Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams
Maia
Popova
 and
Stacey Lowery
Bretz
and
Stacey Lowery
Bretz
 *
*
Miami University, Department of Chemistry & Biochemistry, Oxford, OH, USA. E-mail: bretzsl@miamioh.edu
First published on 16th April 2018
Abstract
The purpose of this study was to elucidate and describe students’ thinking when making connections between substitution and elimination reactions and their corresponding reaction coordinate diagrams. Thirty-six students enrolled in organic chemistry II participated in individual, semi-structured interviews. Three major themes were identified that characterize students’ difficulties with integrating the information from the reactions and the reaction coordinate diagrams: incorrect ideas about the meanings of the reaction coordinate diagrams’ features, errors when examining reaction mechanisms, and an inability to assess the relative energies of reaction species. These findings suggest that students need support for coherence formation between reactions and reaction coordinate diagrams. Implications for teaching to address these student difficulties are suggested.
Introduction and background
Understanding energetics associated with chemical reactions has been identified as an anchoring concept in organic chemistry (Raker et al., 2013). It has been reported, however, that students struggle to understand the energy changes involved in the transformation of reactants into products (Bhattacharyya and Bodner, 2005; Taştan et al., 2010). Reaction coordinate diagrams (RCDs) are one representation to help students visualize the energy changes that occur during chemical reactions. In addition, RCDs provide complementary information about both the thermodynamic and the kinetic considerations that underlie transformations of chemical species depicted in reaction equations (Allinger, 1963; Meek et al., 2016).To date, no research has characterized students’ thinking as they try to forge meaningful connections between organic chemistry reactions depicted as both chemical equations and RCDs. Certainly a wide body of literature has focused on students’ understandings of different reaction mechanisms and on their approaches to solving mechanistic problems (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Rushton et al., 2008; Vachliotis et al., 2011; Grove et al., 2012a, 2012b; Cruz-Ramírez de Arellano and Towns, 2014). It has been reported that students “decorate” with arrows and view the electron-pushing formalism as a meaningless tool to get to the desired product (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012b). Only a few studies have reported students’ ideas about RCDs (Taştan et al., 2010; Csizmar et al., 2013; Morrison et al., 2014; Popova, 2018). For example, as part of a larger study investigating pre-service chemistry teachers’ misconceptions regarding kinetics, Taştan and colleagues asked participants to identify where on an RCD intermediates are encoded, as well as to propose a generic reaction mechanism that would correspond to the given RCD. Approximately 70% of pre-service teachers provided either partially correct or incorrect responses, with the biggest confusion being the conflation of an activated complex with a reaction intermediate (Taştan et al., 2010). In a study designed to examine the use of classroom response systems (“clickers”) in large lecture courses, Morrison and colleagues noted that RCDs depict numerous surface features and that asking multiple choice questions with clickers does not adequately assess students’ understandings of the ideas that are encoded in RCDs (Morrison et al., 2014). Csizmar and colleagues developed a computational activity in which students generated RCDs for both substitution and elimination reactions (Csizmar et al., 2013). They reported that modelling energetic pathways for these reactions was a conceptually challenging task for students.
Several reviews have called for further research on students’ understandings of external representations as related to kinetics and reaction mechanisms in order to investigate possible sources of students’ difficulties with respect to the aforementioned concepts (Kirik and Boz, 2012; Bain and Towns, 2016). A recent study has answered these calls by analyzing organic chemistry students’ understandings of the meanings encoded in the surface features of RCDs, such as the peaks, peak widths, peak heights, the valleys, etc. (Popova, 2018). Findings from this study suggest that organic chemistry students struggle to correctly decode these surface features. The challenges faced by students when interpreting the salient features of RCDs included difficulties with discerning the chemistry concepts encoded in each RCD feature, inaccurately mapping terminology onto RCD features, imposing unintended chemistry concepts upon RCD features, and not being able to differentiate between chemistry concepts that are encoded in RCD features. In the study reported herein, we present the findings of an investigation into the connections that organic chemistry students report between substitution and elimination reactions and the RCDs that correspond to them.
Research question
The objective of this research study was to explore the coherence of students’ thinking about multiple representations of organic chemistry reactions, namely symbolic chemical equations and RCDs. Therefore, the research question that framed this study was what connections do students identify between symbolic chemical equations of organic reactions and reaction coordinate diagrams? Specifically, this research sought to investigate the challenges that students enrolled in organic chemistry have with integrating the information depicted in these two kinds of representations.Theoretical frameworks
Chemical phenomena can be represented either as ‘external models’ or as ‘internal models’ (Kozma and Russell, 2007). Internal models are the mental constructs that are built through the processing of information by the brain to schematize concepts in order to be more readily accessible for human perception and cognition (Kosslyn, 2005; Gilbert, 2007). In order to communicate mental models, scientists commonly utilize multiple external models. One challenge of using external models to learn chemistry is the sheer number of representations that are used to describe abstract ideas: concrete (material) representations such as three dimensional models like ball-and-stick models; symbolic representations such as chemical symbols, formula, and equations; visual representations such as two-dimensional diagrams and graphs; verbal representations such as spoken and written descriptions of the entities that compose a representation and relationships between them; and gestural representations such as body movements to explain the mental model (Gilbert, 2007; Kozma and Russell, 2007; Keehner et al., 2008). Students who lack representational competence focus mostly on the surface features of the representations, or use heuristics that involve the mechanical application of symbolic rules grounded in memorization patterns (Chi et al., 1981; Kozma and Russell, 1997, 2007; Cooper et al., 2010; Mccollum et al., 2014). Students who develop representational competence, however, are able to use multiple representations to describe scientific phenomena, to select or generate representations that accurately explain phenomena, and to use these representations to make predictions, support claims, and communicate scientific ideas (Kozma and Russell, 2007; Stull et al., 2012). RCDs are two-dimensional visual representations. These diagrams are often taught and learned in accordance with chemical reaction equations that are symbolic representations. Thus, in order to make sense of the correspondence between chemical reaction equations and RCDs, students need to be able to translate easily between symbolic and visual representations.
Multiple research studies suggest that the working memory space is limited and can be overloaded when learners need to simultaneously process multiple “chunks” of information (Miller, 1956; Pascual-Leone, 1970; Johnstone, 2006; Cowan, 2010). Therefore, limitations on cognitive abilities can be explained by an overload of the working memory, which impedes the input and storage of the new information into the long-term memory (Pascual-Leone, 1970; Johnstone, 2010). Learners tend to neglect what they think is irrelevant, which might result in low performance in problem solving (Seufert and Brunken, 2004; Repovš and Baddeley, 2006). Given the limited capacity of the working memory, it is possible that students trying to learn organic chemistry that requires multiple representations may focus on some features of the visual and spatial characteristics of the representations and may ignore others they consider less relevant.
