemistry skills and scientific inquiry are relevant to real-world practice, they should be integrated into the core chemistry practices, which include what is important for students to do.
Connections among CKP
To retain CKP and make them meaningful, students should attempt to connect them to both the real world (i.e., applying them) and the conceptual world (i.e., forming a systematic network). In this regard, C-CKP-1 and C-CKP-2 are crucial aspects of chemistry education (Talanquer and Pollard, 2010; Khaddoor et al., 2017; Mahaffy et al., 2017).Several studies noted that connections to real-life contexts (i.e., C-CKP-1) were important in helping students to retain knowledge (e.g., Talanquer and Pollard, 2010; Broman and Parchmann, 2014; Eilks and Byers, 2015). Based on the level of difficulty of questions posed, applications of chemistry CKP can be divided into two levels: low-order and high-order. In most lessons in Chinese secondary schools, applications usually involve the transfer of CKP to a simple question or problem, which is regarded as low-order application. For example, after the teacher introduced the components and mechanism of a primary battery, the teacher provided two redox reactions and students were asked to “design two primary batteries with these two reactions and draw the device diagrams on the draft paper” (teacher of primary battery lesson, video-recorded lesson). This type of application aims to “give students a chance to become familiar with the knowledge we (teachers) have talked about and the students’ performances can be seen as feedback for me to evaluate whether I taught it clearly” (expert teacher, interview).
In terms of high-order applications, one chemistry educator stated that “our daily lessons are full of simple applications instead of high-order and integrated applications…some of the better schools would spend a specific amount of time during a semester designing project-based learning or problem-based learning” (chemistry educator, interview) and high-order applications exist in these particular learning processes. High-order applications, which are an effective “catalyst” for student progress, refer to complex and challenging problems in an unfamiliar situation. For example, the teacher might assign a task requiring students to modify a dry cell battery after introducing the concept of dry cell batteries. Nevertheless, these high-order applications are seldom used in the context of chemistry education in mainland China, and we did not find any high-order applications in the sample lessons used in this study.
Regarding C-CKP-2, a systematic network involves the establishment of relationships in the conceptual world. Previous studies (e.g., Whitehead, 1959; Bodner, 2007; Johnstone, 2009) have indicated that chemistry students face the challenge of “being able to see the woods while avoiding being lost in the trees.” Some students only memorize isolated clusters of chemistry CKP, and thus are less likely to retain the CKP and transfer them to complex problems. In this case, connecting the isolated CKP into systematic networks in classrooms (i.e., C-CKP-2) is useful in enabling students to construct an integrated chemistry world. In Chinese secondary schools, C-CKP-2 generally emerges after students have grasped the CKP, and acts as a supplement or summary that helps to strengthen the students’ understanding. In one of the video-recorded lessons, a summary at the end of the lesson provided a good example.
[Teacher] OK! Let us go back to the question at the beginning. What are the factors influencing the reaction rate?
[Student 1] Temperature and concentration
[Student 2] Pressure and surface area
[Teacher] Anything else?
[Student 2] Catalysts
[Teacher] Ok! Can anybody tell us what the relationships are between these factors?…The occurrence of a chemical reaction can be regarded as a certain amount of particles having a certain energy colliding with each other. The reaction occurs when the collision process reaches the energy required for the reaction to occur…Temperature affects the energy of the particles. These three factors (concentration, pressure, and surface area) influence the number of particles that may participate in the reaction. In terms of the catalyst, it affects the energy required for the reaction to occur. (teacher of reaction-rate lesson, video-recorded lesson)
This episode showed a teacher helping students to understand the relationships between various factors influencing the reaction rate, so that students did not merely remember isolated factors. What should be noted here is that the networks are hierarchical. The example we provided clarified the relationships between five elements involved in one concept (i.e., the factors influencing the reaction rate). High-level networks such as relationships between different concepts (e.g., chemical equilibrium and the reaction rate) or even big ideas (e.g., energy and reactions) were suggested. With all these aspects in mind, assisting students in forming systematic conceptual networks is an indispensable part of teaching in chemistry classrooms.
