Analysing processes of conceptualization for students in lessons on substance from the emergence of conceptual profile zones
Edenia Maria Ribeiro do
Amaral
 *a,
João Roberto
Ratis Tenório da Silva
*a,
João Roberto
Ratis Tenório da Silva
 b and
Jaqueline Dantas
Sabino
b and
Jaqueline Dantas
Sabino
 c
c
aUniversidade Federal Rural de Pernambuco, Departamento de Química/PPGEC, Rua Dom Manoel de Medeiros, S/N, Dois Irmãos, Recife, Pernambuco, Brazil. E-mail: edeniamramaral@gmail.com
bUniversidade Federal de Pernambuco, Núcleo de Formação Docente, Recife, Pernambuco, Brazil. E-mail: joaoratistenorio@gmail.com
cUniversidade Federal Rural de Pernambuco, Postgraduate Program in Science Education – PPGEC, Recife, Pernambuco, Brazil. E-mail: jaquelinedantas@gmail.com
First published on 7th May 2018
Abstract
In this paper, we analyze the process of conceptualization experienced by students in Secondary School when involved in activities in a teaching and learning sequence on the concept of substance, considering the emergence of zones of the conceptual profile. The results point out that the approaches to different modes of thinking of substance enabled the teacher to discuss and confront ideas, leading students to construct or share meanings stabilized in a scientific view. The conceptual profile was an important tool to design activities by creating discursive contexts involving different modes of thinking about substance, which contributed to raising specific discussions involving historical, scientific and social contexts to understand senses and meanings for substances.
Introduction
In this paper, we analyse the process of conceptualization (Mortimer et al., 2014) experienced by students in Secondary School when they studied substance in chemistry lessons throughout different activities structured in a teaching and learning sequence (TLS). The process of conceptualization was approached by taking into account the conceptual profile theory (Mortimer and El-Hani, 2014) and more specifically by using revised zones of the conceptual profile of substance proposed by Silva and Amaral (2013). From the conceptual profile perspective, science learning involves a more complex process than only conceptual change from personal senses to a scientific view on concepts. It is related to a meaning-making process that goes toward the constitution of conceptual thinking (Vygotsky, 1978). Students are considered as part of the larger social environment from which they bring senses and socially stabilized meanings, which play an important role in the meaning-making process for scientific concepts. It seems not appropriate to consider conceptual evolution as a linear progression but a dynamic process in which ideas come and go when students seek to understand and articulate different ideas around scientific concepts. In this way, to plan science teaching and learning sequences, we must open up opportunities for students to express, discuss, confront and compare ideas brought from their experiences and produced in scientific contexts.Substance is an important and structuring concept, which enables students to reach wider learning of chemistry (Gagliardi, 1988). In the literature, there is a large discussion on issues related to the teaching and learning of substance (see De Vos and Verdonk, 1987; Dickinson, 1987; Abraham et al., 1994; Johnson, 1996, 2000, 2002; Stains and Talanquer, 2007; Padilla et al., 2008; Close and Scherr, 2015). Researchers have dealt with different issues to approach substance: discussing epistemological aspects for conceptions on substance and difficulties of learning at different levels (Oliveira, 1995; Hashweh, 1996; Johnson, 2000, 2002; Papagerogiou and Sakka, 2000; Ngai et al., 2014), analysing historical difficulties to understand differences between chemical elements and substances (Oki, 2002), and exploring students’ understanding of different forms of presentation of substances. Some of these authors pointed out the importance of historical and epistemological aspects related to the development of ideas for scientific concepts to overcome learning difficulties and to contribute to the process of making meaning for substance in science teaching and learning (see Oliveira, 1995; Hashweh, 1996; Ngai et al., 2014). In this work, we propose a discussion on epistemological, ontological and axiological commitments to the concept of substance in terms of conceptual profile zones, considering the importance of these aspects to the process of conceptualization.
Mortimer (1995, 1996) introduced conceptual profiles as a tool for modeling the heterogeneity of thinking and speaking in science classrooms. He was initially inspired by Bachelard's ideas on epistemological profile (1940), in which plurality of thought is structured in terms of philosophical schools, and some ideas are considered as obstacles to the understanding of scientific concepts. Differently, Mortimer proposed conceptual profiles for the investigation of science teaching and learning, introducing not only epistemological commitments related to the ideas expressed by individuals, and giving attention to the heterogeneity of thinking in order to make students aware of their own conceptual profiles. In subsequent years, the philosophical bases of the conceptual profile theory moved away from Bachelard's ideas (Mortimer et al., 2014).
The conceptual profile theory is grounded on the idea that people present different ways of seeing and conceptualizing the world, mainly by considering that the social world is not homogeneous, and there are different ways of perceiving and understanding subjective experiences (Schutz, 1932/1967). There is a heterogeneity of thinking, from which different types of verbal thinking can emerge in any culture and in any individual (Tulviste, 1991). Regarding the cognition of individuals, the conceptual profile theory is aligned with the social dimension of human mental processes, such as that proposed by Vygotsky (1978). Mortimer et al. (2014) pointed out that “The fact that those collective constructions are imposed upon individual cognition follows from the development of individual thinking through the construction of an internal plane of functioning by means of cultural tools made available through social interactions” (p. 14). From this perspective, we can share different meanings expressed in different social languages starting from our diverse and multifaceted social experience.
According to the conceptual profile theory, people can exhibit different modes of thinking that are accessed in different contexts, and they represent the ways of conceptualizing for a given experience. In building conceptual profiles, these modes of thinking are modelled as zones that represent ontological, epistemological and axiological commitments, which underlie the meaning-making process for scientific concepts. Conceptual profiles are proposed for a given concept and are constituted by several zones, each one representing a particular mode of thinking about that concept. Each individual presents his or her own individual conceptual profile, but he or she can share different senses and meaning in an appropriate sociocultural context, in which concepts can be applied (Mortimer et al., 2014).
Conceptual profiles are models constituted by zones that represent the relevant aspects of our experience (Mortimer et al., 2014) and individuals in making meaning for scientific concepts can express ideas related to different zones. In the classroom, modes of thinking can be associated with different senses and meanings that students attribute to a scientific concept. From this perspective, in science teaching and learning, it is desirable that the teacher adopts approaches to promote discursive interactions in the classroom, producing a repertoire of ideas that will play an important role in the conceptualization process experienced by the students.
Mortimer et al. (2011) pointed out differences between ideas on concepts and conceptualization, considering that concepts are not physical entities inside people's minds. For the authors, there is a process of conceptualization that is understood as a dynamic process, which is continually subjected to influences from the external experiences lived by the individual (Mortimer et al., 2014). In this sense, in science teaching and learning, we seek to construct meanings for scientific concepts including discussions on situations experienced by individuals. We learn the concepts when we apply them several times in different situations, putting together senses and meanings in order to stabilize and share meanings, in a dynamic and continuous process. Vygotsky (1978) considered that this dynamic process could lead to the constitution of conceptual thinking.
Mortimer et al. (2011) argue that the assumption that individuals “possess” concepts comes from the tendency of conceptual thinking to operate in a similar way when we recognize situations as similar. That is, some meanings stabilize in the process of conceptualization, but it does not imply a static condition of conceptual thinking, which may change in the face of new situations. Another explanation for the idea of permanence of the concept, e.g., the tendency of conceptual thinking to operate in a similar way, lies in the difference between meaning and sense (Vygotsky, 1978). For Vygotsky, sense is understood as a dynamic and personal formation, constructed individually, considering that the sense of a word changes in different contexts. Whereas meaning is elaborated in a sociocultural context and can be considered more stable. For Mortimer et al. (2011), in the process of conceptualization, students can produce different senses for the same word, and these senses may vary according to different discursive contexts. However, collective discussion can lead to the construction and/or sharing of socially accepted and stabilized meanings.
Differently from the conceptual change model for science education (Posner et al., 1982), according to the conceptual profile theory, students do not necessarily have to change their informal conceptions to learn scientific concepts, when non-scientific modes of thinking seem to be pragmatically appropriate to understand situations in quotidian life or specific cultural contexts. Therefore, the process of conceptualization involves the coexistence of these different modes of thinking, and they can be structured in a conceptual profile. In science teaching and learning, it is important to focus on not only changing or substituting ways of thinking in scientific ideas that our everyday language reinforces. Instead, we can promote discussion on cultural diversity and the role played by scientific ideas in social contexts (Mortimer et al., 2011). In this way, science teaching and learning involves making students aware of the diversity of modes of thinking related to scientific concepts, seeking to understand the pragmatic value for them in different contexts and situating the scientific view among others.
In the science classroom, students must be engaged in discussions, analyses and choices related to different situations, where they can compare different modes of thinking applied to them, including scientific views. According to El-Hani et al. (2014), many times students are not aware of the role played by scientific ideas to explain and/or solve particular situations and they intuitively choose informal ideas to approach them. In this case, it could be useful for teachers to lead students to think about both scientific ideas and those emerging from social experience, seeking different ways of approaching a specific problem and leading students to meaningful learning on scientific concepts applied to different situations. In this work, we present a teaching and learning sequence (TLS), planned to teach the concept of substance by considering different modes of thinking structured in terms of conceptual profile zones, in order to analyse the process of conceptualization experienced by students in the classroom.
Silva and Amaral (2013) proposed a conceptual profile for substance considering data obtained from different sources: conceptions on substance in the historical development of the concept, the literature on students’ misconceptions/conceptions about substance, and answers obtained from questionnaires and interviews applied to students and teachers. The authors proposed five zones for a conceptual profile of substance – generalist, essentialist, substantialist, rationalist, and relational. In further studies, the original zones were applied in science classrooms and changes were proposed for the zones (Silva, 2017a; Silva, 2017b; Sabino and Amaral, 2018). In this paper, we have made a critical review of the original zones of the conceptual profile of substance and considered the following zones for data analysis – generalist, pragmatic/utilitarian, substantialist, empirical, rationalist and relational.