Methods
Sample and setting
Prior to beginning the study, an application was submitted to the Institutional Review Board (IRB) to ensure the protection of the student participants’ rights. Thirty-six undergraduates enrolled in organic chemistry II at a medium-sized, liberal arts university in the midwestern United States participated in the study. The students were recruited from two second-semester organic chemistry lecture courses, one for chemistry/biochemistry majors and one for other STEM (non-chemistry/biochemistry) majors. Students were taught by three different instructors, with all the major students taught by one instructor. The nonmajor students were recruited from two different sections that were taught by two different instructors. The textbook for the majors’ course was Organic Chemistry by Jones and Fleming (2014), and the textbook for the non-majors’ class was Organic Chemistry by Klein (2012). RCDs were introduced in organic chemistry I in the majors’ course in a chapter about alkenes and alkynes and in the nonmajors’ course in a chapter that reviewed thermodynamics and kinetics. RCDs were further used in both classes when introducing students to substitution, elimination, and addition reactions. In class and during exams, students were asked to analyze the relative heights of peaks in RCDs and to determine the rate determining step for different reaction mechanisms. RCDs were not commonly used when teaching reaction mechanisms in either organic chemistry II course.The 36 participants in this study were purposefully sampled (Patton, 2002; Bretz, 2008) to ensure diversity in gender (15 males, 21 females), ethnicity (28 white/Caucasian, 8 minority), and prior academic performance (14 students earned a letter grade of “A”, 14 earned a “B”, and 8 earned a “C” in organic chemistry I). The diversity of study participants was representative of the university's population. The sample included 6 chemistry majors and 30 non-majors, with 8 students enrolled in the major's course and 28 students enrolled in the non-major's course (non-major students had the option to enroll in the majors’ course due to scheduling conflicts). Pseudonyms were created for all students in order to protect their identities.
Data collection
Semi-structured, think-aloud interviews were conducted with the sampled students (Drever, 1995; Patton, 2002; Bretz, 2008). This methodology allowed for follow-up questions in order to more deeply probe students’ understandings. For example, when a student selected a particular RCD for a particular reaction without providing a detailed explanation, the interviewer was careful to ask additional questions to discern how the student matched a specific RCD's feature with a specific reaction species or reaction step. The interviews took place during March and April 2016 when participants were enrolled in organic chemistry II. All interviews were conducted by the first author at a mutually convenient time for the interviewer and each student and required, on average, 53 minutes to complete. To aid recruitment, students were given $20 gift cards upon completing the interview to compensate them for their time.Audio and video data were collected, along with real-time note-taking by the first author. The Livescribe™ Smartpen was used to record students’ responses and to capture their writing and drawings (Linenberger and Bretz, 2012). An audio recorder was used as a backup in case the Livescribe™ failed during the interview. The video camera was used to capture students’ gestures that were subsequently used to annotate the transcripts (e.g., clarification of students’ use of “this” or “that” while pointing to specific points on reaction equations or RCDs). Data collection continued until no new ideas were elicited, indicating that data saturation was achieved (Lincoln and Guba, 1985; Patton, 2002).
Description of interview prompts
Each interview began with a general introduction of the nature of the study, a description of the think-aloud protocol, and an explanation of what students were expected to do during the interview. Students were told that they were free to write or draw on the Livescribe™ dot paper in order to provide more detailed descriptions of their thinking processes. Students were given a consent form that described their rights and how the data would be treated to ensure confidentiality. Participants had an opportunity to read the informed consent document and ask questions prior to the start of the interview.The study participants (N = 36) were randomly divided into four groups of 9 students. Each group was interviewed using a different set of reactions and RCDs, to allow for gathering of rich, descriptive data in the context of different reaction mechanisms and RCDs. For example, students in the first group were presented Form I which consisted of both Reactions 1 and 2 (Fig. 1) and RCDs IA, IB, and IC (Fig. 2). Students in the second group were presented Form II (Reactions 3 and 4, along with RCDs IIA, IIB, and IIC), and so on. All the reactions in the study had been taught during organic chemistry I to both the majors and the nonmajors, with the exception of Reaction 5 which was taught to the nonmajor students in organic chemistry II, two weeks before the interviews commenced. Therefore, all students were interviewed after having been taught and tested on these reactions by their instructor(s).
 | ||
| Fig. 1 Substitution and elimination reactions used to elicit students' ideas about connections between reactions and reaction coordinate diagrams. | ||
 | ||
| Fig. 2 Reaction coordinate diagrams used to elicit students' ideas about connections between reactions and reaction coordinate diagrams. For reference, the reactions in Fig. 1 are noted below their corresponding RCDs. | ||
The interview protocol consisted of four phases. Phase I asked questions to ascertain students’ prior knowledge about bonding, stability, and reactivity of simple organic chemistry structures in order to provide insight into the later phases of the interview. Phase II asked students to explain how bonds are formed and broken in each reaction step of the two reaction mechanisms in their assigned Form (Fig. 1). Students were asked to comment on the relative stability of the reaction species in each step of the reaction. Students were also asked in Phase III to examine the three different RCDs in their assigned Form (Fig. 2) and to explain what the specific features of these diagrams represented, such as the peaks, the valleys, etc. Each RCD contained one, two, or three peaks and was generated using Adobe® Photoshop® software (Adobe, 1990) and subjected to expert content validation by three organic chemistry faculty members at the institution. Students were asked in Phase IV of the interview to pair each of their two reactions in Phase II of the interview with one of the RCDs from Phase III of the interview in order to elicit their thinking and reasoning using the symbolic representations of the reactions and the visual representations of the RCDs. Students were told that if they thought that none of the RCDs provided in Phase III matched one of their two assigned reactions, they were free to draw an RCD that, in their mind, matched the reaction more accurately. The data presented in this manuscript is from Phase IV of the interview as students engaged in the task of reasoning how to match the symbolic reaction equations with the visual RCD representations. Findings regarding phases I, II, and III of the interview have been reported elsewhere (Popova, 2018). Colleagues interested in obtaining a copy of the full interview protocol for research purposes should contact the corresponding author.