Scaffolding for CKP
The scaffolding provided by teachers plays a key role in supporting students in their understanding of CKP (Hilton and Nichols, 2011). In this study, they were divided into direct support (i.e., S-CKP-1) and indirect support (i.e., S-CKP-2) of students’ learning based on their degree of relevance to CKP.More specifically, S-CKP-1 contains both evidentiary support and theoretical support. Evidentiary support refers to the data used by teachers to support students’ understanding of CKP. The forms these data take mainly include the process and results of classical experiments (e.g., Rutherford's gold foil experiment), existing data (e.g., the melting points for various metals), and experimental data from actual observations and measurements by students (e.g., the pH of various solutions). These data “can be used as scaffolding to build conceptual understanding in chemistry” (Nichol et al., 2014, p. 1318). For example, a teacher conducted an experiment to support students’ understanding of the properties of sodium. The details were as follows.
[Teacher] What happens to sodium when it encounters water?…Take a small piece of sodium and use filter paper to suck up the kerosene, and then put it into water that has been added to phenolphthalein. The reaction between sodium and water is intense, so we must strictly control the amount of sodium. [Teacher puts the sodium into the water]
[Students] Wow!
[Teacher] What have you observed?
[Student] It swims randomly.
[Teacher] And?
[Student] The water turns red.
[Teacher] Anything else?…Pay attention to the position of the sodium. You can see it floating on the water. And look at its shape. It has melted into a ball…Why did these things happen? (Teacher of the sodium lesson, video-recorded lesson)
Swimming, reddening, floating, and melting were the qualitative data that provided students with a better opportunity to fully understand this reaction and the chemistry involved compared with just a verbal description. In addition to qualitative data, quantitative data are also commonly used in chemistry lessons. For example, Glazier et al. (2010) used data on boiling points to support students’ learning in relation to intermolecular forces. Evidentiary support “offers several significant advantages in assisting teachers to focus on concept development” (Nichol et al., 2014, p. 1318). As for theoretical support, this refers to the fundamental theories used to support students’ learning. For instance, simple collision theory was introduced by a teacher to support students in understanding the factors influencing the reaction rate. In conclusion, these types of support (i.e., S-CKP-1) “are essential for students to construct new concepts” (expert teacher, interview).
S-CKP-2 refers to the content that paves the way for learning CKP, whereby values and roles are designed to help the learning process occur as a smooth transition. For example, the teacher introduced the importance of proteins and the origin of the word “protein” to indirectly support students in learning the properties and structures of the various proteins (i.e., the CKP). The details were as follows.
[Teacher] The food we eat every day not only provides us with sugar, as we learned before, but also supplies us with proteins. Proteins are an essential component of our cells and are necessary for regeneration and functioning. They were first discovered in the 19th century, and scientists regarded them as the essence of life, so they were named after the Greek word “proteions,” meaning first or primary. (teacher of proteins lesson, video-recorded lessons)
This part of the lesson was the prelude, and was labeled as indirect support in this study because it helped students to construct the CKP related to proteins. In this case, compared with direct support (i.e., evidence and basic theories), there is a relatively weak correlation between indirect support and the construction of CKP. With all this in mind, S-CKP-1 is like a “pillar” (expert teacher, interview) and S-CKP-2 is like a “stepping stone” (expert teacher, interview), which together are regarded as scaffolding to support students’ learning.
