Student progression on chemical symbol representation abilities at different grade levels (Grades 10–12) across gender
Shaohui
Chi
 ,
Zuhao
Wang
*,
Ma
Luo
,
Yuqin
Yang
and
Min
Huang
,
Zuhao
Wang
*,
Ma
Luo
,
Yuqin
Yang
and
Min
Huang
Institute of Curriculum and Instruction, Faculty of Education, East China Normal University, Shanghai 200062, China. E-mail: Wangzuhao@126.com
First published on 7th June 2018
Abstract
Chemical symbol representation is used extensively in chemistry classrooms; however, due to its abstract nature, many students struggle with learning and effectively utilizing these symbolic representations, which can lead to ongoing failure in subsequent chemistry learning. Taking the perspective of learning progressions, this study identifies how students’ abilities in chemical symbol representation progress at different grade levels (Grade 10–12), across the genders. A sample of 713 students—254 tenth graders, 262 eleventh graders and 197 twelfth graders—was selected from three senior secondary schools located in Jiangsu, China. A measurement instrument developed in a former study was used to measure students’ chemical symbol representation abilities and students’ raw scores were converted into Rasch scale scores, allowing for direct comparisons of students of different grades. The results of chi-squared tests and analysis of variance (ANOVA) indicated that chemical symbol representation abilities are affected by statistically significant gender and grade effects. Students from higher grades performed better than students from lower grades, and generally, male students obtained a higher mean score than did their female peers. The findings also revealed that there was a statistically significant interaction effect between gender and grade. While male students started out with a much higher mean score in Grade 10, by Grade 11 there was not much of a difference between male and female students’ mean scores, and female students’ mean score was higher than male students’ mean score by Grade 12.
Introduction
Gaining fluency in symbolic representation is an essential key to learning chemistry. Symbolic representation is a highly abstract and specialized chemical language, expressed in the form of symbols, letters, numbers, and signs (Wu et al., 2001). It is used to represent atoms, molecules, ions, compounds, formulas, and structures (Wu and Shah, 2004). However, symbolic representation goes far beyond simply abbreviating the names of molecules or substances; it contains an array of descriptive and conceptual details (Gilbert and Treagust, 2009), and can be used to infer information such as the chemical and physical properties of substances or the potential reaction behavior (Taskin and Bernholt, 2014). Accordingly, chemical symbol representation ability (CSRA)—defined as the competency to identify and understand the meaning of scientific symbols, as well as the ability to utilize those chemical symbols through their expression, inference, and application of those chemical symbols—is a critical component of student's proficiency in chemistry (Wang et al., 2017).Chemical symbol representation is used extensively in chemistry classrooms; however, due to its abstract nature, many students struggle with learning and effectively utilizing these symbolic representations, which can lead to ongoing failure in subsequent chemistry learning (Schmidt, 2000; Wood and Breyfogle, 2006; Musli, 2008). To improve students’ understanding of chemical symbol representation, paving the way to a mastery of their use, before concentrating on instructional approaches, first there is an urgent need for empirical studies, in order to generate diagnostic information on the developmental progression of students (Briggs et al., 2006).
Currently, in science education, learning progressions have garnered growing attention, functioning as curriculum models and assessment frameworks (Sevian and Talanquer, 2014). According to NRC (2007), learning progressions are “descriptions of the successively more sophisticated ways of thinking about a topic that can follow one another as children learn about and investigate a topic over a broad span of time” (p. 214). Learning progression focuses on learning pathways, progressions or trajectories that students formulate and develop knowledge and skills (Duschl et al., 2011). For instance, Smith et al. (2006) developed a learning progression for capturing students’ development of a particle model of matter across the elementary grades (K-8). In chemistry education, learning progressions have been developed in such diverse topics as atomic structure and electrical force (Stevens et al., 2010), carbon cycling (Mohan and Anderson, 2009; Mohan et al., 2009), concept of matter (Liu and Lesniak, 2005; Hadenfeldt et al., 2016), particle model of matter (Smith et al., 2006), concept of substance (Johnson and Tymms, 2011), chemical thinking (Sevian and Talanquer, 2014), chemical reactions (Yan and Talanquer, 2015), and structure of matter (Morell et al., 2017). In chemistry education, developing student proficiency in chemical symbol representation definitely also requires a learning progression perspective. This will effectively provide references for instructors, science educators, curriculum designers, and assessment developers (Duschl et al., 2011).