In the generalist zone, there is an assembly of ideas or conceptions, in which substance is considered as something present in everything, without distinction of the constituents or differentiation among materials, mixture, substance or chemical element. In addition, substance is like the essence or something essential that constitutes and qualifies materials – a common idea in philosophical and historical contexts. These ideas are representative of a generalist view of the concept and represent an epistemological commitment related to an ingenuous realism (Bachelard, 1938/1996), as ideas involve immediate perceptions, sensations, and/or intuitions that guide individuals in the construction of their notions according to the social and historical setting where they live and interact with their peers. It represents a vague and generalized idea that substances can be anything and are found everywhere. Ways of speaking related to this zone are common in everyday situations, in which materials or mixtures are classified as substances or are closely associated with them. For instance, it is common to find people who consider mixtures such as detergent, milk or mineral water as substances, regardless of whether these materials are formed by various chemical compounds or substances.
The pragmatic/utilitarian zone includes ideas on substances defined or characterized by their applications and uses, acquiring importance for possible benefits or damage caused to human beings. In this sense, the properties of substances are addressed, not necessarily in scientific terms, but in a pragmatic sense. For this zone, we identify both epistemological and axiological commitments, considering a pragmatist sense such as that proposed by Putnam (1995), where he considers that there is an interrelationship between empirical situations and a theoretical approach that involves questions of value and interpretation, which lead individuals to construct their notions in accessing the external world. Here, most of the ideas included in the pragmatic/utilitarian zone tend to emphasize values for the use or application of substances, e.g., the utility of them causing benefits or damage to human life or to the environment. For example, often it is recommended to eat protein-containing foods, such as milk, cheese and other animal foods, because of the benefit of protein in maintaining the health of the body. It is also common to recommend the use of medicines, herbal products or food supplements to meet the needs of the body, for example, when someone says, “you need iron to stay strong!” The mention of iron in this case does not necessarily imply the visualization of a substance in the chemical sense but it is associated with the benefit of some product or material in a specific situation (Silva, 2017). In this sense, the understanding of substance comes under an affective aspect (axiological commitment), and a more elaborated comprehension on the properties of substance is not addressed.
The substantialist zone was proposed considering ideas in which a material dimension for substance is emphasized, as a counterpoint to the view discussed by Oliveira (1995) when he took into account Bachelard's ideas on substance as a chemical model. The comprehension of substance as something that keeps materiality even in atomic–molecular dimensions leads students to consider that the properties of a substance extend to its constituents (atoms or molecules). Mortimer (1997) proposed a substantialist zone for the conceptual profile of a molecule, considering that handbooks and dictionaries claim that the molecule is the “smallest unit of matter that can exist by itself and which retains all the properties of the original substance” (Mortimer 1997, p. 203). For Mortimer, this definition is substantialist in the sense that molecules do not retain all the properties of the original substance. The properties are relative to the substances and not to their individual constituents (atoms or molecules). Mortimer considered that substantialism is present in scientific contexts and “for science itself, it is an important zone of the (conceptual) profile, since its automatic and almost unconscious use in chemical language can produce confusion, leading chemists, and especially chemistry students, to make mistakes”. In the same direction, we proposed the substantialist zone by considering ideas on substance as something exclusively material, in which the student does not make a distinction between the properties of the substances and their constituents in the atomic–molecular dimension. For example, many students consider that gold atoms (Au) are a yellow-like substance and that it is yellow because the atoms are yellow (Silva, 2011). Ideas like this can confuse the understanding of the atomic–molecular aspects of substances. The difference in the substantialist zone from the generalist one is that, in a substantialist mode of thinking, there is an idea of substance contained in materials, but this concept is not understood in the atomic–molecular dimension, such as a model to explain the behaviour and properties of the matter.
The comprehension of the atomic–molecular aspects and macroscopic properties of substances is aligned with scientific thinking. According to the scientific perspective on substance, three zones were proposed: empirical, rationalist and relational zones. The empirical zone includes ideas on substance that address the physical and chemical properties of substances as important features for their chemical identification. In this case, the existence of a diversity of substances that constitute materials giving them specific properties is recognized. These properties can be determined or observed macroscopically from the manipulation of materials or substances, and by observing transformations in nature. For example, water is characterized as having a boiling temperature of 100 °C (at an atmospheric pressure of 1 atm); or metals are characterized by their gloss, malleability and hardness. Differently from the pragmatic/utilitarian zone, ideas in the empirical zone are based on theory. For Norman (1998), empiricism is challenged in its central issues by the idea of observation as being theory-laden, highlighting the effort to interpret and to justify what you see. In these terms, we consider that ideas included in an empirical zone are aligned with a scientific view of substance, considering the understanding of this concept from its observable aspects and properties, which could be studied. Here, the students have to deal with an idea about purity of substance, considering that physical and chemical properties are determined for substances extracted from materials in order to characterize them.
In the rationalist zone, we find ideas or conceptions on substance considering scientific models to explain its structures and properties. It is possible to represent and to characterize various types of substances – simple, compound, organic, inorganic, etc. – from the knowledge of the entities that compose materials. The ways of speaking related to this zone represent an understanding of the concept, in which students can go beyond macroscopic and concrete features of materials to interpret many kinds of phenomena and the nature of substances: for example, to know that atmospheric air is composed of several types of gaseous simple substances and compounds; or the differentiation of organic and inorganic substances by the presence of carbon and hydrogen in certain molecules. The rationalistic commitment for this zone is aligned with the applied rationalism proposed by Bachelard (1949/1977), as theory and experiment come together by considering that ideas arising from a theoretical approach tend to get their application and data obtained empirically tend to be organized by theories.
In the relational zone, there are complex ideas on substances when the relational dimension for the properties of the substances is considered. For example, the same substance can behave as an acid or a base under certain conditions, and the acidic properties do not identify the substance itself, but represent the behaviour of the substance when it is dissolved in a specific solvent. In this way, some properties of the substances are associated with the conditions in which they are found and would not be parameters to give them a chemical identity. This is aligned with a complex rationalism, leading to the understanding of the concept of spheres with high level of abstraction. According to Silva and Amaral (2013), in this zone, the concept of substance is considered as a theoretical model for explaining the behaviour of matter, and its existence in the real world is considered a myth (Oliveira, 1995). Thus, the existence of pure substances with well-defined physical and chemical properties stands as a theoretical approximation. In a system, the molecules of a substance are in constant interaction with other species present in the environment and under specific conditions, with a constant energy exchange (Silva and Amaral, 2013).
In this work, these zones of the conceptual profile of substance inspired the proposal of a teaching and learning sequence (TLS), taking into account the ideas proposed by Méheut (2005). According to Méheut, a TLS intends to bring aspects of research into practices in the classroom, and different teaching perspectives may be implicated in their design. The author pointed out four main elements to design a TLS related to each other considering epistemological and pedagogical dimensions – the teacher, the student, the scientific knowledge and the “material world”. The epistemological dimension refers to a genesis of the concept and, for us, it represents to consider modes of thinking ranging from the senses and meanings consolidated in diverse contexts to the scientific view of the concept. For us, the “material world” proposed by Méheut is related to objects and processes that could be represented from sociocultural situations. Therefore, the conceptual profile of substance constitutes a model of structuring these modes of thinking, which may suggest a genesis for the concept.
The TLS proposed sought to promote discussion and articulation among different modes of thinking on substance from different situations. These situations would potentially constitute diverse discursive contexts based on interactions between teacher and students, and students and students, bringing different views and conceptions and favouring the students to be aware of the plurality of ideas that concepts embody (epistemological dimension). The activities were designed to promote active participation of students, interactions and discussions in the classroom, in order to encourage the students to express their conceptions or to comment different ideas about substance (pedagogical dimension). With this, we believe that we have promoted opportunities for a process of conceptualization that involves different modes of thinking on the concept being studied.
Methodology
This research adopted a qualitative approach for the methodology, considering this perspective underpins the role played by researchers to construct and to interpret data from the contact with individuals and the research context (André, 2001; Bogdan and Biklen, 2007). This is aligned with research actions to analyse the students’ process of conceptualization in the science classroom. In addition, a quantitative treatment was applied to the data in order to estimate frequencies in which the conceptual profile zones for substance emerged from the students’ speeches in the classroom discussions.The research involved 13 students aged 14–15 years from year 9 of Secondary School, in Recife, Brazil. We invited students to attend voluntarily lessons in a special schedule offered by the teacher in school. For ethical precaution and considering that the students were minor aged, their parents/guardians were required to sign a term of consent for them to take part in this study. These students had already started studies on chemical content since year 8, and one of the authors of this paper acted as a teacher in years 8 and 9 for this class. To preserve the identity of the students, fictitious names were used. A teaching and learning sequence was planned and applied to the class, and lessons were recorded in video focusing on the discursive interactions among students, and students and teachers. The more relevant recordings for research were transcribed and some pieces analysed in terms of episodes.
Before the beginning of the sequence, students were required to answer a questionnaire in order to identify their previous conceptions about substance. The teaching and learning sequence occurred in three lessons – the first lesson lasted two hours and the other lessons 1 hour each. The activities were carried out seeking to promote discussions on different situations, which encourage students to construct an understanding of the chemical concept of substance. For each activity, an object (content), learning objectives and actions to be carried out by the students were established. A brief summary of the activities developed in the teaching and learning sequence is presented in Table 1.
| Activity | Objective | Object | Actions |
|---|---|---|---|
| Class 1 (2 h) | |||
| Application of questionnaires | To identify students’ informal conceptions | Conceptions on substance | Reading and writing responses |
| Reading of historical text on the Aristotelian view of substance | To discuss epistemological, historical and contextual aspects related to the concept of substance | Historical and philosophical views on the concept of substance | Collective reading of the text |
| Small group discussion and questioning | To promote discussions about conceptions related to the conceptual profile zones | Concepts of substance and chemical element – historical and conceptual aspects | Working in small groups and discussing issues |
| Digital simulation on physical states of substances (https://phet.colorado.edu/pt_BR/) | To discuss concepts of chemical element, substances, and mixtures – molecular and atomic aspects | Constitution – number and types of atoms and molecules – and physical states of some substances | Lecture and computer use for simulation. Debate in the large group |
| Lecture and discussion | To introduce scientific views on substance, and to articulate different zones of the conceptual profile | Atomic–molecular and macroscopic view of substance – concepts of chemical element and substance | Exposition of scientific concepts stimulating debate and student participation |
| Class 2 (1 h) | |||
| Reading thematic text – the use of antibiotics during the war | To approach the concept of substance starting from a theme (contextualization) | Theme on medicines, for discussion on substances | Collective reading of the text and text-oriented debate |
| Lecture and questioning | To introduce scientific ideas and articulate different zones of the conceptual profile | Some macroscopic properties of the substances | To present answers for questions asked in the lecture |
| Class 3 (1 h) | |||
| Analyses of package inserts in small groups | To identify ideas used by students to apply the concept of substance | Substances that constitute medicines | To identify in package insert: substance, element and material |
| Discussion in small groups | To promote discussions between students on package inserts and questions | Macroscopic aspects and molecular atomic aspects of the concept of substance | To answer questions posed on the board |
| Discussion in the large group | To highlight the scientific zones of the profile in articulation with other zones | Macroscopic aspects and molecular atomic aspects of the concept of substance | To share discussions carried out in small groups |
At the end of the three lessons, two students were interviewed in order to verify whether they had a perception of the different modes of thinking of substance involved in the classroom discussions. The criteria for the choice of the two students was that both had very active participation in the lessons – one student presented predominantly ideas aligned with the scientific view of the concept, and the other expressed difficulties in understanding the concept from the scientific point of view.