Expansion of CKP
The CKP presented in the chemistry textbooks are bounded by a certain depth and breadth. Some novice chemistry teachers, particular in China, “rely heavily on textbooks to determine their teaching content” (Chen and Wei, 2015, p. 262), so the CCL in their classrooms do not venture beyond the textbook in terms of both depth and breadth. However, teachers should demonstrate flexibility in expanding the CKP where appropriate to coincide with the needs of their students. On the one hand, teachers are advised to deepen the meaning of CKP, that is, to move “beyond the surface features” (Coppola and Krajcik, 2014, p. 687). On the other hand, a reasonable increase in the breadth of CKP would enrich students’ conceptualization in relation to a given topic. In this case, the expansion of CKP can be divided into two types based on the direction of expansion, i.e., depth or breadth: E-CKP-1 and E-CKP-2, respectively.More specifically, E-CKP-1 means that teachers promote students’ deeper understanding of CKP. The format for this type of CCL mainly includes explanations or illustrations by the teacher. For example, textbooks define an electrolyte as any compound that conducts electricity when melted or dissolved in water, but one teacher explained an electrolyte as “any compounds that break their chemical bonds and form ions owing to the energy released by melting or dissolving in water” (teacher of ionization lesson, video-recorded lesson). The definition in the textbooks captures the electrolyte from a macroscopic perspective, whereas the teacher explained it at the microscopic level. These kinds of explanations are useful for promoting and deepening students’ understanding of CKP. Since chemistry always adopts various models and theories to explain empirical data (Carr, 1984; Taber and Watts, 2000) or uses microscopic entities to explain the macroscopic world (Johnstone, 2000; Tümay, 2016), insightful explanations or illustrations often appear in chemistry classrooms. As shown in Table 2, the percentage of E-CKP-1 was 19.86% over the six video-recorded lessons. Teachers “pay great attention to explanations or illustrations” (expert teacher, interview) for promoting students’ learning.
E-CKP-2 refers to reasonable extensions of CKP that are not highlighted by the curriculum standards but are nonetheless conducive to students’ understanding of CKP. For example, after learning about the differences between reactions involving sodium and oxygen under different conditions (i.e., room temperature and higher temperatures), the teacher added that “the product of a reaction between lithium and oxygen is always lithium oxide, regardless of whether the reaction occurs at room temperature or at higher temperatures” (teacher of the sodium lesson, video-recorded lessons). The lack of differences in the reaction between lithium and oxygen under different temperatures is not mentioned in the standards document but can be used as a comparison with the outcomes of reactions between sodium and oxygen to enrich students’ learning in terms of reactions between alkali metals and oxygen, which is considered a reasonable extension. This type of CCL is appropriate for enriching students’ conceptual breadth.
Meaningless content in relation to CKP
In contrast with the four groups mentioned above, this group includes content that is considered meaningless for students wanting to understand CKP, including inappropriate content (i.e., MC-CKP-1) and unreasonable extensions (i.e., MC-CKP-2). MC-CKP-1 was mentioned by the interviewees based on their experiences and MC-CKP-1 was separated from E-CKP-2* in the process of revising the initial version of the CCM. More specifically, MC-CKP-1 occurs when content is either too difficult for students to learn or too simple to be useful. For example, when discussing the reaction between chlorine and water, the teacher stated that the reaction between chlorine and water was a disproportionation reaction, which exceeds the requirements of the curriculum standard and is too difficult for students to understand.As for MC-CKP-2, this involves unreasonable extensions that do not help students to understand CKP. For instance, following the experiment that involved heating sodium, one student noticed that the resulting yellow product was accompanied by some black material and asked the teacher what it was. In responding, the teacher paid too much attention to explaining the insufficient combustion of kerosene. The aim of the experiment was to provide qualitative data for students to support their understanding of the properties of sodium, and the teacher should simply have responded to the student's question by, for example, stating that “the black material is the impurities from the kerosene combustion, and students who are interested in this can investigate it further after class,” instead of providing an elaborate explanation of the process of producing the black material, which was regarded as an unreasonable extension.
Conclusions and discussion
One of the basic competences of teaching is to know how to select and organize lesson content that is appropriate to the students’ learning requirements (Cai and Wang, 2006). Thus, the classification of content is a fundamental requirement for analyzing and designing lessons. This study developed a CCM that classifies the CCL into 12 types based on their roles and values. Previous studies investigating lesson content (e.g., Roth et al., 2006) have only focused on the integrated science curriculum, rather than paying attention to subject-specific scenarios. This study is the first attempt to analyze lesson content in chemistry classrooms. Further, previous studies have classified content into various types based on a static rather than a dynamic perspective. For example, Roth et al. (2006) divided types of science knowledge into six categories: canonical knowledge, knowledge of real-life issues, procedural and experimental knowledge, classroom safety knowledge, nature of science knowledge, and metacognitive knowledge. This classification captured the content that students should know and what they should be able to do, similar to the CKP in this study. However, we classified the CCL by paying attention to the dynamic process of classrooms and identifying the various roles and values in the students’ learning process. Nevertheless, compared with the work of Roth et al. (2006), it should be noted that the CCM does not include nature of science and metacognition, which are regarded as two important components in general science education, as well as chemistry teaching (Demirdöğen and Uzuntiryaki-Kondakçı, 2016). This study is limited to chemistry classrooms in Chinese high schools, so this is evidence that these classrooms lack these two elements. Thus, professional development programs should make greater efforts to improve teachers’ conceptualization in regard to nature of science and metacognition and encourage them to engage these two aspects in their classrooms (Deng et al., 2014; Wang and Zhao, 2016).With all this in mind, a discipline-specific and dynamic perspective is the highlight of this study. Chemistry educators can use the CCM as an analytic tool to analyze the content of chemistry lessons, while chemistry teachers, especially those who only have a little teaching experience, can use the CCM to guide them in selecting and organizing the CCL.