Researchers often use grade as a time unit to identify students’ progress in learning. For instance, Kermen and Méheut (2009), in exploring the reasons that students provided when asked to explain why a chemical change might remain incomplete, found that the interpretations of students from Grade 8 to Grade 12 gradually deepened. Özdem et al. (2010) investigated the scientific literacy levels of Turkish elementary students, and their findings showed that 8th grade students differed significantly in their scientific literacy levels, as compared to 6th and 7th grade students. In addition, empirical studies have indicated that there are interaction effects between gender and grade in chemistry learning (e.g., Andreous et al., 2006), suggesting that students’ development varies depending on gender. However, to our knowledge, existing studies on learning progression have only focused on broad chemistry learning outcomes or motivation; no prior study has considered student learning progression at different grade levels across the genders, as regards the symbol representation abilities of students.
To this point, instead of focusing on the development and evaluation of specific instructional activities or curriculum materials, this present study falls into a specific line of learning progression research, aiming to identify how students’ chemical symbol representation abilities progress at different grade levels (Grade 10–12) across the genders. In doing so, this study will provide empirical evidence for learning progression research. In addition, given that the ability to understand and use symbol representation is an essential component of key chemistry competency, this study would not only throw a spotlight on male and female students’ progressions in learning chemistry, but would also inform the development of better aligned instruction, curriculum, and assessments.
Literature review
Over the past three decades, one of the most powerful and productive ideas in chemical education is that chemical knowledge and a chemistry-based understanding of the world is generated, expressed, taught, and communicated at macroscopic, submicroscopic and symbolic levels (Talanquer, 2011). This symbolic level, as opposed to a discrete level of chemistry knowledge, can act as a bridge or means to connect the other two levels, as it simultaneously represents both the macroscopic and submicroscopic levels and facilitates shifts between these levels in explanations (Taber, 2013). Chemical symbol representation is a symbolic system in international academic circles, characterized by unified regulation, expressing chemical composition, structure, and chemical processes in the discipline of chemistry (Wang et al., 2017). The ability to move fluently between any two of the three representations—macro, micro, and symbolic—to reason through and solve chemistry problems is precisely the key to adequate chemical representation abilities (Treagust et al., 2003). However, many students have persistent difficulty in mastering symbol representations. For instance, students often find it difficult to translate between symbolic and submicroscopic representations of substances or of chemical reactions (Sanger, 2005). Taskin and Bernholt (2014) reviewed 38 articles and found that students’ common difficulties concerning chemical formulae were mainly composed of the following issues: (1) understanding the meaning and functioning of chemical symbols; (2) setting up empirical and molecular formulae; (3) interpreting empirical and molecular formulae and (4) linking empirical and molecular formulae to submicroscopic diagrams.Despite decades of inclusionary efforts, women are still underrepresented in areas of the hard sciences, namely chemistry and physics (Ziegler and Stoeger, 2004; Nosek et al., 2009). Gender has been found to be a significant factor in chemistry learning and instruction (Bunce and Gabel, 2002; Devetak and Glažar, 2014; Pazicni and Bauer, 2014; Karatjas and Webb, 2015; Boz et al., 2016; Vincent-Ruz et al., 2018). For example, Devetak and Glažar (2010) found that there is a statistically significant difference between male and female students in solving problems which include reading or drawing submicro-representations. The 2007 findings of the Advanced Placement Program (APP) for American high school students revealed that male students outperformed their female peers on 35 tests, including chemistry tests. While 18% of the male students received a score of 5 (i.e., ‘Extremely well qualified’) in the APP chemistry test, the percentage of female students who received the same score is 11% (cited in Veloo et al., 2015). Obrentz (2012) found that male college students performed better, on a statistically significant scale, than did female students, in terms of their final chemistry grades.