In lesson 1, initially, a questionnaire was applied to investigate students’ previous conceptions about the concept of substance. After answering the questionnaire, students read and discussed the text “History of chemistry: Alchemy” (Fonseca, 2007). After reading, students were divided into three small groups to discuss questions such as “Nowadays, how does the concept of an element present in Aristotle's age look to you?” and “Do you agree with Aristotle's ideas on the composition of matter?” The intention was to promote a discussion on historical, epistemological and contextual aspects related to the concept of substance. Next in the lesson, the teacher used a digital tool for simulations on computers (https://phet.colorado.edu) to present a scientific view about the atomic–molecular aspects of chemical elements, substance and mixtures. Throughout lesson 1, the teacher tried to stimulate student–student interactions, and teacher–student interactions, mediating debate and raising questions to instigate students to participate and express their ideas.
In lesson 2, the activities were carried out in two moments: first, the students read the text Miracle Medicines from the book Napoleon's Buttons,† proposed by the teacher to present the theme on medicines, and second, the teacher presented content about the macroscopic aspects of the concept of substance. From this book, among others, the text had an approach on the importance of the antibiotic properties of substances, used mainly during the First World War. Upon reading, the teacher intended to start a discussion about substances used to produce medicines, and ideas about natural and synthesized substances that emerged from the classroom discussion. It was also possible to explore ideas on the macroscopic properties of the substances, benefits or damage that products can cause in people, highlighting ideas about substances and products and their applications in various situations.
In lesson 3, the teacher brought back discussions held in the previous lessons and it promoted a very fruitful debate, in which students were free to express their ideas and to make known the difficulties they had to understand the concepts studied in lessons 1 and 2. Next in the lesson, the teacher asked students working in small groups to identify chemical elements, substances and mixtures in package inserts. The purpose of the activity was to find out whether the students were able to apply the concepts studied in previous lessons and whether they were able to relate the information contained in the package insert with some properties and effects caused by the substances on the human body. The students were invited to answer some questions posed by the teacher, such as “Are the medicines substances or mixtures?”, “What substances and chemical elements are present in the medicine?”. At the end of the lesson, teachers and students engaged in an open discussion with the whole class, when each group presented their answers.
In the planned teaching and learning sequence, at least two different contexts were approached: the sociocultural context – Aristotle's ideas, use of antibiotics in war, medicine package inserts; and scientific context – presentation and simulation of scientific models for substance, discussions on the properties of substances and composition of materials. We expected to analyse the students’ process of conceptualization by considering the dynamics for emergence of different zones of the conceptual profile in the classroom discussion.
Data were organized in three parts for analysis: the answers to the questionnaire, the students’ and teachers’ speeches in the classroom discussion and interviews with two students. Answers to the questionnaire were categorized taking into account the conceptual profile zones for substance: generalist, pragmatic/utilitarian, substantialist, empirical, rationalist and relational. Students and teachers’ speeches were analysed in episodes extracted from the transcripts of the videos. The transcripts were made using standard punctuation marks seeking to highlight pauses with commas, questions marks for interrogations, slash to indicate interruption for speech, and parentheses to make explanation or complementation for elocution. The episodes were defined as pieces of discursive interactions that bring a meaningful set of ideas expressed by students and teachers (Amaral and Mortimer, 2007), and they were organized in turn to allow the identification of different ways of speaking that can be related to modes of thinking on substance.
Mortimer and Wertsch (2003) sought to establish relationships between ways of speaking and modes of thinking that are representative of zones in a conceptual profile. According to the authors, different modes of thinking are interwoven with different forms of speech characterized in terms of social languages and discourse genres (Bakhtin, 1981, 1986). According to Bakhtin, this heterogeneity of speech exists because a discourse consists of several different social voices, considered as specific points of view about the world, the ways of conceptualizing the world in words, specific perspectives of the world, each one characterized by its own objects, meanings and values. As such, they can all be juxtaposed to one another and coexist in the consciousness of real people.
From this point of view, the analysis of speeches produced in the classroom was oriented to the identification of ways of speaking of the students about substance that presented the characteristics described in the proposed zones for the conceptual profile of substance, thus seeking to associate ways of speaking and modes of thinking about this concept. Identifying the emergence of conceptual profile zones in students’ speeches is not always an easy task, considering that the ideas representative of one or another zone are not necessarily expressed in an isolated and well-defined way, e.g., students often express ideas in which different zones of the profile may be articulated. In analysis, we tried to visualize a set of elocutions to interpret modes of thinking presented by students. In Table 2, we summarize the zones of the conceptual profile for substance used in the analysis.
| Zones | Brief description |
|---|---|
| Generalist | Substance considered as something present in everything. There is no differentiation of materials, mixture, substance or chemical element. In addition, substance is like the essence or something essential that constitutes and qualifies materials |
| Pragmatic/utilitarian | Substance defined or characterized by their applications and uses, acquiring importance for possible benefits or damage caused to human beings |
| Substantialist | Substance understood as something material more than a chemical model, even in atomic–molecular dimensions leading students to consider that the properties of a substance extend to its constituents (atoms or molecules) |
| Empirical | Substance addressed by physical and chemical properties, which are important for its chemical identification. It is recognized that there are many substances constituting materials and giving them specific properties |
| Rationalist | Substance understood by scientific models to explain its structures, properties, and classification in different types – simple, compound, organic, inorganic, etc. – depending on the entities that compose them |
| Relational | Substance understood in a complex view when the relational dimension for the properties are considered. Properties do not identify the substance itself, but represent the behaviour of the substance when it is associated with specific conditions |
Different ways of speaking associated with different modes of thinking on substance, represented by zones of the conceptual profile of substance, were identified in the questionnaire and episodes extracted from the lessons. They were framed on a spreadsheet (Microsoft Excel®), in which lines represented the conceptual profile zones, emerging from each of the lessons, considering the individual responses and speeches for each student. A main spreadsheet was organized with different arrangements that enabled us to estimate frequencies in which the zones of the conceptual profile emerged in the speech of each student, and for the entire data set analysed. Different graphs were generated from the main spreadsheet and they favoured a visualization of changes in the emergence of zones in the data set organized for analysis. It is important to emphasize that the values for frequency cannot be considered as absolute values.
Results and discussion
The results are presented in three parts. Firstly, we present an analysis of the students’ answers to the questionnaire taking a glance at the possible zones of the conceptual profile of substance that could be associated with the responses, before the application of the TLS. In the second part, we show an analysis of the episodes extracted from lesson 1 and an overview of lessons 2 and 3, from which we identify the emergence of zones of the conceptual profile in the discursive interactions, at different moments of each lesson, seeking to characterize conceptualization processes. In the third part, we present an analysis of the interviews with two students, after the application of TLS, in which we seek to understand details on the conceptualization process for these students.Analysis of the questionnaire
The questionnaire applied in lesson 1 had six questions, from which we looked for previous conceptions of the students to take a glance at possible zones of the conceptual profile that could be implied in these conceptions. Before the first question, the students read a short poem exalting chemistry that is present in various situations – water we drink, breathing, human metabolism and medicines.Questionnaire applied to the students
POEM: Chemistry, a central science (Magda R. S. Vieira)
Chemistry is a science/Very active and special/It is present in our life/In different and unique ways/As we look around/We cannot deny/Chemistry is all near us/At all times and places/In the water we drink/In our breath/Also in our metabolism/We see Chemistry in action/Developing medications/Creating new technologies/Beyond the limits/From the pioneer Alchemy/For those who still/Find Chemistry apart/Look inside you/It joins us all the time.
(1) According to the poem, Chemistry is actively present in our daily life. So, substances surround us. For you, what is a substance?
(2) Give some examples for: (a) chemical elements; (b) substances; (c) materials.
(3) Aristotle was a Greek philosopher who lived between 384 and 322 BC, and he asserted that four primordial elements constituted all things: water, earth, fire and air. For him, different combinations of these four elements constituted everything. Do you agree with this philosophical view (Aristotelian)? Is it similar to the chemical view of substance we have today? Justify.
(4) Evaluate the statements if they are correct or not and justify your answer: (a) gold is a metal made up of atoms of gold. Atoms are small particles and they form substances. Gold is yellow, so gold atoms are yellow; (b) regular consumption of apple can prevent or reduce high cholesterol as well as many other health benefits. An apple contains several substances.
(5) What do you mean by pure substance? Give an example.
(6) In the previous lesson, we study the properties of matter. We have seen that there are general properties such as mass, volume, and inertia, and specific properties such as density and boiling point. How important is it to know the properties of substances?
In general, the students presented short responses to the questions and we were only able to make approximations to the zones of the profile. We did not analyze question 5 from the questionnaire because the students’ responses were out of the scope of this paper. In question 1, the poem was mentioned and the teacher asked students: for you, what is a substance? Some of the students’ responses were:
It is something that is present everywhere and everywhere on the earth; It's all around us;
Substance is something made up of atoms;
(It) is a set of molecules, which may form some things;
For me, substance is all that has volume, matter;
Substance is all that has chemical composition.
In question 2, the students were asked to give examples of (a) chemical elements, (b) substances and (c) materials. Some of the students’ responses:
(a) chemical elements:
Set of atoms with different atom numbers e.g. mineral water;
Gases;
Hydrogen;
O, H, N;
Chlorine, sodium chloride;
Water; H 2 O.