Data analysis
The interviews were transcribed verbatim, and students’ verbal descriptions, gestures, writings, and drawings were used to augment the transcripts, ensuring greater fidelity of the final interview transcript. The transcript data were inductively coded and managed using the NVivo 11 software (Patton, 2002; Creswell, 2003; Bazeley and Jackson, 2013; QSR International Pty Ltd, 2015). An organizational data framework was created during the data analysis where similar pieces of data were combined together into larger codes (i.e., all the data describing how students matched a specific reaction to a specific RCD were stored in the qualitative data management software as one encompassing code). This process was followed by dividing the larger codes into smaller codes that captured students’ thinking with respect to how and why they matched a specific reaction to a specific RCD. The emergent codes from this descriptive qualitative analysis consisted of meaningful words and phrases. Two types of codes were generated: in vivo codes (wording that participants use in the interview) and constructed codes (codes created by the researcher to summarize a common idea expressed by the study participants) (Bradley et al., 2007). Once all the data were coded, constant comparative analysis was used to organize codes into meaningful categories and themes that reflected patterns in students’ thinking (Bradley et al., 2007). The process of coding and theme generation was accompanied by writing reflective memos in order to capture the researcher's thoughts about the raw data, which aided in mapping research activities and in the communication between the researchers (Birks et al., 2008). To ensure the trustworthiness of the coding process, the first and second authors conducted weekly meetings during which codes were discussed and revised. In addition, the confirmability and credibility of the results were established through periodic external debriefing sessions with other chemistry education researchers at the institution who were uninvolved with the project (Lincoln and Guba, 1985; Creswell, 2003).Results and discussion
Of the 36 students who were interviewed, only 6 students (2 majors and 4 non-majors) accurately paired an RCD with the chemical equation for both of the reactions that they were assigned. Some students (n = 16) correctly matched one of their assigned reactions to an RCD, while more than one–third of the sample (n = 14) was unable to correctly match either of their assigned reactions to an RCD. No significant differences were identified in the reasoning and performance of chemistry/biochemistry majors when compared to non-major students, as both majors and non-majors made incorrect inferences when matching reactions and RCDs.Three themes (Fig. 3) were identified from the data analyses that explain the erroneous thinking of students when trying to identify connections between the symbolic reactions and the visual RCD representations. As can be seen in Fig. 3, some students faced multiple challenges when trying to identify similarities between reactions and RCDs. For example, six students sit at the intersection of Theme I and Theme III, because their reasoning incorporated both of these difficulties. Each theme is described in detail below.
 | ||
| Fig. 3 Themes that capture students' challenges with forging connections between chemical equations and reaction coordinate diagrams. | ||
Theme I: meanings of reaction coordinate diagrams’ features (n = 17)
Seventeen students were unable to correctly match their assigned reactions to an RCD because they did not understand the meaning encoded in one or more features of an RCD (Table 1). Seven distinct codes were captured under Theme 1. Note that the total number of instances in Table 1 (n = 27) is greater than the total number of students in Theme I in Fig. 3 (n = 17), because one student could be confused about multiple features of an RCD and therefore be assigned to multiple codes under Theme I in Table 1.| Codes | n |
|---|---|
| 1. Peaks represent intermediates | 10 |
| 2. Incorrect interpretations of valley | 6 |
| 3. Halves of peaks have meaning | 4 |
| 4. One intermediate is represented by a peak, the other by a valley | 3 |
| 5. “Transition state” and “intermediate” used interchangeably | 2 |
| 6. Starting point represents activation energy | 1 |
| 7. “Counting parts” strategy | 1 |
The most common mistake (n = 10) in students’ interpretations of the surface features of the RCDs was the idea that peaks represent reaction intermediates (Table 1, code 1). For instance, when third-year kinesiology major Vera attempted to choose an RCD for Reaction #3, she said that none of the RCDs in Form II were correct because they had either one peak or three peaks. Instead, she drew an RCD with two peaks (Fig. 4) and explained:
 | ||
| Fig. 4 Vera's drawing showing that the peaks represent intermediates and the valley represents an activated complex. | ||
“I am not sure that this reaction has three peaks. I guess I will just assign the two intermediates depending on energy. I think this [second intermediate] is going to be higher in energy.”
When drawing her own RCD, Vera assigned each of the two reaction intermediates to its own peak. When asked about the meaning of the valley in respect to Reaction 3, she drew a structure that resembled an activated complex, namely, a carbocation intermediate connected to a water molecule with a dashed line (Fig. 4). Vera was one of several students (n = 6) who incorrectly interpreted the meaning of a valley (Table 1, code 2). This is also evident in the third-year biology major Larisa's response, where she matched Reaction 8 with RCD IVB and commented that the circled area in her drawing (Fig. 5a) represented a transition state:
 | ||
| Fig. 5 Larisa's drawing showing that (a) peaks represent intermediates and (b) the valley represents an activated complex. | ||
“[Referring to Fig. 5a ]: So these are your reactants [label R], and this is the cation [first intermediate assigned to the first peak], and this is the next molecule [second intermediate assigned to the second peak], and this is your product [label P]… And here [circled area] is the transition state, which is part of the reaction where bonds are forming in order to resolve in this intermediate product… [Referring to Fig. 5b ]: It is kind of not yet formed, so I will draw a dotted line. So it's like in the stage of forming this bond.”
There were multiple additional interpretations regarding the meaning of a valley, including the idea that a valley represented the “depletion of resources” after one reaction step was over and another was about to start. This inaccurate idea is related to the previously reported alternative idea that “valley represents reaction slowing down” (Popova, 2018). Two students also reported that a valley depicts the “time in between reaction species react[ing]” (Victor, third-year chemical engineering major) because they interpreted the x-axis to represent time, and therefore, the width of the valley represented the time it takes for the next reaction step to start. Previous research has shown that students also perceive the widths of peaks as representative of the amount of time it takes for each reaction step to occur (Popova, 2018). These interpretations are consistent with Elby's findings that students use intuitive reasoning to attribute meaning to the most prominent visual attributes of representations (e.g., the shape of a peak can be considered to have the properties of a hill, a straight line depicts constancy, etc.) (Elby, 2000).