Recently, the interactive effects of age and gender have garnered increasing attention in science education (Yang et al., 2016). For instance, Rubin et al. (2018) found that age is a positive predictor of both surface and deep learning, while gender could moderate the age effect in the case of deep learning. Cheung (2009) found that male Hong Kong students in Secondary 4 and 5 (approximately 16 or 17 years of age) liked chemistry theory lessons more than their female counterparts, yet male students’ liking for chemistry laboratory work declined once they had progressed from Secondary 4 to Secondary 7, whereas no significant deterioration in attitude toward chemistry laboratory work was found in females. Other studies found that female students improved, in regard to ability and attitude, when they moved on to higher grades (e.g., George, 2006). Additionally, gender differences in spatial ability were also found to diminish with age (Linn and Petersen, 1985). However, previous studies of the interaction between gender and age mainly focused on affective variables, e.g., learning satisfaction (González-Gómez et al., 2012) and self-esteem (Bleidorn et al., 2016). The research exploring grade and gender interaction effects on students’ learning progressions, as regards certain specific scientific knowledge or practice, has been limited.
Corcoran et al. (2009) proposed five essential elements of learning progressions: (1) learning targets or clear end points; (2) progress variables; (3) levels of achievement or stages of progress; (4) learning performance; (5) assessment. Informed by these elements, we first developed an assessment framework for measuring chemical symbol representation abilities. This framework includes levels of achievement/stages of progress that students are expected to pass through, and learning performances in terms of chemical symbol representation, or what students’ understanding and skills would look like at each stage of progress (details in Wang et al., 2017). Following this framework, we then developed an assessment instrument for measuring students’ chemical symbol representation abilities, at different grades and across genders. Based on the results of the assessment, we identified female and male students’ development progress by grade. The findings of this study will support the coordination of teaching, instructional resources, and assessment with cognitive and metacognitive practices, so that learning builds coherently and gender differences are reduced. In addition, this study will inform ongoing efforts for efficient curriculum development, improving student chemistry learning on a variety of fronts. Specifically, this study will answer the following research questions:
(1) How do students’ chemical symbol representation abilities develop from Grade 10 to Grade 12?
(2) Is there an interaction effect between gender and grade on students’ chemical symbol representation abilities?
Methodology
Chinese chemical education background
In China, every child must undergo compulsory school education lasting nine years, including six years of primary school and three years of junior secondary school. Subsequently, some students go on to three years in ordinary senior secondary schools, preparing to enter universities or colleges while the other students go on to vocational schools or specialized secondary schools.In primary school, students attain some basic chemistry knowledge in nature studies (studying oxygen and nitrogen). More systematic chemical instruction begins in the third year of junior secondary school (Grade 9). In the first two years of senior secondary school (Grades 10 and 11), chemistry is a required course and also a component of the Minimum Competency Test. In the third year of senior secondary school (Grade 12), chemistry is an optional course taken only by students who are ready to participate in the National Entrance Examination for science, engineering, agriculture, and medical science. Table 1 lists the principles of chemistry content knowledge and practices that students should have at different grade levels in senior secondary school.