The analysis of CCL include three main aspects: frequency (i.e., the proportion of each type of CCL in lessons), arrangement (i.e., the sequence of different types of CCL), and time allocation (i.e., the time spent on different types of CCL). With the aim of exploring the appropriate allocation of time to different CCL, future research can be undertaken wherein a large sample of high-quality (i.e., award-winning) lessons is collected and analyzed in terms of time allocation. In addition, these high-quality lessons can be used to investigate patterns in terms of frequency and sequencing to identify whether there are more effective ways of selecting and organizing the CCL. Any patterns that emerge from a study of these high-quality lessons will not only support chemistry teachers in their lesson design but also provide a benchmark for chemistry educators when evaluating lessons.
In terms of future research, after developing the CCM, one question that was raised in the Introduction section remains unanswered: what kinds of teaching behavior are appropriate for various types of content? This is a vital factor influencing the effectiveness of teaching (Zheng et al., 2014; He et al., 2016). Using the classifications in this CCM, the relationship between teaching behaviors and types of CCL should be investigated in future studies with the aim of identifying the most effective mode of classroom teaching.
Moreover, future studies should apply this CCM to analyzing the links between content patterns of chemistry lessons and various factors such as culture, teaching experience, curriculum materials, and the level of the teachers’ pedagogical knowledge.
In summary, this study developed a CCM that enables the analysis of the CCL in Chinese high schools from a subject-specific and dynamic perspective. The development and validation process used to create this CCM might be applicable to other science disciplines such as physics and biology.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper is based upon work supported by National Education Sciences Planning Program (General Category) under Grant No. BHA170131, which titled “Research on Promoting Teachers’ Competency-based Classroom Teaching Ability”. Any opinions, findings, and conclusions or recommendations expressed in the materials are those of the authors and do not necessarily reflect the views of the National Education Sciences Planning Program. In addition, the authors would like to thank Prof. Xiufeng Liu for his insightful suggestions to the research described in this paper.References
- Abd-El-Khalick, F., Summers, R., Said, Z., Wang, S. and Culbertson, M., (2015), Development and large-scale validation of an instrument to assess Arabic-speaking students' attitudes toward science, Int. J. Sci. Educ., 37(16), 2637–2663.
- Anderson, R. D., (2002), Reforming science teaching: What research says about inquiry, J. Sci. Teach. Educ., 13(1), 1–12.
- Arends-Tóth, J., Van de Vijver, F. J. and Poortinga, Y. H., (2006), The influence of method factors on the relation between attitudes and self-reported behaviors in the assessment of acculturation, Eur. J. Psychol. Assess., 22(1), 4–12.
- Belova, N. and Eilks, I., (2015), Learning with and about advertising in chemistry education with a lesson plan on natural cosmetics–a case study, Chem. Educ. Res. Pract., 16(3), 578–588.
- Benny, N. and Blonder, R., (2018), Interactions of chemistry teachers with gifted students in a regular high-school chemistry classroom, Chem. Educ. Res. Pract., 19(1), 122–134.
- Bodner, G. M., (2007), Strengthening conceptual connections in introductory chemistry courses, Chem. Educ. Res. Pract., 8(1), 93–100.