Some students (n = 3), when examining reactions that involved multiple intermediates, assigned one intermediate to a peak and another intermediate to a valley (Table 1, code 4), as was the case with Klava (third-year nutrition major):
Klava: “Okay, so this [Reaction 3] is going to have one intermediate which is the carbocation intermediate. So I think this [second intermediate in Reaction 3] is going to be a transition state here [label B inFig. 6]. I think it would look something like this where you start with reactants [label 1 inFig. 6], you have one intermediate [label A inFig. 6] and then the products [label C inFig. 6]. And, so, thus it wouldn’t be any of these [given] graphs [referring to the RCDs in Form II]. So this point here [circled area under label A inFig. 6] is going to be the carbocation intermediate.”
 | ||
| Fig. 6 Klava's drawing showing that the carbocation intermediate (label A) in Reaction 3 corresponds to the valley, whereas the oxonium intermediate (label B) in Reaction 3 corresponds to the peak. | ||
Interviewer: “Can you explain how you distinguish between A and B [inFig. 6]?”
Klava: “Only just because of the carbocations called carbocation intermediate.”
Klava explained that the reason why she assigned one intermediate to the valley and the other to the peak is because she considers only the carbocation to be an intermediate, as signified by how chemists name this species. Klava considered the second intermediate to be a transition state, suggesting that she had a shallow understanding of how these two concepts differed. Two additional students had another difficulty with communicating what species are encoded at peaks and valleys as they used the terms “transition state” and “intermediate” interchangeably (Table 1, code 5). This is not surprising as the lexical semantics of both of these terms mean “something in between”.
Another interpretation of a peak in an RCD included attribution of different meanings to the left half and the right half of one peak (Table 1, code 3). Students (n = 4) reported that the left side of a peak represented the acquisition of the necessary conditions for the process of bond breaking to start:
“I feel like for that [bond breaking] to happen, there has to be some sort of conditions that allows for that to happen and, I guess, that is what I consider the areas before the peak, on the left side of the peaks. Something needs to occur that allows for this [bond breaking] to happen.” (Lev, second-year biochemistry major)
whereas the right side represented the actual bond breaking process, as explained by Raisa (third-year biology major) when she examined Reaction 2:
“A [label A in Fig. 7b ] is just your starting material. Um, B [label B in Fig. 7a and b ] is removing the bromine group. So you had, so taking this [bromine] off would be B [drew arrow towards Br in Fig. 7a ]. So that would be from here to here [drew the line segment in Fig. 7b ].”
 | ||
| Fig. 7 Raisa's drawing showing (a) the loss of the leaving group and that (b) the right side of the first peak represents the loss of the leaving group. | ||
One student incorrectly considered the starting point in an RCD to represent the activation energy (Table 1, code 6), while another student employed a “counting parts” strategy when making connections between reactions and RCDs (Table 1, code 7). This strategy involved breaking a reaction equation into its component parts (reactants, intermediates, and products) and then assigning each part to a different feature of an RCD. For example, when analyzing Reaction 8, Arina (third-year kinesiology major) suggested that the reaction “has four [parts]”, by which she meant (1) reactants, (2) first intermediates, (3) second intermediates, and (4) products (Fig. 8a). She then explained why an RCD would contain these four parts:
 | ||
| Fig. 8 Arina's (a) identification of four parts in Reaction 8; (b) initial alternative RCD; (c) final alternative RCD. | ||
“I am not sure because this [Reaction 8] has four [parts] and that ruins my strategy… [ Fig. 8a ]. I don’t think you can stop at an intermediate… [ Fig. 8b , point 4]. So I am not sure how to exactly like draw… it would be like, hypothetically, I know this isn’t right, but it would be like this [ Fig. 8c ]… So this is a question mark [question mark label above the second peak in Fig. 8c ]. I guess that is my final answer.”
Interestingly, the act of drawing her own RCD forced Arina to realize that identifying four parts ruined this strategy that had previously “worked” when she chose an RCD for Reaction 7. In Reaction 7, Arina had identified three “parts” (reactants, intermediates, and products) that she easily assigned to the features of RCD IVA i.e., reactants at the starting point, intermediates at the peak, and products at the ending point. However, for Reaction 8, she was uncomfortable with the final product being at the top of the peak (4 in Fig. 8b) because she said “I don’t think you can stop at an intermediate…” (n.b. that Arina also has the misconception that peaks represent intermediates). Arina realized that her strategy did not work for a reaction with four “parts” and attempted to adjust her initial drawing (Fig. 8b) to something more “meaningful” (Fig. 8c). However, her revised RCD now contained one too many features, as there were not enough reaction “parts” to assign to the second peak, so Arina settled for labelling the second peak with a question mark (Fig. 8c).
Theme II: reaction mechanisms (n = 10)
In order to successfully pair reactions with their corresponding RCDs, students had to carefully examine not only the RCDs, but also the reactions. However, as students “pushed” arrows (Kermack and Robinson, 1922), they (n = 10) either proposed additional steps that generated unlikely intermediates or omitted a reaction step. Two distinct codes were captured under Theme II (Table 2).| Codes | n |
|---|---|
| 1. Added extra step | 7 |
| 2. Omitted a step | 3 |
For example, several students (n = 7) failed to recognize that Reaction 1 and Reaction 2 in Form I proceed through a concerted mechanism. Instead, they proposed their own mechanisms for these two reactions (Table 2, code 1). Therefore, when choosing RCDs, they tended to select RCDs with the number of peaks equal to the number of arrows in their proposed mechanisms. This approach was evident in second-year biochemistry major Lev's explanation for why he incorrectly chose RCD IB for Reaction 1 (Fig. 9):
“I guess I would say… I don’t think it's diagram C [RCD IC] because I think it shows too many steps. This is a more simple reaction. But also I don’t know about diagram A [RCD IA] because, in my opinion, it's not happening in one step. So I guess I would have to go with diagram B [RCD IB]. Step A [label A in Fig. 9a and b ] would be that lone pair attacking carbon and step B [label B in Fig. 9a and b ] would be, um, the chlorine group leaving to form the chloride ion, yielding the product.”
As can be seen in Fig. 9b, Lev assigned the two curved arrows in his mechanism to the two peaks because he failed to recognize that Reaction 1 proceeds through a concerted SN2 mechanism. As a result, he proposed instead that this reaction proceeds in two steps through an implausible intermediate that features a pentavalent carbon atom and does not account for the conservation of charge (Fig. 9a). A similar approach was used by Anton (third-year biology major) who matched Reaction 2 with RCD IB (Fig. 10):
 | ||
| Fig. 10 Anton's (a) proposed mechanism for Reaction 2; (b) re-drawn RCD IB showing that Reaction 2 proceeds through an intermediate (label “I” under the valley); (c) proposed intermediate structure. | ||
“Okay, this seems to be a mechanism that would happen in two steps, with the OH attacking and then the Br leaving in the second step [ Fig. 10a ]. So we would have, um, another intermediate form [ Fig. 10c ] and two transition states.”