| Grade | Content knowledge and practices |
|---|---|
| 10 | Chemical elements; chemical formulas and chemical equations; principles of chemical stoichiometry; atomic structures; periodic law of elements and chemicals; chemical reactions; energy; primary battery bonds; structures and properties of the simplest organic compounds (i.e., methane, ethylene, benzene, and ethanol). |
| 11 | Principles of chemical reactions, chemical reaction rates; Le Chatelier's principle; electrolyte solution; functional groups of organic compounds; mutual transformations between organic compounds; organic compounds; properties of materials at the microstructure. |
| 12 | Combining production and living phenomena to refine chemical knowledge; using chemical knowledge to explain practical problems in production and life; using chemical principles to explain important chemical production processes; addressing specific issues by designing experimental investigation schema; solving the problems through experiments; comprehensively applying chemical knowledge to solve new complex problems. |
Participants
The sample was drawn from senior secondary school students (Grades 10–12) in Jiangsu, China. On account of the practical benefits of convenience sampling, three senior secondary schools, including eleven classes from three different grades (10–12), were purposefully selected. All three schools are four-star public high schools accredited by the Jiangsu Agency for Educational Evaluation Authorities, which signifies that they were appraised as being of the highest level, with first-class school conditions, teaching staff, and management. In accordance with ethical principles, all of the participants were informed about the aim of the test and the procedures, and they were told that their participation was voluntary, and that their anonymity would be ensured. In addition, the participants were told that they would have no bearing on their academic performance. All participants were requested to sign a consent form indicating their willingness to participate. Ultimately, a total of 713 students (268 females, 445 males; 254 tenth graders, 262 eleventh graders and 197 twelfth graders) consented to participate in the test (as shown in Table 2).| School | Grade 10 | Grade 11 | Grade 12 | Total | |||
|---|---|---|---|---|---|---|---|
| Age 15.43 (SD = 0.57) | Age 16.37 (SD = 0.54) | Age 17.41 (SD = 0.48) | |||||
| Male | Female | Male | Female | Male | Female | ||
| A | 66 | 30 | 58 | 49 | 39 | 16 | 258 |
| B | 74 | 41 | 51 | 49 | 61 | 34 | 310 |
| C | 32 | 11 | 28 | 27 | 36 | 11 | 145 |
| Total | 172 | 82 | 137 | 125 | 136 | 61 | 713 |
Instrument
In our previous study (Wang et al., 2017), an assessment framework of chemical symbol representation abilities for senior secondary school students, based on Bloom's taxonomy (Bloom et al., 1956; Anderson and Krathwohl, 2001) and SOLO taxonomy (Biggs, 2011), had been constructed. This framework contained four levels: (1) connecting chemical symbols with the macro-representation, (2) understanding the submicro meaning of chemical symbols, (3) understanding and interpreting the transformation between macro- and submicro-representation of chemical symbols and (4) using chemical symbols for reasoning in chemistry problems.Based on this framework, we developed an instrument assessing students’ chemical symbol representation abilities. This instrument consists of 17 multiple-choice items (S1–S17) and three constructed-response items (S18–S20). Through two rounds of tests, the results of Rasch measurements (Linacre, 2011) demonstrated good reliability and validity of instrument measures based on the framework (details of the development and validation of the instrument can be found in Wang et al., 2017).
In this present study, we used this instrument to measure students’ chemical symbol representation abilities (CSRA); students’ raw scores were converted into Rasch scale scores, allowing for direct comparison among students of different grades (Chi et al., 2017). All of the participants were required to respond to the items individually within a limited time (45 minutes). As shown in Table 3, the mean score (measured by the Rasch logit scale) for each item in this study has been calculated, and the average value of the items belonging to each level was used as the threshold value. For instance, a student would be considered having achieved Level 4 of CRSA when his or her ability measure is higher than 1.89 (the threshold value of Level 4).
| Items and measures | Threshold value | |
|---|---|---|
| Level 1 | S01 (−2.85), S02 (−1.46), S03 (−1.66), S04 (−1.41), S05 (−1.55) | −1.78 |
| Level 2 | S06 (−0.63), S07 (−1.01), S08 (−0.97), S09 (−0.73), S10 (−0.96) | −0.47 |
| Level 3 | S11 (−0.19), S12 (0.93), S13 (0.44), S14 (0.69), S15 (0.82), S16 (1.81) | 0.75 |
| Level 4 | S17 (1.87), S18 (1.26), S19 (1.92), S20 (2.51) | 1.89 |
Data analysis