(b) substances:
Set of chemical elements ex: sparkling water;
Water, air (H 2 0, CO 2 );
Water (H 2 O), carbon (C);
NaCl, CO 2 .
(c) materials:
Gold, plastic;
Pen, pencil;
Elements in different physical states e.g. ice;
H;
Beaker, graduated cylinder, Erlenmeyer flask, Petri dish, watch glass
The responses in these two questions suggest that the students understand substance in different ways and there are misconceptions in relation to the chemical view. According to the responses, the students considered substance as something present in everything, and there is no distinction among materials, substances, and chemical elements; or substance has materiality (volume and matter); or substances have atoms or molecules in their constitution, presenting a chemical composition. The examples proposed by the students reflect the heterogeneity of thinking and suggest that most students do not know how to distinguish chemical elements, substances and materials. Although it was not possible to know details of the students’ ideas from their short answers, it is possible to consider that some of the ideas are related to different modes of thinking about the concept, and consequently they should be associated with different zones of the conceptual profile such as generalist, substantialist, pragmatic/utilitarian and rationalist.
The ideas proposed by Aristotle on elements and substances were mentioned in question 3, when the students were challenged to compare the views from philosophical and current contexts. Most of the students disagreed with the Aristotelian view, but they did not present clear ideas on atoms, elements and substances or the distinction between them. Some answers to this question were:
The composition of matter is made up of atoms that constitute all things;
(Matter is) Composed of atoms of various substances;
I do not (agree);
Everything is composed of chemical elements, water, fire, etc. Everything is made of substances and it is divided into different levels; I think matter is all made up of atoms and can have one or more elements.
One student responded: I agree with him (Aristotle). In bringing up Aristotle's ideas, again we find the heterogeneity of thinking on substance and elements expressed by the students. For this question, the students’ responses suggested ideas associated with the generalist and rationalistic zones of the conceptual profile on substance.
In question 4, the students were invited to remark on given propositions: (a) Gold is a metal made up of atoms of gold. Atoms are small particles and they form substances. Gold is yellow, so gold atoms are yellow; (b) regular consumption of apple can prevent or reduce high cholesterol as well as many other health benefits. An apple contains several substances. For item (a), we found most of the students denying the proposition (Wrong, because the color of a substance does not define the color of its atoms), but three students agreed with the sentence (True, because the atoms together form the gold). Here, we found clues of the substantialist mode of thinking on substance, from which the students do not differentiate the properties of atoms, molecules and substances. Here, the sentences informed clearly that gold is a substance made up of atoms, and we were seeking to verify if students would extend the properties of the substance to its constituting atoms.
For item (b), most of the students gave one-word responses (yes or true) confirming what the proposition stated. One student tried to explain (True, it must contain one substance for each benefit – Laura), and two students denied the proposition without justification. In this sense, it was not possible to make a deeper analysis of this question and analyzing the modes of thinking, but the answer from Laura suggests an understanding of substance associated with the benefits that it can promote for health (pragmatic/utilitarian zone).
In question 6, we asked the students about the properties of the substances. Many students presented vague or nonsense answers and some of them presented answers such as: To know how to apply the substances by knowing their properties; to be able to identifysubstances; to know in which physical state it (the substance) is. From these answers, we suggest that some students seemed to recognize the importance of the properties of substances for different applications (a pragmatic/utilitarian view) and as a way of identification of the substances (empirical or rationalistic view).
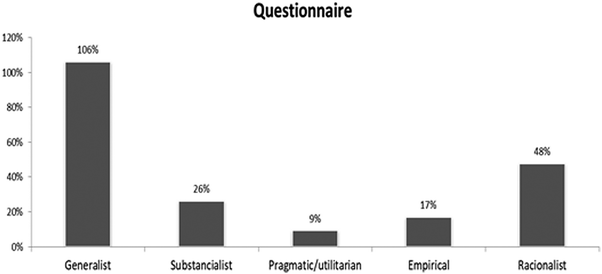
The answers of the students to the questionnaire present ideas that suggest approximations to the different zones of the conceptual profile, with the exception of the relational zone, which was not identified in the answers. Considering an approximation of the students’ responses to the zones of the conceptual profile for substance, we estimated frequencies to these zones for the whole class, as shown in Fig. 1. The students often expressed different modes of thinking in their responses and the sum of the percentages overpassed 100%.
According to Fig. 1, on the one hand, the students’ previous conceptions seemed to be representative of the intuitive or informal ideas, probably from perceptions built in the sociocultural context (generalist and substantialist zones – 132%), perhaps because of the lack of familiarity of the students with chemistry studies, at this stage of schooling. On the other hand, it is interesting that we also found answers that seemed to represent ideas aligned with the scientific view for substance (empirical and rationalist zones – 65%). This seems to be associated with previous studies of the concept (year 8), when some students reached scientific aspects to understand substance. The analysis of the discursive interactions in the classroom was more effective in identifying the emergence of the conceptual profile zones on substance.
Analysis of the emergence of zones of the conceptual profile of substance in lessons
The emergence of zones of the conceptual profile of substance was analyzed from the transcripts of the three lessons that were video recorded, from which relevant episodes were extracted. We will present the detailed analysis for lesson 1 and the synthesis of the analysis for lessons 2 and 3.After reading the text, the teacher posed some questions similar to those posed in the questionnaire: (a) Nowadays is there the concept of element as stated in the time of Aristotle? and (b) Do you agree with Aristotle's ideas on the composition of matter? The students discussed these questions in small groups and the teacher intervened when she had demands or perceived it was necessary. Small group work favored the interactions between students for discussion and elaboration of ideas. Episode 1.1 (Table 3) represents a discussion in a specific group.
| 1. | Teacher: For you, what has changed about the concept of chemical elements and substance? (from the historical context to nowadays) |
| 2. | Miguel: We discovered that the chemical element is like the atom of the substance… |
| 3. | Valentina: Nowadays, the element is constituted by atoms… |
| 4. | Miguel: Nowadays, element is as if it was the atom of matter |
| 5. | Valentina: Before, it was the substance, the element… |
| 6. | Miguel: It was fire, earth, air… |
| 7. | Valentina: Then! They were the substances! |
| 8. | Miguel: Yes, they were the substances of today… |
| 9. | Valentina: What about the substance? |
| 10. | Miguel: Nowadays, it is the set of elements. |
In episode 1.1 (Table 3), Miguel and Valentina expressed an understanding that suggests the differences between the concepts of chemical elements and substances in the historical and current contexts. For them, nowadays we have been considering the idea of atoms as constituting elements (turn 3), and previously there was the idea that matter was constituted of four elements (turns 5 and 6), and these elements had a status of substance (turns 7 and 8). The students expressed that current ideas on atoms, elements and substances are organized as follows: elements are like an atom for the substance (turn 2) or matter (turn 4), and substances are constituted by a set of elements (turns 9 and 10). They seemed to understand that explanatory models of matter have changed throughout history; however, we do not have evidence that they understood what a chemical element is, when they spoke of elements as atoms that constitute substance or matter. From this analysis, it is not appropriate to consider that the students presented a rationalist view of the concept of substance, even though they reasoned based on the atomic–molecular dimension. In this sense, we consider that they presented the ideas that represent the generalist zone of the profile in this episode, considering that they do not differentiate materials, substances, chemical elements and atoms. Araújo et al. (1995) argue that most of the textbooks still present Aristotelian definitions of substance and highlight that problems can appear when teacher and students deal with these views. Silva and Amaral (2013) found that students do not seem to have a clear idea of how substances compose materials and they are constituted by chemical elements.
Episode 1.2 (Table 4) was extracted from discussion with the whole class during the activity of digital simulation, when the teacher was presenting the scientific view of substance by showing simulations of the atomic–molecular composition and dynamics for substances – water, oxygen, neon and argon – in different physical states (gaseous, liquid and solid). The software displayed the images of small colored balls representing atoms that constitute three simple substances (oxygen, neon and argon) and one compound (water). The grouping of balls changed by heating or cooling simulating different physical states for each substance. These images were shown to the whole class with a projector. The teacher's purpose was to discuss the differences between elements, substances and mixtures, confronting ideas from historical and scientific contexts.
| 1. | Teacher: The air, is what? |
| 2. | Laura: There are several substances… |
| 3. | Teacher: And when you have several substances, what do you call it? |
| 4. | Rafaela: Element? |
| 5. | Jose: No!!! Mixed substance |
| 6. | Laura: So the air is a mixture? |
| 7. | Miguel: Oxygen, carbon dioxide, water… |
| 8. | Teacher: And what are oxygen, carbon dioxide and water? |
| 9. | Miguel: Chemical elements |
| 10. | Laura: No, substances! No? Element? Ahhh I do not understand anything at all! |
In episode 1.2 (Table 4), the three students seemed to understand the (atmospheric) air as a mixture, but they did not express well-defined ideas about the differences between substance and chemical element. Laura and Jose indicated the air as a mixture (turns 2, 5 and 6), and Miguel specified some components of it (turn 7), but he got confused defining these components as chemical elements (turn 9) and also Rafaela (turn 4). In the activity of digital simulation, students visualized substances such as neon and argon – constituted for one atom each – in an atomic–molecular dimension and it seemed to interfere with conflicting ideas presented by them. Also, Laura clearly expressed her difficulties in differentiating substance and element (turn 10).
In episode 1.2 (Table 4), we observed advances in the students’ speeches, which appeared more elaborate than in episode 1.1 (Table 3). It is important to highlight that the activities dealt with two different contexts – historical and nowadays – to discuss the concepts, creating opportunities for the students to visualize them in diverse situations. Although there is no evidence that they put together ideas from the different contexts, we can consider that students seemed to look for shared meanings for these ideas, performing their own trajectories in a process of conceptualization (Mortimer et al., 2011). In terms of conceptual profile zones, we realize a movement from generalists to a more rational way of speaking on substances, even though there are persistent ideas in a generalist zone.