Anton failed to recognize that Reaction 2 proceeds through a concerted E2 mechanism. Just like Lev, he generated an intermediate (Fig. 10c) that he assigned to the valley in RCD IB. Both Lev and Anton were focused upon making the desired product and used curved arrows to create mechanisms to “get-to-the-product” (Bhattacharyya and Bodner, 2005).
Other students in this theme (n = 3) proposed mechanisms that omitted one of the reaction steps (Table 2, code 2). For example, Inna (third-year microbiology major) proposed an incorrect mechanism for Reaction 3 in which a bromide leaving group left in the first step and simultaneously attacked a proton in the water molecule (Fig. 11). Due to drawing this incorrect first step, Inna thought that the overall reaction proceeded in two steps and did not correspond to any of the RCDs in Form II (IIA, IIB, and IIC), as these diagrams depicted either one-step or three-steps reactions.
 | ||
| Fig. 11 Inna's drawing showing that as leaving group leaves in the first step of Reaction 3, it simultaneously attacks the proton. | ||
Theme III: relative energies of reaction species (n = 14)
The codes that describe students’ thinking under Theme III (Table 3) are grouped into two categories either pertaining to charge (codes 1–3) or concerning conformers and functional groups (codes 4–6). The total number of participants in Table 3 (n = 21) is greater than the total number of students in Theme III in Fig. 1 (n = 14), because one student could be confused about multiple aspects of charge and structure as they relate to energy and would therefore be assigned to multiple codes in Table 3. Note that some of the codes (i.e., codes 4 and 5) are specific to a particular reaction (Reactions 6 and 5, respectively), whereas the rest of the codes pertain more broadly to multiple reactions and were identified in students’ reasoning in regards to several reactions.| Categories | Codes | n |
|---|---|---|
| Charge | 1. Comparison of charged atoms based on electronegativity | 5 |
| 2. Charged products are highest in energy and will keep reacting | 4 | |
| 3. Carbocation is a transition state because it is very high in energy | 2 | |
| Conformers and functional groups | 4. Axial and equatorial chairs are equivalent in energy | 6 |
| 5. Little energy is needed to open up an epoxide because it is very reactive | 2 | |
| 6. The product of an elimination reaction is less stable because it contains a double bond | 2 | |
“To distinguish this reaction, I would compare first two intermediates and see, um, which one took more energy to form and which intermediates have lower energy compared to each other. If I were to guess I would say that this reaction [Reaction 3] coordinates with C [RCD IIC].” (Nika, second-year biology major)
Even though Nika's approach to solving this problem was procedurally correct, she chose an incorrect RCD (RCD IIC) for Reaction 3. When asked to explain how she made her choice, Nika explained:
“Um, well in this reaction [Reaction 3] you have, your first intermediate is a carbocation and, um, your second intermediate is a, um, you have a loss of a leaving group. So to me, it's more favourable to have that plus [positive] charge on carbon than on oxygen, because oxygen is more electronegative and doesn’t like having a positive charge and would rather have its own electrons. So because this first intermediate [pointing at the first/lower valley in RCD IIC] has a lower energy than the second, I would assign plus charge on the carbon to be here [first/lower valley in RCD IIC] and oxygen to be here [second/higher valley in RCD IIC].”
Nika reasoned that, to her, an intermediate that contains a positively charged oxygen is less stable than a carbocation intermediate, because she knew the relative electronegativity values of oxygen and carbon atoms (Table 3, code 1). In total, five students disregarded that the first intermediate lacked an octet and, instead, invoked the unrelated property of electronegativity to draw inferences about the relative stability and reactivity of the intermediates. This is consistent with previous research that reported that when students make claims about the feasibility of reactions, they view the presence of highly electronegative atoms as indicative of high reactivity (Weinrich and Talanquer, 2015).
Two students who analyzed reactions that proceed through a carbocation intermediate noted that carbocations are very high in energy and, therefore, should be encoded in RCDs at the peak because these species are in fact highly energetic transition states (Table 3, code 3). Using this reasoning, Daniil, a second-year biology major, incorrectly chose RCD IVA for Reaction 7 because he mapped the reactants to the starting point, the intermediates to the peak, and the products to the ending point:
“Carbon carrying a [positive] charge would be pretty high in energy. You would not see that isolated. You really can’t isolate transition state… With intermediates you are able to some extent.”
Unlike many other students in this study, Daniil understood the relative difference in energy between an intermediate and a transition state, and yet, despite his understanding that carbocations are high in energy, he logically, but incorrectly, mapped the carbocation intermediate to a peak in RCD IVA. This shows that Daniil was not able to correctly identify intermediates encoded in reaction equations.
Students readily attended to the surface feature of charge not only when thinking about relative stabilities of intermediates, but also when comparing the relative energies of reactants and products. When examining reactions that contained no charged species among the reactants but did contain charges in the products, students (n = 4) disregarded all of the possible exergonic RCDs and instead suggested that the reactions would better match with an endergonic RCD (Table 3, code 2). Consider Inga (second-year biology major), who proposed her own RCD for Reaction 7 (Fig. 12):
“Reactants are stable because they don’t have a formal charge on them… Products are [stable], at least this one [2-methyl-2-butene]. But the chloride and H 3 O + [in products] are unstable and would probably react with some other molecules in the area to, um, gain a neutral state… I would say reactants are more stable and that is why you need heat to give energy to move it to different state… I think I would draw my own [RCD]. I think it would be something more like, um, this ( Fig. 12 ). It's not an exothermic reaction.”
Inga considered the charged products to be the most energetic species in Reaction 7. She also thought that achieving neutrality was the main driver for a chemical reaction, so, therefore, the ions in the products would continue to react in order to eventually become neutral or uncharged. A similar idea was expressed by Efim (second-year chemistry major) who initially correctly chose RCD IVC for Reaction 8, but later on suggested that RCD IVC required some modification to better match Reaction 8:
“The only change that I would make to this [RCD IVC], maybe draw more peaks and valleys to represent that these [iodide and hydrogen ions in products] will continue reacting.”
Inga and Efim failed to realize that the individual ions in products would form electrostatic interactions with the solvent molecules, which is not surprising considering that these interactions are typically omitted from the symbolic representations.