Using Bond & Fox Steps (version 1.0) software for Rasch measurement (Linacre and Wright, 2000), the raw score of the test was converted and estimated on a logit scale. The score values presented in the Findings section are all logarithmically scaled. The chi-squared test was used to determine whether there is a significant difference among the three grades surveyed, in terms of the distribution of chemical symbol representation ability levels. Given the fact that the F-test is robust in contending with moderate violations of normality and homogeneity assumptions (Liu and Boone, 2006), analysis of variance (ANOVA) was conducted, using students’ chemical symbol representation abilities as the dependent variable, and grade and gender as the independent variables, to test the statistical significance of development by grade and by gender.Findings
Students’ chemical symbol representation abilities (CSRA) development along the grades
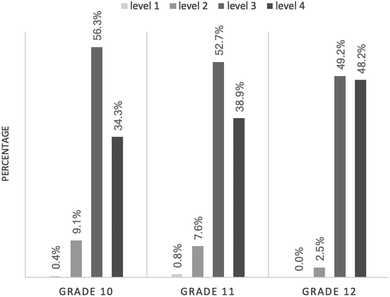
Fig. 1 shows the student percentage of each CSRA level, by grade level. In Grade 10, more than half of the students (56.3%) performed at Level 3, and a third of the students (34.3%) achieved Level 4. In Grade 11, the percentage of students who attained Level 3 or higher was nearly equivalent to that of the students in Grade 10; 52.7% of the students achieved Level 3 and 38.9% of the students were at proficiency Level 4. In Grade 12, 97.5% of the students attained Level 3 or higher; the percentage of twelfth graders at Level 4 increased by 10%, as compared with the percentage of eleventh graders. Overall, the difference in proportions of students at each level, from Grade 10 to Grade 12, was significant—χ2 (6, N = 713) = 15.638, p = 0.016—though the practical significance was small—Cramer’ V = 0.105.Students’ performance in each CSRA level at different grades
In the following section, we purposefully selected four items (one item from each CSRA level) to explore how students’ chemical symbol representation abilities develop and deepen from Grade 10 to Grade 12. Note that Chinese-language items from the original version of the test have been translated into English for reporting in the journal (all of the four items were reproduced from Wang et al. (2017), with permission from the Royal Society of Chemistry).Level 1. Connecting chemical symbols with the macro–representation
S02. Which of the following sequences represents the same substance in name, common name, and chemical formula?
A. Copper sulfate crystal, Blue vitriol, CuSO4 B. Potassium hydroxide, Caustic soda, KOH
C. Calcium hydroxide, Quicklime, Ca(OH)2 D. Sodium bicarbonate, Baking soda, NaHCO3
This item belonged to CSRA Level 1, which tended to examine whether students can associate a given chemical formula with the corresponding substance and its common name. Table 4 shows the distribution of responses in each grade. The percentage of correct responses by the three grades was 84.3%, 85.5% and 86.3%, indicating that most of the participants could correctly connect the chemical formula with its corresponding chemical substance well. For each grade, around 6% of students failed to connect copper sulfate crystal (the substance name) with CuSO4·5H2O (chemical formula), and around 3–4% students could not figure out that caustic soda represents NaOH (or had mistaken quicklime for Ca(OH)2).
Level 2. Understanding the submicro meaning of chemical symbols
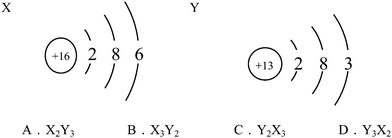
S09. The structure diagrams of two particles, X and Y, are shown below. Which is the chemical formula of the compound formed by X and Y?
This item belonged to CSRA Level 2, which tended to examine whether students can understand the meaning of atomic structure diagrams. Students at this level not only need to understand the meaning of each part of the atomic structure diagrams, but should also be able to understand that the outermost electrons of the metal atoms are generally less than four, and that those of the nonmetal atoms are generally more than four. The relationship between the outermost electrons and chemical valence should be understood as well. As shown in Table 5, among the incorrect responses, more students (in all surveyed grades) chose B than A and D, indicating that although those students could correctly recognize the chemical valence via the atomic structure, but they did not notice that the element with positive valence was not presented first (leftmost in orientation) in the chemical formula. Noticeably, more tenth graders (15.0%) chose B than did students in Grade 11 (10.7%) and Grade 12 (9.6%), demonstrating that the tenth-grade students exhibited more misunderstanding regarding chemical valence. Overall, for this level, students from Grade 12 performed better than those from Grades 10 and 11.
Level 3. Understanding and interpreting the transformation which occurs between macro- and submicro-representation of chemical symbols
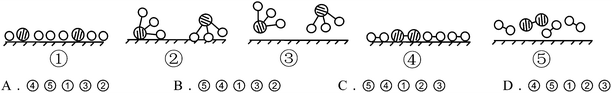
S15. In the presence of selected catalysts, nitrogen and hydrogen can synthesize ammonia under conditions of high temperature and high pressure. N2, H2 and NH3 are respectively represented by 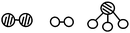 . Please consider the following figures. Which order is in line with the ammonia synthesis process at the surface of the catalyst?