In order to analyze how the students were making meaning for the concepts, we extracted another piece of the discursive interactions following on the activity of digital simulation. In episode 1.3 (Table 5), we intend to illustrate a moment in which the students expressed ideas closer to the scientific view of substance.
| 1. | Teacher: What is the difference between oxygen and water? |
| 2. | Jose: Because the water is made of two different atoms |
| 3. | Carlos: Because the water molecule has three atoms and oxygen has two |
| 4. | Gabriela: Is a set of different molecules and elements |
| 5. | Teacher: What are the elements that make up water? |
| 6. | Jose: Hydrogen and oxygen |
In episode 1.3 (Table 5), Jose expressed a good understanding of the constitution of water (turns 2 and 6) and Carlos pointed out the difference between water and oxygen from the different number of atoms in both molecules (turn 3). Gabriela still presented ideas of a generalist view when she did not show indications for which molecules and elements are different in the two substances, oxygen and water (turn 4). At this point, Jose and Carlos seemed to differentiate the concepts of atoms, elements, and molecules, and we can consider they presented the ways of speaking associated with the rationalist zone of the conceptual profile, since they point to an understanding of water (substance), considering its constitution by two chemical elements, from an atomic–molecular dimension.
In summary, in lesson 1, several activities were carried out with distinct objects and objectives that sought to explore the concept of substance under different aspects. The engagement of the students in carrying out these activities played an important role in the meaning-making process for concepts. The small groups work favored the expression of ideas by the students. The reading of text with issues related to the historical development of the concept and the subsequent discussion were important for the perception and understanding of the sociocultural aspects associated with the meaning attributed to the concept of substance. All these features – expressing ideas, reading the text to confront ideas, and analyzing digital simulations – contributed to make meaningful the classroom discussion and consequently to expand the repertoire of ideas in the process of meaning making for the scientific concept.
Some of the students emphasized the importance of substances to human beings, following ideas given in the text, which highlighted the use of antibiotics for the treatment of infections in soldiers injured at the front in the war. Therefore, the reading stressed the ideas about the macroscopic properties and applications of substances, and the students were invited to extend their ideas on substance considering differences other than from elements and mixture (lesson 1). The text favored the emergence of ideas included in the pragmatic/utilitarian zone of the conceptual profile, when they highlighted relationships between substances and their application, for example, as Carlos claimed that knowing the properties of substances is important “to know whether it (the substance) causes benefits or damage”. Nevertheless, ideas associated with the other zones of the conceptual profile emerged in the classroom discussion.
The students expressed ideas representing the empirical and rationalist zones of the conceptual profile, especially when the teacher presented scientific models to explain the theme. For example, when referring to the properties of substances, Manuela claimed that they are “extremely well defined”, which tends to be associated with an empirical mode of thinking. Also, Jose pointed out that a pure substance “has only one type of molecule”, and “when it (the substance) has one type of atom it is a simple substance”, making clear the difference between pure and simple substances by using a scientific model to characterize the types of substances (rationalist zone). The teacher and the students discussed the classification of substances, when they try to understand the properties of the substance that constitutes medicines or other products, and the process of extraction of these substances from natural sources. It is possible to extract a pure substance from a mixture of them (natural substances), but it is also possible to synthetize chemical substances in laboratories (synthetized substances). For this specific point, students get confused about the difference between the concepts of pure substance (out of the mixture or material) and simple substance – a substance made up of just one type of element.
Ideas representing the substantialist zone of the conceptual profile also emerged when the teacher and the students held a discussion about substances obtained by extraction from plants to produce medicines. For example, when Jose said “Most of the time it (the substance) has to be modified. If you take a substance out of a plant, for example, you have to change something”. The student seemed to consider substance as something material inside of the plant, since it must be modified in the process of extraction, in spite of many times the appropriate process of extraction is chosen according to the properties of the substance. For example, it is common to extract essences from plants by vaporization and condensation. Although it was not the purpose of the teacher, ideas on pure, natural or synthetized substances emerged in the discussions and the students found it hard to manage and to understand these issues. These difficulties went beyond our aims in this work as they focused on differences between natural and artificial substances.
In lesson 2, we see that the type of activity led the classroom discussion through ways of speaking about the sources and properties of substances, favoring the emergence of different modes of thinking mainly associated with the applications and effects of the substances in human life. It is possible to point out ontological shifts in these modes of thinking, considering that it was predominantly ideas on substance more as concrete materials than a model for explanation of the behavior of matter. It is important to highlight that both of the ontological categories (Chi, 1992) – material and abstract – could be aligned with the scientific view for the concept of substance, depending on the articulations constructed between macroscopic and atomic–molecular dimensions. This could be a big challenge to teach the concept of substance in chemistry lessons.
Most of the students were not able to articulate ideas discussed in previous lectures to the analysis of the package inserts. Silva and Aguiar (2011) verified that students (year 8) did not use definitions of substance in a conscious and adequate way in an activity on the importance of minerals on human nutrition, which involved the understanding of the concepts of chemical elements and substance. In this work, the students seemed to understand that several substances constitute medicines and these medicines when ingested by the people can provoke desired reactions (cure diseases) and/or side effects. For them, the effect caused by these substances in our organism depends not only on the nature of the constituents but also on the amount in which the medicines are ingested. However, they have struggled to translate their ideas in terms of atoms, chemical elements, and molecules that make up the medicines.
With regard to the emergence of the conceptual profile zones, we observed the ways of speaking representative of four zones: generalist, pragmatic/utilitarian, substantialist and rationalist. To illustrate the ways of speaking associated with the generalist zone, we look at the speeches by Jose: “it does not matter the substance, depending on amount and intensity, substances can cause benefits or damages to the human body”, and by Laura: “the elements are secnidazole and excipients, and the substances are what constitute them, it is cellulose”. Jose seemed to reproduce common knowledge about substances and their effects, without a deep reflection on chemical concepts. Laura presented very confused ideas related to the names read in the package inserts when she considered the active principle of the medicine, secnidazole (synthetic derivative of the group of nitroimidazoles with antiparasitic activity effects) and excipient as elements made up of cellulose (substance).
Ideas associated with the pragmatic/utilitarian zone can be illustrated by the following speech by Laura: “I think the natural substance is likely a chemical substance (both) that can cause benefits or damage”, and by Miguel: “I think they (the medicines) are made up of some substances that together will cause effects”. Bernardo expressed the ideas associated with the substantialist zone when he said, “What are medicines made up of? From compressed substances”. Rationalist ideas were considered when the students recognized substances described in the package inserts regardless of whether they were represented by chemical formulas, for example, when Rafaela said: “…if we know the molecular formula of the substances, so we could know the elements” and Valentina said: “cellulose, silicon, sodium starch glycolate…those are substances”. In this way, the students tried to answer the issues posed by the teacher – to identify chemical elements, substances and mixtures in the package inserts – but other ideas emerged in the discussions in both small groups and the whole class.
In the analysis of the three lessons, we observed variations in the emergence of zones of the conceptual profile of substance. The emergence of the conceptual profile zones for the whole class in the episodes extracted from each lesson and in the questionnaire is illustrated in Fig. 2.
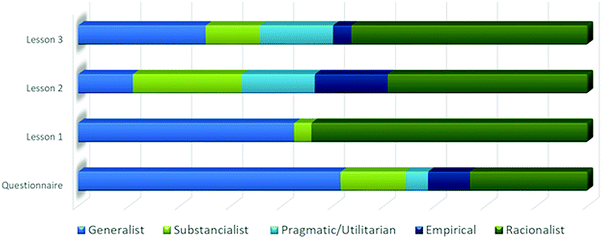 | ||
| Fig. 2 Dynamics of the emergence of the conceptual profile zones in the three lessons and questionnaire for the whole class. | ||
The students expressed ideas associated with all zones, except for the relational zone, which represents a complex way of understanding the substance. The ideas related to the relational zone were not expected for this level of schooling. In Fig. 2, we can observe a large variation in the emergence of the conceptual profile zones throughout the three lessons and in the questionnaire. We attribute these variations to several factors, such as the different types of questions and didactic situations to which the students were exposed, and the use of a theme in which the concept of substance has a specific use related to human health. In spite of the texts and theme guiding the discussions in a specific direction, in general, we can see that students presented more elaborate ideas than the informal conceptions found in the responses to the questionnaire, throughout the lessons (see Fig. 2). Nevertheless, students’ informal conceptions emerged in discussions of new situations even if they seemed to advance in a scientific understanding of substance, for example, when they work with package inserts.
Fig. 2 shows generalist ideas that are more evident in the questionnaire, lesson 1 and again in lesson 3. We agree that the students did not abandon their informal conceptions in the process of teaching and learning of scientific concepts, but we can see that they advanced in expressing ideas related to the scientific zones, extending their particular conceptual profiles (Mortimer et al., 2014). Besides, they expressed more diversified ideas in lessons 2 and 3, suggesting that scientific ideas were discussed in articulation with other modes of thinking.
The TLS design played an important role in promoting discussions and bringing out diverse conceptions that can be contrasted and reflected from the school content to be studied. Therefore, in this work we seek to promote an approximation of scientific knowledge with the sociocultural context from a theme, a way to contemplate the epistemic dimension of the teaching and learning sequence (TLS), according to the terms proposed by Méheut (2005). From the point of view of the pedagogical dimension, we found that the teacher played an important role in favoring the communication of the ideas by the students, seeking to raise and capture different conceptions and visions about the concept being worked on. A broader domain of possible sense and meanings that the concept can acquire in different contexts or situations seems to make the teacher more aware and engaged in the task of promoting debate and interactions in which students’ ideas are considered (Silva and Amaral, 2010). It points to the potential use of the conceptual profile theory in the formation and professional development for science teachers.
In the analysis of the emergence of the zones of the conceptual profile from the discursive interactions in the classroom, each student went through a particular conceptualization process, presenting different rhythms and pathways in making meaning to the contents studied. We consider that it is important to analyze learning pathways experienced by two students, seeking to understand in depth the different aspects of the process of conceptualization. As previously mentioned, learning pathways were analyzed in terms of the variations in the emergence of conceptual profile zones for each student in each lesson.