“For this one [Reaction 6], we had the flip, but that is not really a part of a reaction. So the reaction hasn’t really progressed. So I don’t think that that really shows up on the progress of reaction axis. I feel like this [second step] is the only step, so I would go with A [RCD IIIA] for this one.”
Similarly, Aleksei (second-year premedical studies co-major) when explaining why he matched Reaction 6 to RCD IIIA, labelled the equatorial and axial chairs as A1 and A2 and assigned both of these species to the starting point in RCD IIIA, signifying that both of the chairs have the same energy (Fig. 13).
 | ||
| Fig. 13 Aleksei's drawing of RCD IIIA that he chose for Reaction 6, placing both the equitorial and axial chair conformers at the same energy and ignoring the chair-flip as a step in the mechanism. | ||
Yana (second-year biology major) explained that the axial chair conformation was not an intermediate in Reaction 6:
“It [Reaction 6] doesn’t proceed through an intermediate, it all happens at once and then you get your final product.”
Students did not recognize the importance of the chair flip step during which the hydrogen atom becomes anti and periplanar in respect to the bromide leaving group; the orbitals can only overlap to form the π bond in the product when the carbon–hydrogen σ bond and the carbon–bromine σ bond lie in nearly the same plane. Students’ difficulties with understanding the anti-periplanar proton abstraction in alkyl halide reactions have been previously reported (Cruz-Ramírez de Arellano and Towns, 2014) but the impact of this difficulty on interpreting RCDs is novel here.
Reaction 5 presented an additional challenge for students as they focused upon the epoxide structure. Two students incorrectly chose RCD IIIC because they believed that an epoxide is a very unstable structure and therefore “little energy is needed to open up an epoxide” (Table 3, code 5):
“I think it won’t take a lot to break this angle strain molecule. So I think it would not be a lot of energy to go over this hump ( Fig. 14 ) [first peak in RCD IIIC], because the three-membered rings, they want to break, because there is a lot of angle strain. It's a lot of work to try to keep an angle this close, compared to like even the five-membered ring.” (Alla, second-year biology major)
 | ||
| Fig. 14 Alla's annotation of RCD IIIC for Reaction 5 to show the structure of the transition state at the first peak. | ||
“Um, the epoxide is going to react pretty easily. So that means that its activation energy would be quite small.” (Lidia, second-year microbiology major)
These students did not consider that the next step in Reaction 5 is a deprotonation which is a less energetic step. In their explanations, they drew inferences about stability by focusing only on the epoxide structure and not comparing the first and second reaction steps. This is consistent with previous research regarding the complex cognitive task of integrating multiple representations. Learners often concentrate on only parts of the given information or on a single representation to reduce cognitive costs (Seufert, 2003; Seufert and Brunken, 2004). In this case, students considered only one structural feature (epoxide) when drawing inferences about the energetics of an entire reaction mechanism.
Finally, two students focused on the structural feature of double bonds. These students considered endergonic RCDs to be better representations of elimination reactions because they reasoned that products that contained a double bond are less stable than reactants that contained only single bonds (Table 3, code 6). For example, when Vera (third-year kinesiology major) chose an RCD for Reaction 4, she rejected all three of the RCDs provided to her in Form II and instead proposed her own RCD (Fig. 15). Vera disregarded the RCDs in Form II for two reasons. First, she had the misconception that peaks represent intermediates (Popova, 2018) and because there are two intermediates in Reaction 4, her proposed RCD (Fig. 15) includes two peaks. Second, Vera believed that Reaction 4 is best represented by an endergonic RCD. As can be seen from Vera's drawing, she initially drew an exergonic RCD with two peaks. However, she decided that her initial RCD was incorrect, and she scribbled out the curved line at the ending point and drew a new curved line that ended above the starting point as she explained:
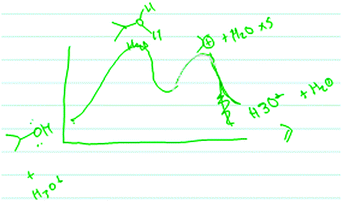 | ||
| Fig. 15 Vera's drawing of her proposed RCD for Reaction 4 as endergonic because the product contained a double bond but the reactants contained only single bonds. | ||
“I think they [products] are less stable than original reactants. That is, just looking, I am trying to compare the functional groups [in reactants and products].”
Mihail (second-year biology major) expressed a similar idea:
“I think the products would be high in energy, because I think, since we have no double bonds [in reactants] and we have a double bond in products, I think that [double bond in products] is associated with a higher energy level.”
Conclusions
This study investigated students’ thinking as they sought to make connections between the symbolic representations of organic chemistry reactions and the visual representations of those same reactions in the form of RCDs. Of 36 student participants, only 6 students were correctly able to match an RCD to each of their two assigned reactions (one substitution reaction and one elimination reaction). Three themes that describe students’ difficulties with the task were identified.The first theme captures students’ ideas about the meanings encoded in RCD features, which prevented nearly 40% of the students in this study from making proper connections between the symbolic representation of the chemical reaction and its RCD. Students demonstrated confusion about the meanings of peaks and valleys in RCDs, which is related to their confusion regarding the terms “transition state” and “intermediate” (Popova, 2018).
The second theme encompasses students’ thinking when extracting meaning from the symbolic representations of the reaction mechanisms themselves. Nearly 30% of the study participants identified non-existent connections between reactions and RCDs because they could not interpret the symbolic representation of a concerted reaction mechanism and instead tried to create a multi-step mechanism. When drawing these mechanisms, students proposed additional steps, generated implausible intermediates, and used the electron-pushing formalism of curved arrows to get to the desired product, similar to findings previously reported for how graduate students propose mechanisms for organic chemistry reactions (Bhattacharyya and Bodner, 2005). The findings from the study reported herein point to important implications of the multiple studies that have reported that students “decorate” reaction equations with arrows, but do not understand the predictive and explanatory functions of this formalism (Ferguson and Bodner, 2008; Rushton et al., 2008; Grove et al., 2012b).
The third theme captures students’ thinking about the relative energies of reaction species. Almost half of the students in this study made incorrect connections between reactions and RCDs because they focused primarily on the surface features of organic chemistry structures when making inferences about stability and relative energies. Mental integration of multiple representations is a complex cognitive task. To reduce the costs of cognitive processing, learners often concentrate only on parts of the given information or on a single representation (Seufert, 2003; Seufert and Brunken, 2004; Repovš and Baddeley, 2006; Johnstone, 2010). This explains the tendency of students to focus on specific structural features of reaction species rather than on the overall mechanism. Students identified structural features such as charge or single vs. double bonds as the most salient characteristics to match to RCDs. This finding is consistent with previously published research (Chi et al., 1981; Weinrich and Talanquer, 2015).