. Please consider the following figures. Which order is in line with the ammonia synthesis process at the surface of the catalyst?
This item belonged to CSRA Level 3, which tended to examine whether students can transition fluently between chemical objects, macro phenomenon, submicro structure and theories, and whether they can explain the principles of macro phenomena, or progress from the point of the submicro structure or process. The above principle of the process of ammonia synthesis at the surface of the catalyst should be interpreted thusly: molecules first have random movement, and then are attached to the surface of the catalyst and divided into atoms; the atoms are then recombined into new molecules, and finally leave the surface of the catalyst. As shown in Table 6, more Grade 12 students correctly chose C (71.1%) than did students from Grades 10 (61.8%) and 11 (66.0%), indicating that the twelfth graders explained the principles of macro progress from the point of submicro progress better than students from the other two grades.
Level 4. Using chemical symbols for reasoning in chemistry problems
As mentioned before, in Grades 10 and 11, while more than half of the students performed at Level 3, only a third of the students achieved Level 4. In Grade 12, the percentage of students who attained Level 4 increased to 48.2%. Therefore, Level 4 might be considered as a threshold to determine whether or not students fully understand chemical symbol representation. We took S19 as an example in the analysis of different grade students’ responses.
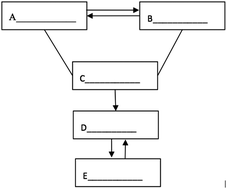
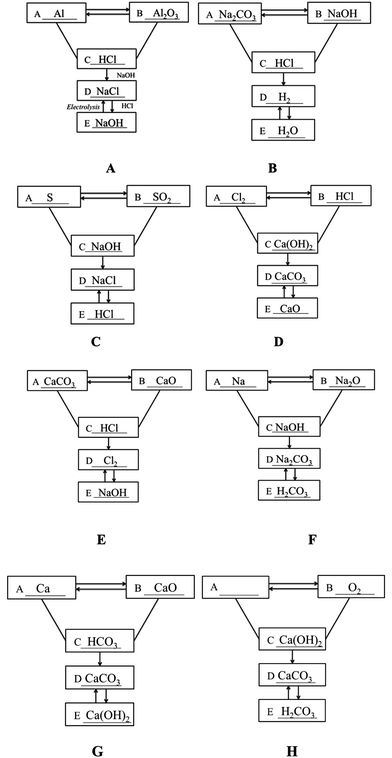
S19. The following figure depicts the relationship between the five different types of substances: simple substance, oxide, acid, alkali and salt. A “—” (line) means that the two substances connected can react, and a “ →” (arrow) means that one substance can be converted into another. Please fill in the blanks with the chemical formulae of specific substances within the five categories.
Item S19 examined whether students can use symbols to represent the inner connection of chemical concepts or to represent mutual transformation of matter. The transformation relationship in the figure can fall into four categories: (1) mutual transformation between A and B, (2) C reacts with A and B, (3) the transformation of C to D, and (4) mutual transformation between C and D. Answers could be assigned partial credit, with responses that got all categories right receiving four points (Fig. 2A and B), responses that got three categories right receiving three points (Fig. 2C and D), responses that got two categories right receiving two points (Fig. 2E and F), and responses that got one category right receiving one point (Fig. 2G and H). As shown in Table 7, more than 20% of students in each grade failed to establish the mutual transformation relationship between the different types of substances. The percentage of students who got full credit in Grade 12 (28.4%) turned out to be higher than that the percentage of students in Grade 10 (13.8%) and Grade 11 (15.6%). Moreover, the mean scores for Grade 10 (1.81) and Grade 11 (1.85) were approximately the same, and both of them were lower than that of Grade 12 (2.01).