Process of conceptualization of the students – Laura and Jose
We present a detailed analysis of the conceptualization process for two students – Laura and Jose – who actively participated in all of the lessons. This analysis would be unreliable for students who participated in a limited way because we do not have enough evidence for how they dealt with previous and new ideas focused in the classroom discussions. The expression of ideas that we identified as associated with the zones of the conceptual profile was different for both students, which endorses the individual dimension of the meaning-making process for scientific concepts, although social interactions play a crucial role in it.Analysis of Laura's conceptualization process
Laura expressed ideas and raised questions in most of the classroom discussions. In her speech, she explicitly recognized her difficulties with different aspects of the concepts of substance and chemical elements. In Laura's responses to the questionnaire, we were able to identify conceptions that suggest approximations to the three zones of the conceptual profile, at least. She pointed to the importance of knowing the properties of the substances “because from it we can classify and divide them into groups and facilitate the study (on substance)”, suggesting an empirical view of the concept. In replying to the proposition about apples’ benefits and composition, she expressed “true, as it should contain one substance for each benefit”, highlighting a pragmatic view on substance. Laura considered false the statement about the yellow color for gold because the atoms are yellow, “false because I think atoms are very small and their color has no influence”. In spite of the proposition being considered false, she seemed to state the color for atoms, a macroscopic characteristic, suggesting an approximation to the substantialist commitment.Throughout the three lessons, different ways of speaking were identified in Laura's speech, when conceptions and ideas were exposed, confronted, compared and reflected, in the classroom discussions. In lesson 1, Laura expressed difficulties in differentiating chemical elements, simple substances, compounds and mixtures, for example, in the discussion about air, she seemed to be surprised because air is a mixture, and she felt confused to classify its components: “No, substances! No? Element? Ahhh I do not understand anything at all” (episode 1.2 (Table 4), turn 10). In addition, she presented misunderstandings about pure substance and simple substance: “I think pure (substance) is when you have only one type of element”. These are the ideas representative of the generalist zone of the conceptual profile, and they often emerged in her speech. It did not seem to restrict her in stating the ideas representative of the other zones, for example, for the rationalist zone, she claimed “nowadays water is a substance, but before it was an element! That substance nowadays, in the case of water, is formed by hydrogen and oxygen”.
In lesson 2, during the discussion about the acquisition and application of natural and synthesized substances, Laura stated that there is no difference between these two types of substances – “I think it's the same thing” – suggesting an understanding of the relation between the identity of substances and their properties, regardless of how they were obtained. Ideas like that are representative of the empirical zone of the substance concept. In some moments of the lesson, Laura referred to substances as something that might cause some benefit or damage to the human body – “it (the substance) can be changed to cause benefit to your body, or it can be changed to injure it, depending on the quantity…”. For her, the amount of substance plays an important role for the action of substances. In this case, the use and effects of the substances seemed to guide the comprehension of the concept, promoting the emergence of the pragmatic/utilitarian zone of the conceptual profile. The ideas representative of the substantialist zone emerged in this lesson, for example, when Laura admitted that an isolated water molecule has an equal boiling point at 100 °C.
In lesson 3, the teacher's purpose was to encourage students in applying the concepts studied to analyze package inserts. Laura appeared to realize that medicines have several substances in their composition and presented a more structured speech: “I think the medicine is a mixture, which is made of substances that are excipients and secnidazole”. It is quite probable that she read this information on the package insert, but she did not know the meaning for technical names, including excipients like a substance. Conflicting ideas about the differences between substances and molecules emerged when she requested “So molecule is substance?” and she replied herself “but it is small”, showing difficulties in differentiating macroscopic and atomic–molecular dimensions of substances, which often implies in assigning the properties of the substance to the molecule. In this case, the ideas included in the substantialist zone emerged in Laura's speech, in this lesson.
According to the analysis shown above, from Laura's speech, the ideas representative of the zones of the conceptual profile were placed in an Excel® spreadsheet, in order to generate a visualization of the emergence of zones for the student throughout the TLS. The emergence of the zones is not considered from the absolute values of the frequency, but it represents a proportional analysis of the dynamics of zones observed during the three lessons and in the questionnaire.
In Fig. 3, we can see the heterogeneity of the ways of speaking by Laura at different moments in the lessons and in the questionnaire, which makes it possible to consider that different modes of thinking were interwoven in her conceptualization process (Mortimer and Wertsch, 2003; Mortimer and El-Hani, 2014). In general, it seems reasonable to expect that after the student expressed ideas aligned with the scientific view, the emergence of the nonscientific modes of thinking should be overcome. Nevertheless, Fig. 3 shows an oscillation of the emergence of the scientific and nonscientific zones of the conceptual profile. For example, even though generalist ideas emerged in lesson 1 and they were less frequent in lesson 2, as the student followed constructing more structured ideas, generalist ways of speaking persist in Laura's speech when she was faced with applying these ideas in a new situation (insert packages – lesson 3).
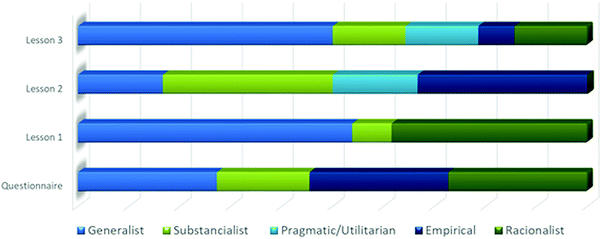 | ||
| Fig. 3 Emergence of the conceptual profile zones in the three lessons and the questionnaire for Laura. | ||
In this dynamic process, stabilized meanings could be part of the students’ repertoire of ideas. We consider that the scientific ideas of substance were incorporated to the senses and meanings presented by Laura, which seems to be aligned with the objectives of educational aims at school. Scientific and nonscientific ideas play important roles in the teaching and learning process of making meaning for scientific concepts. In this case, we reinforce the conceptualization process such as a continuum in which senses and meanings oscillate depending on the experiences of individuals (Mortimer et al., 2011).
In the interview with Laura, after the TLS, our expectation was to identify details of the conceptualization process experienced by the student. Initially, we asked her to define the concept of substance, and episode IL1 (Table 6) shows a piece of the interview at this moment.
| 1. | Teacher: Define substance |
| 2. | Laura: Every time I feel confused about element and substance. I know that element is the whole set (…) element is a kind of general, and substance I do not know well what it is, because… it has the atom of something. For example, the substance oxygen would be in the case O2, the substance would be O2, and an “O” would be an atom and a lot of O2together is an element, I think. |
| 3. | Teacher: Do you think a lot of O2is an element? In the periodic table, what do we find? |
| 4. | Laura: Elements. |
| 5. | Teacher: Is there oxygen in the periodic table? |
| 6. | Laura: There is. |
| 7. | Teacher: But we see the representation in the periodic table only with an “O”, right? |
| 8. | Laura: Yeah! But the element would be “O” because it would be “O” along with all its variations. Like that… “O” isotopes would all be together when putting the “O” element there. And like that… what's the point? Substance right? Substance would be, for example, O2the substance oxygen. |
| 9. | Teacher: You defined the substance using an example and speaking in microscopic terms. |
| 10. | Laura: So if I was defining it in words other than with examples, I would say that substance is a set of atoms. |
In episode IL1 (Table 6), Laura was able to express in detail her conflicting ideas about chemical elements and substance (turn 2), using words belonging to the scientific language. This suggests a kind of appropriation she had during the lessons and an indication that she is advancing in the understanding of the scientific meaning of substance. She correctly exemplified a substance (O2), identified the atom that forms that substance (O), but defined an element as “a lot of O2”. The teacher intervened seeking to stress the senses presented by Laura for substance (turns 3–7), by evoking chemical symbols presented in the periodic table. In turn 8, Laura recognized O as an element representative of its different isotopes and she compared it to the substance O2. The teacher suggested that she is putting together macroscopic and atomic–molecular (“microscopic”) dimensions for the concept (turn 9). Then she defined substance as being “a set of atoms” (turn 10). We consider that despite the advances in the use of the scientific language, Laura yet did not embody stabilized meanings for chemical substance.
In another moment of the interview, a macroscopic dimension was mentioned to investigate her comprehension of substance properties, mainly considering the activity with the package inserts. This moment is shown in episode IL2 (Table 7).
| 1. | Teacher: How important is it to know the properties of substances? |
| 2. | Laura: I think it's important for you in differentiating one from the other, so you know more about the substance. For me, it does not matter, but for certain people who… |
| 3. | Teacher: Why do you think it does not matter, you do not have substances in your daily life? |
| 4. | Laura: Yes, but I kind of do not care what property of the substance I “catch”. |
| 5. | Teacher: Because you are only thinking about the melting point, boiling… you are not thinking of the smell, flavor… |
| 6. | Laura: It is that whenever I think of properties I remember more of melting point and boiling point, things like this |
In episode IL2 (Table 7), Laura recognized the properties as important to identify substances mainly by addressing melting and boiling points for them (turns 2 and 5), a very common approach found in chemistry textbooks. However, she did not realize the role played by these properties in materials or substances present in our life or social context (turn 4). Vogelezang (1987) stated that students admitted the existence of substances (or materials) around everything, but usually they do not associate them with contents studied in lessons of chemistry. At another moment of the interview, the teacher expressed the following statement: “Substance is everything that is around us. Almost everything we see in the world is a chemical substance”. And Laura commented: “I think kind of everything we see is composed of substances, which does not mean that this is a type of substance (…) a pen is made up of several substances, but that does not mean a pen is a type of substance… no! One or more substances make up a pen”. She seemed to understand the difference between material (mixture) and substance. When the teacher asked her to comment on another sentence “The smell of a perfume is due to a substance present in the material”, Laura stated “I think the smell is a property like this… and what will come of it… maybe, I think, that the perfume is a mixture, and I think the smell is a property of this. The smell will be a property of the substance present in there. The smell is not a kind of substance. The substance has properties like melting and boiling points, color, smell, taste… I think this is also a property of the substance. So I think the smell is a property”. Here, Laura seemed to get confused about the smell as a sensation and as the property of a specific substance composing the perfume.
These results suggest that Laura presented elaborate ideas about substance, but she did not stabilize meanings in terms of a scientific view, and (personal) senses still played an important role in her conceptualization process. It appears from such evidence that the process of conceptualization occurs by oscillation between senses and meanings, in a continuum movement toward the stabilized meanings, which are shared socially. Laura advanced in the understanding of substance by acquiring the domain in the use of the scientific language but the meaning-making process is not conclusive, and she would be faced with challenges in her learning pathway as soon as a new situation is engendered. From this perspective, it is not so easy to measure progression for learning.