- Broman, K. and Parchmann, I., (2014), Students' application of chemical concepts when solving chemistry problems in different contexts, Chem. Educ. Res. Pract., 15(4), 516–529.
- Cai, J. and Wang, T., (2006), US and Chinese teachers' conceptions and constructions of representations: A case of teaching ratio concept, Int. J. Sci. Math. Educ., 4(1), 145–186.
- Carr, M., (1984), Model confusion in chemistry, Res. Sci. Educ., 14(1), 97–103.
- Cervetti, G. N., Barber, J., Dorph, R., Pearson, P. D. and Goldschmidt, P. G., (2012), The impact of an integrated approach to science and literacy in elementary school classrooms, J. Res. Sci. Teach., 49(5), 631–658.
- Chen, B. and Wei, B., (2015), Examining chemistry teachers' use of curriculum materials: in view of teachers' pedagogical content knowledge, Chem. Educ. Res. Pract., 16(2), 260–272.
- Chinese Ministry of Education, (2017), National chemistry curriculum standard for junior high school, Beijing: Beijing Normal University Press, (in Chinese).
- Çiçek, Ö. and Ilhan, N., (2017), Evaluating interest in acids–bases: development of an acid–base interest scale (ABIS) and assessment of pre-service science teachers' interest, Chem. Educ. Res. Pract., 18(4), 630–640.
- Clarke, D., Emanuelsson, J., Jablonka, E. and Mok, I. A., (2006), Making connections: Comparing mathematics classroom around the world, Rotterdam, The Netherlands: Sense Publishers.
- Clough, M. P., Berg, C. A. and Olson, J. K., (2009), Promoting effective science teacher education and science teaching: A framework for teacher decision-making, Int. J. Sci. Math. Educ., 7(4), 821–847.
- Coppola, B. P. and Krajcik, J. S., (2014), Discipline-centered post-secondary science education research: Distinctive targets, challenges and opportunities, J. Res. Sci. Teach., 51(6), 679–693.
- Crawford, B. A., (2007), Learning to teach science as inquiry in the rough and tumble of practice, J. Res. Sci. Teach., 44(4), 613–642.
- Crujeiras-Pérez, B. and Jiménez-Aleixandre, M. P., (2017), High school students' engagement in planning investigations: findings from a longitudinal study in Spain, Chem. Educ. Res. Pract., 18(1), 99–112.
- DeBoer, G. E., (2011), The globalization of science education. J. Res. Sci. Teach., 48(6), 567–591.
- Demirdöğen, B. and Uzuntiryaki-Kondakçı, E., (2016), Closing the gap between beliefs and practice: Change of pre-service chemistry teachers' orientations during a PCK-based NOS course, Chem. Educ. Res. Pract., 17(4), 818–841.
- Deng, F., Chai, C. S., Tsai, C. C. and Lin, T. J., (2014), Assessing South China (Guangzhou) high school students’ views on nature of science: A validation study, Sci. Educ., 23(4), 843–863.
- do Amaral, E. M. R., da Silva, J. R. R. T. and Sabino, J. D., (2018), Analysing processes of conceptualization for students in lessons on substance from the emergence of conceptual profile zones, Chem. Educ. Res. Pract., 19(4), 1010–1028.
- Eilks, I. and Byers, B. (ed.), (2015), Innovative methods of teaching and learning chemistry in higher education, Cambridge: Royal Society of Chemistry.
- Fay, M. E., Grove, N. P., Towns, M. H. and Bretz, S. L., (2007), A rubric to characterize inquiry in the undergraduate chemistry laboratory, Chem. Educ. Res. Pract., 8(2), 212–219.
- Glazier, S., Marano, N. and Eisen, L., (2010), A closer look at trends in boiling points of hydrides: Using an inquiry-based approach to teach intermolecular forces of attraction, J. Chem. Educ., 87(12), 1336–1341.
- Gulacar, O., Overton, T. L., Bowman, C. R. and Fynewever, H., (2013), A novel code system for revealing sources of students' difficulties with stoichiometry, Chem. Educ. Res. Pract., 14(4), 507–515.
- Harshman, J. and Yezierski, E., (2015), Guiding teaching with assessments: high school chemistry teachers' use of data-driven inquiry, Chem. Educ. Res. Pract., 16(1), 93–103.