| 0 | 1 | 2 | 3 | 4 | Mean | |
|---|---|---|---|---|---|---|
| Grade 10 | 66 (26.0%) | 50 (19.7%) | 40 (15.7%) | 63 (24.8%) | 35 (13.8%) | 1.81 |
| Grade 11 | 53 (20.2%) | 58 (22.1%) | 67 (25.6%) | 43 (16.4%) | 41 (15.6%) | 1.85 |
| Grade 12 | 40 (20.3%) | 45 (22.8%) | 41 (20.8%) | 15 (7.6%) | 56 (28.4%) | 2.01 |
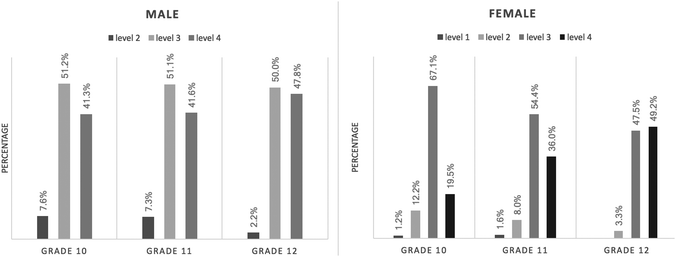
Student CSRA development along the grades across gender
Fig. 3 shows the percentage of each CSRA level by grade and across the genders. All of the male students performed above Level 2. The distribution of male students in the three CSRA levels was nearly the same from Grade 10 to Grade 11; about half of the male students attained Level 3, and two in five male students attained Level 4. In Grade 12, the percentage of male students who attained Level 2 decreased by 5%, while the percentage of male students at Level 4 increased by 6%, as compared with that in the lower grades. Ultimately, the difference in the proportions of males at each CSRA level, from Grade 10 to Grade 12, was not significant—χ2 (4, N = 445) = 5.352, p = 0.253.For the female participants, about 1% were at Level 1 in both Grade 10 and Grade 11. In Grade 10, two-thirds of the female students achieved Level 3, while one out of five attained Level 4. In Grade 11, as compared with Grade 10, the percentage of female students at Levels 2 and 3 decreased. In Grade 12, with the exception of the 3.3% female students at Level 2, all performed above Level 3. For female students in the higher grades, a higher proportion achieved Level 4. The percentage of female students at Level 4, from Grades 10 to 12, was 19.5%, 36.0%, and 49.2%. The difference in the proportions of females at each Level, from Grade 10 to Grade 12, was statistically significant—χ2 (6, N = 268) = 16.279, p = 0.012. The effect size (Cramer’ V = 0.174) suggested a medium to large practical significance.
For Grades 10 and 11, the percentage of female students at Levels 2 and 3 was higher than for male students, while more male students attained Level 4 than did female students. Yet for Grade 12, the percentage of female students at Level 4 was higher than that of male students.
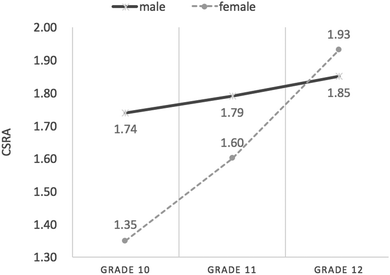
Interaction effect between gender and grade on student CSRA
Fig. 4 shows male and female students’ CSRA mean Rasch measures from Grade 10 through Grade 12. It can be seen that both female and male students’ CSRA mean Rasch measures increased from the lower grades to the higher grades. In Grade 10, male students’ mean Rasch measures (M = 1.74 logits, SD = 0.90) were much higher than those of their female peers (M = 1.35 logits, SD = 0.64). However, the gender difference had been reduced by Grade 11 (Mmale = 1.79 logits, SD = 0.88; Mfemale = 1.60 logits, SD = 0.83), and by Grade 12, male students (M = 1.85 logits, SD = 0.67) scored lower than females (M = 1.93 logits, SD = 0.77).The results of ANOVA are presented in Table 8. From Table 8, it can be seen that a statistically significant effect of grade (F(2, 707) = 8.75, p < 0.01) emerged from the data; that is, as students progressed through the grades, their chemical symbol representation abilities significantly increased, though the effect was weak (η2 = 0.02). Overall, male students performed better than females on a statistically significant scale (F(1, 707) = 6.84, p < 0.01), but the gender difference was weak (η2 = 0.01). Additionally, the interaction effect between gender and grade on chemical symbol representation abilities was statistically significant, though this was also weak (F(2, 707) = 4.10, p < 0.05, η2 = 0.01), indicating that students’ development of chemical symbol representation abilities as they progressed through the grades significantly varied with gender.