Limitations
In order to focus this study, boundaries were set in respect to selecting the breath of content investigated during the interviews. Due to interview time constraints, this study was focused on students’ understandings of unimolecular and bimolecular substitution and elimination reactions that study participants were taught during organic chemistry I. The interviews did not extend to other types of reactions introduced in the course. Additionally, students were interviewed only about exergonic RCDs with differing number of peaks; the interview guide did not include questions about endergonic RCDs. Despite the gathering of rich data, qualitative methodology does not allow for the generalization of these findings to wider populations (Patton, 2002).Implications for teaching and research
Implications for teaching
The findings reported herein suggest that students need support for coherence formation between reactions and RCDs. Students’ abilities to visualize molecular structures and translate between different domain-specific representations have been shown to improve through instruction (Stieff, 2010; Stieff et al., 2012). Therefore, helping students master the use of domain-specific strategies for translating between different representations – in this case between the visual representations of RCDs and the symbolic representations of chemical equations and mechanisms – should benefit students in their learning. In order to assist students with integrating information from these two different representations, instructors should be explicit about each of their corresponding elements, both surface features and the deep structure level (Seufert and Brunken, 2004). One way to support students’ understanding of the surface features would be to use color coding, redundant text, and/or draw redundant structures. For example, instructors could color code the structures that comprise reactants, intermediates, and products in a chemical equation or reaction mechanism and display these color coded structures above the starting point, valley(s), and ending point of RCDs. This has the potential to reduce the cognitive cost of processing the translation back and forth between the symbolic reactions and the visual RCD representations. To ensure more than a narrow focus on merely matching colour coded surface features between these representations, students also need to be assisted in the semantic analysis of the relationships between reactions and RCDs, i.e., coherence formation on a deep structure level. Faculty need to emphasize the difference between the terms “intermediate” and “activated complex,” where these species are encoded in reaction equations, and where they are encoded in RCDs. Faculty should also emphasize that in order for students to make connections between reaction representations and RCD representations, the students need to focus on the system of reactants, intermediates, and products, instead of drawing conclusions based on a single chemical species (e.g., epoxide, chair conformation) or a single structural feature (e.g., charge, double bond, electronegative atom, etc.).A useful in-class activity that could promote students’ coherence formation would be tasking students to generate their own RCDs for specific reactions. Students need opportunities to practice recognizing and decoding both kinetic and thermodynamic information encoded in RCDs. They can then engage in peer discussion and feedback to evaluate and reflect upon the accuracy of their representations through guided questions. The level of instructional assistance must account for students’ prior knowledge (Renkl et al., 1998; Ainsworth, 2006).
Implications for research
This study provides evidence that most students in our sample struggled with the cognitively demanding task of coherence formation between multiple representations of chemical equations and RCDs. Research regarding instructional techniques that could reduce extraneous and intrinsic load on the working memory (DeLeeuw and Mayer, 2008) would be valuable.Research studies that investigate how students construct mental models of chemical reactions could provide insights regarding how students identify similarities and differences among features from differing types of symbolic and visual representations. Additional research regarding students’ understanding of both the kinetic and thermodynamic parameters encoded in RCDs would also be important.
All the RCDs in this study were exergonic. Future research studies should explore students’ coherence formation in the context of endergonic RCDs and their corresponding reactions. It would be particularly interesting to investigate what differences exist, if any, with regard to how students think about the thermodynamic ideas encoded in exergonic RCDs vs. endergonic RCDs while identifying connections to reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Volwiler Family Endowment to the Miami University Department of Chemistry and Biochemistry and by Grant No. 1432466 from the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors thank the students and instructors of the organic chemistry courses.References
- Adobe, (1990), Adobe Photoshop CC. Accessed February 15, 2018, accessed at: https://www.adobe.com/products/photoshop.html?promoid=PC1PQQ5T&mv=other.
- Ainsworth S., (2006), DeFT: A conceptual framework for considering learning with multiple representations, Learn. Instr., 16, 183–198.
- Allinger N. L., (1963), Energy relations in teaching organic chemistry, J. Chem. Educ., 40(4), 201–202.
- Baddeley A., (2003), Working memory and language: An overview, J. Commun. Disord., 36, 189–208.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract., 17, 246–262.
- Bazeley P. and Jackson K., (2013), Qualitative Data Analysis with NVivo, in Seaman J. (ed.), 2nd edn, Thousand Oaks, CA: SAGE Oublications Ltd.
- Bhattacharyya G. and Bodner G., (2005), It gets me to the product: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Birks M., Chapman Y., and Francis K., (2008), Memoing in qualitative research: Probing data and processes, J. Res. Nurs., 13(1), 68–75.
- Bradley E. H., Curry L. A., and Devers K. J., (2007), Qualitative data analysis for health services research: Developing taxonomy, themes, and theory, Health Serv. Res., 1758–1772.
- Bretz S. L., (2008), Qualitative research designs in chemistry education research, in Bunce D. M. and Cole R. S. (ed.), Nuts and Bolts of Chemical Education Research, Washington, DC: ACS Symposium Series, pp. 79–99.
- Chi M. T. H., Feltovich P. J., and Glaser R., (1981), Categorization and representation of physics problems by experts and novices, Cogn. Sci., 5, 121–152.
- Cooper M. M., Grove N., Underwood S. M., and Klymkowsky M. W., (2010), Lost in lewis structures: An investigation of student difficulties in developing representational competence, J. Chem. Educ., 87(8), 869–874.
- Cowan N., (2010), The magical mystery four: How is working memory capacity limited, and why? Curr. Dir. Psychol. Sci., 19(1), 51–57.
- Creswell J. W., (2003), Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, in Laughton C. D. (ed.), 2nd edn, Thousands Oaks: Sage Publications.
- Cruz-Ramírez de Arellano D. and Towns M. H., (2014), Students’ understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- Csizmar C. M., Daniels J. P., Davis L. E., Hoovis T. P., Hammond K. A., McDougal O. M., and Warner D. L., (2013), Modeling SN2 and E2 reaction pathways and other computational exercises in the undergraduate organic chemistry laboratory, J. Chem. Educ., 90, 1235–1238.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: Diagrams, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, The Netherlands: Springer, pp. 169–191.