- Hashweh, M. Z., (1996), Effects of science teachers' epistemological beliefs in teaching, J. Res. Sci. Teach., 33(1), 47–63.
- He, P., Liu, X., Zheng, C. and Jia, M., (2016), Using Rasch measurement to validate an instrument for measuring the quality of classroom teaching in secondary chemistry lessons, Chem. Educ. Res. Pract., 17(2), 381–393.
- Herrington, D. G., Yezierski, E. J., Luxford, K. M. and Luxford, C. J., (2011), Target inquiry: Changing chemistry high school teachers' classroom practices and knowledge and beliefs about inquiry instruction, Chem. Educ. Res. Pract., 12(1), 74–84.
- Hiebert, J., Gallimore, R., Garnier, H., Giwin, K. B., Hollingsworth, H. and Jacobs, J., et al., (2003), Teaching mathematics in seven countries: Results from the TIMSS 1999 video study, Washington, DC: U.S. Department of Education.
- Hilton, A. and Nichols, K., (2011), Representational classroom practices that contribute to students’ conceptual and representational understanding of chemical bonding, Int. J. Sci. Educ., 33(16), 2215–2246.
- Johnstone, A. H., (2009), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Johnstone, A. H., (2000), Teaching of chemistry-logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Khaddoor, R., Al-Amoush, S. and Eilks, I., (2017), A comparative analysis of the intended curriculum and its presentation in 10th grade chemistry textbooks from seven Arabic countries, Chem. Educ. Res. Pract., 18(2), 375–385.
- Kulatunga, U., Moog, R. S. and Lewis, J. E., (2013), Argumentation and participation patterns in general chemistry peer-led sessions, J. Res. Sci. Teach., 50(10), 1207–1231.
- Lee, M. N., (1992), School science curriculum reforms in Malaysia: World influences and national context, Int. J. Sci. Educ., 14(3), 249–263.
- Liu, X., Zhang, B., Liang, L. L., Fulmer, G., Kim, B. and Yuan, H., (2009), Alignment between the physics content standard and the standardized test: A comparison among the United States-New York State, Singapore, and China-Jiangsu, Sci. Educ., 93(5), 777–797.
- Mahaffy, P. G., Holme, T. A., Martin-Visscher, L., Martin, B. E., Versprille, A., Kirchhoff, M., and Towns, M., (2017), Beyond “inert” ideas to teaching general chemistry from rich contexts: Visualizing the chemistry of climate change (VC3), J. Chem. Educ., 94(8), 1027–1035.
- McNeely, M. E. (ed.), (1997), Guidebook to Examine School Curricula, Washington, DC: U.S. Department of Education, Office of Educational Research and Improvement.
- Ngai, C., Sevian, H., and Talanquer, V., (2014), What is this Substance? What Makes it Different? Mapping Progression in Students' Assumptions about Chemical Identity, Int. J. Sci. Educ., 36(14), 2438–2461.
- Nehring, A., Päßler, A. and Tiemann, R., (2017), The complexity of teacher questions in chemistry classrooms: an empirical analysis on the basis of two competence models, Int. J. Sci. Math. Educ., 15(2), 233–250.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States, Washington, DC: The National Academies Press.
- Nichol, C. A., Szymczyk, A. J. and Hutchinson, J. S., (2014), Data First: Building Scientific Reasoning in AP Chemistry via the Concept Development Study Approach, J. Chem. Educ., 91(9), 1318–1325.
- Olson, J. K., Bruxvoort, C. N. and Vande Haar, A. J., (2016), The impact of video case content on preservice elementary teachers’ decision-making and conceptions of effective science teaching, J. Res. Sci. Teach., 53(10), 1500–1523.
- Philipp, S. B., Johnson, D. K. and Yezierski, E. J., (2014), Development of a protocol to evaluate the use of representations in secondary chemistry instruction, Chem. Educ. Res. Pract., 15(4), 777–786.
- Ramnarain, U. and Schuster, D., (2014), The pedagogical orientations of South African physical sciences teachers towards inquiry or direct instructional approaches, Res. Sci. Educ., 44(4), 627–650.