Analysis of Jose's conceptualization process
Jose presented active participation in the classroom discussion and, differently from Laura, most of the time he expressed structured ideas in a scientific view in the responses to the questionnaire and throughout the three lessons. In this way, he seemed to understand the different aspects of the substance concept – differentiating elements, substances and mixtures, and the classification of pure and simple substances, in both macroscopic and atomic–molecular dimensions.In lesson 1, Jose presented predominantly ideas from a scientific view, when debating in the diverse activities and discursive contexts. For example, he expressed ideas such as: “H2O is a substance and H is an element; What changed? Now, I know what it is. Chemical element is atom; Substances are atoms together; H2O is made up two different atoms; (pure substance) is when it has only one type of molecule; (simple substance) because it is constituted of only one type of atom”. Jose showed the ways of speaking aligned with a rationalist mode of thinking on substance, considering atomic–molecular dimensions to explain the constitution of matter. Nevertheless, at some moments, he seemed to show other commitments related to this concept, for example, Jose explains to a colleague – “molecule is substance, the smallest part of the substance is a molecule” – it sounds like a substantialist way of understanding the concept, when macroscopic and atomic–molecular dimensions are not clearly distinguished. Also, in discussing Aristotle's ideas, he did not give attention to the difference between meanings related to elements and substance in the historical context and nowadays – “There were four elements and today there are more (of them)” – presenting a generalist way of speaking.
In lesson 2, the discussion on the use of substances to produce medicines and natural and synthetized substances appeared to promote the emergence of different modes of thinking from Jose's speech. He expressed the following idea “I think that chemical substances are created in the laboratory”, from which he associated substance to the processes of synthesis and industrialization of products (generalist view) – and “Most of the time it (substance) has to be modified. If you take a substance out of a plant, for example, you have to change something” – considering substance as something material contained in plants (substantialist mode of thinking). We realize that the approach of the macroscopic aspects for substance stressed some of the structured ideas presented in the previous lesson, leading to the emergence of the ways of speaking representative of generalist and substantialist modes of thinking about the concept. Jose also claimed that it is important to know the properties of substances to “know the amount and intensity of substance (for use)” at the same time that he pointed out “the color, smell” as some of these properties (an empirical mode of thinking of substance). However, when appropriate in the discussion, he confirmed the scientific ideas previously presented (rationalist mode of thinking). For us, it marks the dynamics of his process of conceptualization, when he is invited to express ideas in a continuous movement of seeking for a wide understanding of the concept.
In lesson 3, we see more diversity of ideas in Jose's speech, which suggests that the discussion on the package insert allowed posing new ways of speaking about substance, engaging the student in a new challenge to articulate ideas. From reading the text in the package inserts, Jose was able to identify the name of the components of the medicine, but the lack of symbols for chemical elements lead him to say “I do not think there is any element here”. He considered that it was not possible to get information about chemical elements without the chemical formula for substances. In the analysis about the properties of the substances, he stated “it does not matter the substance itself, it depends on the amount and intensity of this substance (it) can cause benefits or damage to the human body”. He seemed to evoke a very common sense idea about good practices in the use of some products – salt, sugar, coffee, etc. – and, as in the previous lesson, he named “intensity” as a feature to evaluate the use of substances, expressing a way of speaking representative of the generalist mode of thinking. Jose also presented conflicting ideas related to natural and synthetic substances, when he stated “(natural substances) as the name says (they) are found in nature and do not have to suffer any chemical industrial process and, as I said before, depending on the amount (it) can bring benefits or damage to the organism (body)”. Here, he expressed a naive view about natural substance pointing the amount as the reason for the effects it causes in the human body, showing a way of speaking that highlights a pragmatic/utilitarian mode of thinking of substance. On the other side, differently than in lesson 2, he pointed out that substances’ properties can be useful to identify substances – “When you know the properties of a substance, you can identify it in the environment” – a way of speaking representative of an empirical mode of thinking. The emergence of conceptual profile zones from Jose's speech is shown in Fig. 4.
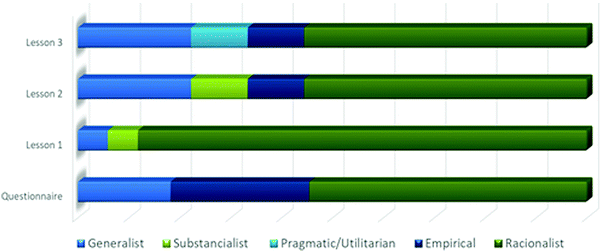 | ||
| Fig. 4 Emergence of the conceptual profile zones in the three lessons and the questionnaire for Jose. | ||
In Fig. 4, we did not see significant changes in the emergence of the conceptual profile zones from Jose's speech throughout the three lessons and the questionnaire. He continued to express ways of speaking representative of the rationalist modes of thinking of substance in the whole TLS; however, other ways of thinking emerged from discursive contexts resulting from activities carried out in lessons 2 and 3, including ideas that are more intuitive. In this case, we highlight the importance of encouraging discussion on different situations in the science classroom in order to bring together different ways of thinking from which the students draw meaning to understand the scientific view for concepts in the face of the different meanings stabilized for them in nonscientific contexts.
These results lead us to a positive expectation toward the understanding of the concept of substance in Jose’'s interview, when a closer conversation would allow us to perceive details in his process of conceptualization. First, the teacher asked him to define substance and he claimed, “Something is made up of molecules (…) if it is pure, it is made up of only one type of molecule. If I am not mistaken, it (a substance is) composed (is constituted) of more (?)”. First, he presented an atomic–molecular view of the substance, and then he seemed to be confused about the classification of pure substance and compound, a difficulty not expressed in the lessons. At another moment, the teacher asked him about the statement that a molecule has the properties of the substance, for example, that a water molecule would have a boiling point in 100 °C. Jose argued, “This is wrong because the molecule has no boiling point, the substance does”, reinforcing his response in the questionnaire.
Following the interview, the teacher showed some statements and asked Jose to comment on them. In Table 8, we show the statements and comments from Jose.
| 1. Substance can be understood as a compound made up of a single element or by more than one element. |
| Jose 1: Yes, a substance is made up of molecules, which are formed of one or more chemical elements. Therefore, when analyzing a substance, it is noticed that it is fundamentally constituted by one or more chemical elements. |
| 2. Pure substance has all the properties distributed equally. A way of verifying whether the substance is pure or not is by freezing or heating it. If the melting and boiling points remain constant, the substance is pure. |
| Jose 2: No, because this also occurs in compounds. The variation (of properties) is observed only for mixtures. |
| 3. Substance is everything around us. Almost everything we see in the world is a chemical substance. |
| Jose 3: No, most of what is around us are mixtures. |
| 4. The pure substance is that which is found in nature and when it undergoes a chemical process, it becomes impure. |
| Jose 4: No, pure substance is that made up of molecules of only one chemical element. |
| 5. The smell of a perfume is due to a substance present in the material |
| Jose 5: No, for the “smell” itself is not a substance, but a reaction of our bodies to a substance present in the perfume (mixture). |
In Table 8, Jose confirmed previous ideas related to the scientific view of substance, nevertheless the conflicting ideas on pure substance and compounds persisted (see items 2 and 4). In item 2, he disagreed with the statement taking into account that compounds (constituted by more than one chemical element) can also present constant melting and boiling points, not only pure substance. He claimed that the pure substance has only one chemical element (item 4), a definition given for a simple substance. In this case, it seemed that understanding of substance in the atomic–molecular dimension is not aligned with the macroscopic comprehension, as purity is related to the separation of substances from mixtures or materials. In lessons 2 and 3, we realize Jose's difficulties in describing the extraction of substances from natural sources. Another interesting point is when Jose did not relate smell to a property of substance present in perfumes but to a reaction that our body presents to the substance (item 5). In lesson 2, he had listed smell as one of the substance properties, and it reinforces that Jose understands the atomic–molecular model to explain the relations among atoms, chemical elements, substance and mixture, however he has difficulties in understanding the macroscopic aspects of this concept.
In summary, we consider that Jose marked mainly the scientific ideas in the classroom discussion, but it does not mean that he did not defend other modes of thinking of substances, since they emerged sometimes. We suppose that Jose has a predominant commitment to scientific views in the school context. In this sense, we claim the importance in bringing discursive contexts associated with different modes of thinking of scientific concepts to the classroom discussions; the teachers could contribute to reach a wider and more meaningful learning, by allowing the students to confront the scientific and the other modes of thinking. In Jose's case, some difficulties to understand diverse aspects on the concept of substance emerged as specific situations were presented in classroom discussion and we realize it more clearly in the interview. The discussion about different modes of thinking seemed to be useful in helping him to make connection between scientific knowledge and real situations.
Laura and Jose showed different processes of conceptualization and learning pathways. On the one hand, Laura presented difficulties to understand substance in an atomic–molecular dimension, and she sought for articulation between macroscopic and atomic–molecular dimensions. On the other hand, Jose presented stabilized meanings related to the atomic–molecular dimension for the concept of substance, but he seemed to have difficulties in articulating this view with macroscopic aspects and applications of the substances.
Finally, we point out that each student must present a particular process of conceptualization with different learning demands. This is an important point to consider when teachers and researchers make proposals for science teaching. In addition, it is relevant to the evaluation of science learning, when advances in the understanding for scientific concepts occur without necessarily overcoming informal conceptions or neglecting meanings stabilized in sociocultural contexts. In this sense, an evolution of the conceptual thinking could be evaluated taking into account the expansion of the repertoire of ideas expressed by students, in which ideas accepted in a scientific view for a specific concept could be confronted to different ways of understanding this concept that reach sense or meaning in other contexts.
Final comments
In this paper, we analyze the process of conceptualization experienced by students when involved in activities in a TLS on the concept of substance, considering the emergence of zones of the conceptual profile. The results showed that the approaches on different modes of thinking of substance enabled the teacher to discuss and confront ideas, leading students to construct or share meanings stabilized in a scientific view. The conceptual profile was an important tool to design activities by creating discursive contexts involving different modes of thinking about substance, which contributed to raising specific discussions involving historical, scientific and social settings to understand senses and meanings for substances. Thus, we mark the relevance of the use of conceptual profile zones as a tool to science teaching and learning by structuring different modes of thinking indicating epistemological, ontological and axiological commitments for them.The students’ process of conceptualization was dynamic and continuous, and dependent on the individual characteristics of students, their experiences and participation in the proposed activities in a TLS. The students’ participation in discursive interactions is crucial to the articulation of senses and stabilized meanings for scientific concepts. Therefore, it is quite important that teachers start discussions in the classroom, and they act as mediators in this process, in order to make the students aware of the heterogeneity of ideas existing in the learning of scientific concepts. It is important to highlight that students expressed different modes of thinking in different moments in the TLS, and some of these ideas persist during the process of understanding the scientific view for the concept of substance. It appeared relevant to address these ideas in the classroom discussion, because many times they come from the experiences lived by students and have value in real life for them, assuming an important role in the meaning-making process for scientific concepts.