- DeLeeuw K. E. and Mayer R. E., (2008), A comparison of three measures of cognitive load: Evidence for separable measures of intrinsic, extraneous, and germane load, J. Educ. Psychol., 100(1), 223–234.
- Drever E., (1995), Using semi-structured interviews in small-scale research. A teacher's guide, Edinburgh: The SCRE Centre.
- Elby A., (2000), What students’ learning of representations tells us about constructivism, J. Math. Behav., 19, 481–502.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Friel S. N., Curcio F. R., and Bright G. W., (2001), Making sense of graphs: Critical factors influencing comprehension and instructional implications, J. Res. Math. Educ., 32(2), 124–158.
- Gilbert J. K., (2007), Visualization: A metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in Science Education, The Netherlands: Springer, pp. 9–27.
- Grove N. P., Cooper M. M., and Cox E. L., (2012a), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89, 850–853.
- Grove N. P., Cooper M. M., and Rush K. M., (2012b), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7(2), 49–63.
- Johnstone A. H., (2010), You can’t get there from here, J. Chem. Educ., 87(1), 22–29.
- Jones M. J. and Fleming S. A., (2014), Organic Chemistry, in Fahlgren E. (ed.), 5th edn, W. W. Norton & Company: New York, NY.
- Keehner M., Hegarty M., Cohen C., Khooshabeh P., and Montello D. R., (2008), Spatial reasoning with external visualizations: What matters is what you see, not whether you interact, Cogn. Sci., 32, 1099–1132.
- Kermack W. O. and Robinson R., (1922), An explanation of the property of induced polarity of atoms and an interpretation of the theory of partial valencies on an electronic basis, J. Chem. Soc., 121, 427–440.
- Kirik O. T. and Boz Y., (2012), Cooperative learning instruction for conceptual change in the concepts of chemical kinetics, Chem. Educ. Res. Pract., 13, 221–236.
- Klein D., (2012), Organic Chemistry as a Second Language, in Recter P. (ed.), 3rd edn, John Wiley & Sons, Inc.: Hoboken, NJ.
- Kosslyn S. M., (2005), Mental images and the brain, Cogn. Neuropsychol., 22(3/4), 333–347.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R. and Russell J., (2007), Students becoming chemists: Developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education. Dordrecht, The Netherlands: Springer, pp. 121–146.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park, CA: Sage Publications, Inc.
- Linenberger K. J. and Bretz S. L., (2012), A novel technology to investigate students’ understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- Mccollum B. M., Regier L., Leong J., Simpson S., and Sterner S., (2014), The effects of using touch-screen devices on students’ molecular visualization and representational competence skills, J. Chem. Educ., 91(11), 1810–1817.
- Meek S. J., Pitman C. L., and Miller A. J. M., (2016), Deducing reaction mechanism: A guide for students, researchers, and instructors, J. Chem. Educ., 93, 275–286.
- Miller G. A., (1956), The magical number seven, plus or minus two: Some limits on our capacity for processing information, Psychol. Rev., 63(2), 81–97.
- Morrison R. W., Caughran J. A., and Sauers A. L., (2014), Classroom response systems for implementing interactive inquiry in large organic chemistry classes, J. Chem. Educ., 91, 1838–1844.
- Pascual-Leone J., (1970), A mathematical model for the transition rule in Piaget's developmental stages, Acta Psychol., 32, 301–345.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, 2nd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Popova M., (2018), Organic chemistry students' understandings of stability and reactivity: Challenges with interpreting concepts encoded in structural formulas, reactions, and reaction coordinate diagrams, Miami University.
- Prins G. T., (2010), Teaching and Learning of Modelling in Chemistry Education Authentic Practices as Contexts for Learning, The Netherlands: Leersum.
- QSR International Pty Ltd, (2015), NVivo qualitative data analysis Software, Version 11, accessed March 1, 2018, accessed at: http://www.qsrinternational.com/.
- Raker J., Holme T., and Murphy K., (2013), The ACS exams institute undergraduate chemistry anchoring concepts content map II: Organic chemistry, J. Chem. Educ., 90, 1443–1445.
- Renkl A., Stark R., Gruber H., and Mandl H., (1998), Learning from worked-out examples: The effects of example variability and elicited self-explanations, Contemp. Educ. Psychol., 23, 90–108.
- Repovš G. and Baddeley A., (2006), The multi-component model of working memory: Explorations in experimental cognitive psychology, Neuroscience, 139, 5–21.
- Rouse W. B. and Morris N. M., (1986), On looking into the black box: Prospects and limits in the search for mental models, Psychol. Bull., 100(3), 349–363.
- Rushton G. T., Hardy R. C., Gwaltney K. P., and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Seufert T., (2003), Supporting coherence formation in learning from multiple representations, Learn. Instr., 13, 227–237.
- Seufert T. and Brunken R., (2004), Supporting coherence formation in multimedia learning, in Gerjets R. J. P., Kirschner P. and Elen J. (ed.), Instructional design for effective and enjoyable computer-supported learning: Proceedings of the first joint meeting of the EARLI SIGs Instructional Design and Learning and Instruction with Computers, Tubingen, Germany: Knowledge Media Research Center, pp. 138–147.
- Seufert T. and Brünken R., (2006), Cognitive load and the format of instructional aids for coherence formation, Appl. Cogn. Psychol., 20, 321–331.
- Stieff M., (2010), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry, Sci. Educ., 95(2), 310–336.
- Stieff M., Ryu M., Dixon B., and Hegarty M., (2012), The role of spatial ability and strategy preference for spatial problem solving in organic chemistry, J. Chem. Educ., 89, 854–859.
- Stull A. T., Hegarty M., Dixon B., and Stieff M., (2012), Representational translation with concrete models in organic chemistry, Cognition and Instruction, Taylor & Francis Group, LLC, pp. 404–434.
- Taştan Ö., Yalçinkaya E., and Boz Y., (2010), Pre-service chemistry teachers’ ideas about reaction mechanism, J. Turkish Sci. Educ., 7(1), 47–60.
- Vachliotis T., Salta K., Vasiliou P., and Tzougraki C., (2011), Exploring novel tools for assessing high school students’ meaningful understanding of organic reactions, J. Chem. Educ., 88(3), 337–345.
- Weinrich M. L. and Talanquer V., (2015), Mapping students’ conceptual modes when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 16, 561–577.
| This journal is © The Royal Society of Chemistry 2018 |