- Roth, K. J., Druker, S. L., Garnier, H. E., Lemmens, M., Chen, C., Kawanaka, T. and Gallimore, R., (2006), Teaching science in five countries: Results from the TIMSS 1999 video study, Washington, DC: National Center for Education Statistics.
- Sadler, T. D., (2004), Informal reasoning regarding socioscientific issues: A critical review of research, J. Res. Sci. Teach., 41(5), 513–536.
- Sawyer, R. K. (ed.), (2005), The Cambridge handbook of the learning sciences, Cambridge: Cambridge University Press.
- Schmidt, W. H., Wang, H. C. and McKnight, C. C., (2005), Curriculum coherence: An examination of US mathematics and science content standards from an international perspective, Journal of Curriculum Studies, 37(5), 525–559.
- Schuster, D., Cobern, W. W., Adams, B. A., Undreiu, A. and Pleasants, B., (2018), Learning of Core Disciplinary Ideas: Efficacy Comparison of Two Contrasting Modes of Science Instruction, Res. Sci. Educ., 48(2), 389–435.
- Schwab, J., (1969), The practical: A language for curriculum, Sch. Rev., 78(1): 1–23.
- Schwab, J., (1971), The practical: Arts of the eclectic, Sch. Rev., 79(4): 493–542.
- Schwab, J., (1973), The practical: Translation into curriculum, Sch. Rev., 81(4): 501–522.
- Seng, M. G. J. and Hill, M., (2014), Using a dialogical approach to examine peer feedback during chemistry investigative task discussion, Res. Sci. Educ., 44(5), 727–749.
- Stigler, J. W., Gonzales, P., Kawanaka, T., Knoll, S. and Serrano, A., (1999), The TIMSS videotape classroom study: Methods and findings from an exploratory research project on eighth-grade mathematics instruction in Germany, Japan, and the United States, Washington, DC: USA Department of Education, National Center for Education Statistics.
- Taber, K. S., (2016), Learning generic skills through chemistry education, Chem. Educ. Res. Pract., 17(2), 225–228.
- Taber, K. S. and Watts, M., (2000), Learners’ explanations for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Talanquer, V. and Pollard, J., (2010), Let's teach how we think instead of what we know, Chem. Educ. Res. Pract., 11(2), 74–83.
- Tümay, H., (2016), Reconsidering learning difficulties and misconceptions in chemistry: emergence in chemistry and its implications for chemical education, Chem. Educ. Res. Pract., 17(2), 229–245.
- van der Lans, R. M., van de Grift, W. J. and van Veen, K., (2018), Developing an instrument for teacher feedback: using the rasch model to explore teachers' development of effective teaching strategies and behaviors, J. Exp. Educ., 86(2), 247–264.
- Vhurumuku, E., (2011), High school chemistry students' scientific epistemologies and perceptions of the nature of laboratory inquiry, Chem. Educ. Res. Pract., 12(1), 47–56.
- Wang, J. and Zhao, Y., (2016), Comparative research on the understanding of nature of science and science inquiry between science teaching from Shanghai and Chicago, J. Balt. Sci. Educ., 15(1).
- Wang, Z., Chi, S., Luo, M., Yang, Y. and Huang, M., (2017), Development of an instrument to evaluate high school students' chemical symbol representation abilities, Chem. Educ. Res. Pract., 18(4), 875–892.
- Whitehead, A. N., (1959), The aims of education, Daedalus, 88(1), 192–205.
- Yezierski, E. J. and Herrington, D. G., (2011), Improving practice with target inquiry: High school chemistry teacher professional development that works, Chem. Educ. Res. Pract., 12(3), 344–354.
- Yuriev, E., Naidu, S., Schembri, L. S. and Short, J. L., (2017), Scaffolding the development of problem-solving skills in chemistry: guiding novice students out of dead ends and false starts, Chem. Educ. Res. Pract., 18(3), 486–504.
- Zheng, C., Fu, L. and He, P., (2014), Development of an instrument for assessing the effectiveness of chemistry classroom teaching, J. Sci. Educ. Technol., 23(2), 267–279.
| This journal is © The Royal Society of Chemistry 2019 |