From the results in this work, we could point out some issues. It seemed desirable that in science teaching and learning we take into account discussions on scientific and nonscientific ideas in the classroom, in order to promote a meaningful understanding on scientific concepts and models, which could be articulated to other forms of seeing and conceptualizing the world. In this sense, science learning could be aligned with sociocultural contexts contributing to inserting scientific knowledge in real situations experienced by individuals.
The process of conceptualization is characterized as a dynamic process in which different modes of thinking come and go in a collective and individual search to share stabilized meanings. It points to the importance of planning and designing teaching and learning sequences seeking for promoting discussions on diversity of ideas/situations in the classroom, mainly allowing students to express their conceptions and experiences. The evaluation of the learning does not seem to follow a linear or progressive movement of conceptual change, but a more complex and dynamic process in which continuously new ideas are constructed in articulation with the previous ones.
Finally, the conceptual profile theory provides a consistent basis to plan the TLS, to analyze the process of conceptualization and to guide the teacher in leading discussion in the classroom.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
To CNPq (Support for Research Project (478519/2011-7))/CAPES (Postgraduate Scholarship) for financial support to this research.Notes and references
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross-age study of the understanding of five chemistry concepts, J. Res. Sci. Teach., 31(2), 147–165.
- Amaral E. M. R. and Mortimer E. F., (2007), Uma metodologia para estudar a dinâmica entre as zonas de um perfil conceitual no discurso da sala de aula [A methodology to study the dynamics among conceptual profile zones in the classroom discourse]. A pesquisa em ensino de ciências no Brasil e suas metodologias, Ijuí: UNIJUÍ, pp. 239–296.
- André M., (2001), Pesquisa em educação: Bucando rigor e qualidade, Cadernos de pesquisa, 113, 51–64.
- Araújo D. X., Silva R. R. and Tunes E., (1995), O conceito de substância química apreendido por alunos do ensino médio, Quim. Nova, 1(18), 80–90.
- Bachelard G., (1938/1996), A formação do espírito científico, Rio de Janeiro: Contraponto Editora.
- Bachelard G., (1940), A filosofia do não [The philosophy of no], Coleção Os pensadores, São Paulo: Editora Abril Cultural.
- Bachelard G., (1949/1977), O racionalismo aplicado [The applied racionalism ], Rio de Janeiro: Zahar Editors.
- Bakhtin M., (1981), The dialogic imagination, in Holquist M. (ed.), Austin: University of Texas Press.
- Bakhtin M., (1986), Speech genre and other essays, Austin: W. McGee.
- Bogdan R. and Biklen S. K., (2007), Qualitative research for education, Needham Heights (Massachusetts): Allyn and Bacon.
- Chi M. T. H., (1992), Conceptual change within and across ontological categories: examples from learning and discovery in science, in Giere R. (ed.), Cognitive models of science: Minnesota Studies in the philosophy of Science, Minnesota: University of Minnesota Press.
- Close H. and Scherr R., (2015), Enacting conceptual metaphor through blending: Learning activities embodying the substance metaphor for energy, Int. J. Sci. Educ., 839–866.
- De Vos W. and Verdonk A., (1987), A new road to reactions: the substance and its molecules, J. Chem. Educ., 64, 692–69.
- Dickinson D., (1987), The development of a concept of material kind, Sci. Educ., 71(4), 615–628.
- El-Hani C. N., Silva Filho W. J. and Mortimer E. F., (2014), The Epistemological Grounds of the Conceptual Profile Theory, in Mortimer E. F. and El-Hani C. N., Conceptual Profi les: A Theory of Teaching and Learning Scientific Concepts, New York: Springer, pp. 35–66.
- Fonseca M. R., (2007), Quimica – Meio Ambiente – Cidadania, São Paulo, Brasil: FTD, vol. 1.
- Gagliardi R., (1988), Cómo utilizar la historia de las ciencias en la enseñanza de las ciencias, Enseñanza de las ciencias: revista de investigación y experiencias didácticas, 291–296.
- Hashweh M., (1996), Effects of science teachers’ epistemological beliefs in teaching, J. Res. Sci. Teach., 47–63.
- Johnson P., (1996), What is a substance? Educ. Chem., 33, 41–42.
- Johnson P., (2000), Children's understanding of substances, part 1: recognizing chemical change, Int. J. Sci. Educ., 719–737.
- Johnson P., (2002), Children's understanding of substances, part 2: explaining chemical change, Int. J. Sci. Educ., 1037–1054.
- Méheut M., (2005), Teaching–learning sequences tools for learning and/or research, in Boersma K., Goedhart M., de Jong O. and Ejkelhof H. (ed.), Research and the quality of science education, Dordrecht: Springer, pp. 195–208.
- Mortimer E. F., (1995), Conceptual change or conceptual profile change? Sci. Educ., 4, 265–287 DOI:10.1007/BF00486624.
- Mortimer E. F., (1996), Construtivismo, mudança conceitual e o ensino de ciências: Para onde vamos? [Constructivism, conceptual change and teaching of science: Where do we go?], Investigações em Ensino de Ciências, 1, 20–39.
- Mortimer E. F., (1997), Beyond chemical boundaries: a conceptual profile for molecule and molecular structure, Quim. Nova, 20(2), 200–207.
- Mortimer E. F. and El-Hani C. N., (2014), Conceptual Profiles: A Theory of Teaching and Learning, New York: Springer Science & Business Media.
- Mortimer E. F. and Wertsch J. V., (2003), The architecture and dynamics of intersubjectivity in science classrooms, Mind, Culture, and Activity, vol. 10, pp. 230–244.
- Mortimer E. F., Scott P. and El-Hani C. N., (2011), Bases teóricas e epistemológicas da abordagem dos perfis conceituais, TED – Tecné, Episteme y Didaxis, 111–125.
- Mortimer E. F., Scott P., Amaral E. M. and El-Hani C. N., (2014), Conceptual Profiles: Theoretical-Methodological Bases of a Research Program, in Mortimer E. F. and El-Hani C. N., Conceptual Profiles: A Theory of Teaching and Learning, New York: Springer Science & Business Media, pp. 3–34.
- Moura M. O., Araújo E. S., Moretti V. D., Panossian M. L. and Ribeiro F., (2010), Atividade orientadora de ensino: unidade entre ensino e aprendizagem, Revista Diálogo Educacional, 10(29), 205–229.
- Ngai C., Sevian H. and Talanquer V., (2014), What is this substance? What makes it different? Mapping progression in students’ assumptions about chemical identity, Int. J. Sci. Educ., 2438–2461.
- Norman A., (1998), Seeing, semantics and social epistemic practice, Stud. Hist. Philos. Sci., 29, 501–513.
- Oki M. d., (2002), O conceito de elemento da antiguidade à modernidade, Quim. Nova Esc., 21–25.
- Oliveira R. J., (1995), O mito da Substância, Quim. Nova Esc., 8–11.
- Padilla K., Ponce-de-Leo A. M., Rebado F. M. and Garritz A., (2008), Undergraduate professor's pedagogical content knowledge: the case of ‘amount of substance’, Int. J. Sci. Educ., 30(10), 1389–1404.
- Papagerogiou G. and Sakka D., (2000), Primary School Teachers’ Views on Fundamental Chemical Concepts, Chem. Educ. Res. Pract., 237–247.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: Toward a theory of conceptual change, Sci. Educ., 211–227.
- Putnam H., (1995), Pragmatism: an open question, Oxford and Cambridge: Blackwell Publishers.
- Sabino J. D. and Amaral E. M. R. d., (2018), Utilização do perfil conceitual de substância no planejamento do ensino e na análise do processo de aprendizagem, Investigação em Ensino de Ciências, 23(1), 245–265.
- Schutz A., (1967), The Phenomenology of the Social World, Evanston: Northwestern University Press.
- Silva J. R., (2011), Um perfil conceitual para o conceito de substância, Dissertação de mestrado, Recife, Brasil: Universidade Federal Rural de Pernambuco.
- Silva, F. C. V., (2017a), Análise de diferentes modos de pensar e formas de falar o conceito de ácido/base em uma experiência socialmente situada vivenciada por licenciandos em química, Tese de Doutorado, Recife: Universidade Federal Rural de Pernambuco.
- Silva J. R., (2017b), Diversos modos de pensar o conceito de substância química na história da ciência e sua visão relacional, Ciência & Educação, 707–722.
- Silva J. R. and Amaral E. M., (2010), Uma análise sobre concepções de alunos e professores de química relativas ao conceito de substância, Anais do XV Encontro Nacional de Ensino de Química (XV ENEQ), Brasília-DF.
- Silva J. R. and Amaral E. M., (2013), Proposta para um Perfil Conceitual de substância, Revista Brasileira de Pesquisa em Educação em Ciências, 53–72.
- Silva N. S. and Aguiar O., (2011), O uso dos conceitos de elemento e substância por estudantes do ensino fundamental: uma perspectiva de análise sociocultural, Revista Brasileira de Pesquisa em Educação em Ciências.
- Stains M. and Talanquer V., (2007), Classification of chemical substances using particulate representations of matter: an analysis of student thinking, Int. J. Sci. Educ., 29(5), 643–661.
- Tulviste P., (1991), The cultural-historical development of verbal thinking, Commak: Nova Science Publishers.
- Vogelezang M. J., (1987), Development of the concept ‘chemical substance’: some thoughts and arguments, Int. J. Sci. Educ., 9(5), 519–528.
- Vygotsky L. S., (1978), Mind in society: The development of higher mental process, Cambridge: Havard Universit Press.
Footnote |
| † Remédios Milagrosos in Os botões de Napoleão: as 17 moléculas que mudaram a história. Penny Le Couteur, Jay Burreson. Translated by Maria Luiza X. Borges. Rio de Janeiro: Zahar, 2006. (Miracle Remedies. Napoleon's Buttons: How 17 Molecules Changed History). |
| This journal is © The Royal Society of Chemistry 2018 |