| Source | Level | M (SD) | df | F | η 2 | p |
|---|---|---|---|---|---|---|
| Grade | 10 | 1.61 (0.84) | 2 | 8.75 | 0.02 | 0.00 |
| 11 | 1.69 (0.86) | |||||
| 12 | 1.87 (0.70) | |||||
| Gender | Male | 1.79 (0.83) | 1 | 6.84 | 0.01 | 0.01 |
| Female | 1.60 (0.79) | |||||
| Grade × Gender | 2 | 4.10 | 0.01 | 0.02 | ||
| Error | 707 |
Discussion and limitations
Taking the perspective of learning progressions, this present study identified how students’ abilities in chemical symbol representation progress at different grade levels (Grade 10–12), across the genders. This study's results showed that, overall, students in the sample developed their chemical symbol representation abilities as they progressed in the grades. Students in the higher grades performed significantly better than students in the lower grades; in particular, students in the higher grades tended to achieve significantly higher CSRA levels. Although the overall trend of students’ symbol representation abilities showed increases along the grades, growth was not demonstrated in any sudden spikes. For instance, Grade 10 to Grade 11, the percentage of students who achieved Level 3 and Level 4 only increased by one percentage point, indicating that students’ progressions of “understanding and interpreting the transformation between macro and submicro representation of chemical symbols” (Level 3) and “using chemical symbols for reasoning in chemistry problems” (Level 4) are much slower. Even by Grade 12, there were still over 20% of students who were unable to establish the mutual transformation relationship between different types of substances. These findings indicated that developing student chemical symbol representation abilities must be looked upon as a long-term effort. It follows that it may be preferable for chemical symbol representation to be introduced from an early grade, such as by Grade 7, and for a continued focus on developing students’ understanding of chemical symbol representation to persist in all subsequent grades.Furthermore, the results of the present study revealed that students who did not reach the highest CSRA level are contending with obstacles in problem-solving processes and lack sufficient scientific reasoning abilities. Therefore, we must holistically strengthen students’ problem-solving skills and reasoning competencies through the use of chemical symbols, transforming their acquired knowledge within and across various representational forms—for instance, at the macro and submicro levels (Jaber and BouJaoude, 2012)—leading to the development of more integrated conceptual knowledge in chemistry, as well as higher levels of scientific thinking (i.e., making interpretations and inferences). In terms of current science standards, not only should the kinds of knowledge and skills that students at different grade levels are expected to have be specified; the expected knowledge and associated skills must be organized systematically around existing, coherent knowledge and skills, and should avoid being fragmentary and disconnected.
It should be added that, overall, there was a significant gender difference in terms of CSRA. While male students had significantly higher mean scores than did their female peers, it should be cautioned that this main effect result belies the true state of affairs. The most important insight gained from this study is that there was a significant interaction effect between gender and grade on student chemical symbol representation abilities. The results demonstrated that while male students started out with a much higher mean score of chemical symbol representation abilities in Grade 10, there was not much of a difference in scores by Grade 11, and by Grade 12, females ended up achieving a higher mean score. Female students demonstrated a dramatic increase in terms of chemical symbol representation abilities from Grade 10 to Grade 12, whereas male students did not show as drastic a change, though they continued to show improvement as well.
In light of the above findings, both grade and gender should be taken into consideration during instruction. For instance, educators could design targeted learning activities and use different instructional strategies for male and female students at different grade levels. In addition, this study provides evidence of gender differences in the development of symbol representation abilities, showing that while female students may fall behind their male peers at earlier grade levels, they can make significant progressions in later grades, as compared with male students. In accordance with these findings, teachers may have to keep in mind that even if their female students might exhibit a slow start in learning chemical symbol representation, they may end up progressing even farther than their male peers. Also, teachers may need to be more patient with their female students, providing them with sufficient support and helping them to enhance their self-efficacy, particularly when learning abstract and complicated concepts.
Relatedly, regarding the potential impact of the findings on curriculum, curriculum designers should consider introducing this manner of highly abstract material in the earlier grades, as the limited timeline for learning these more abstract concepts may deter female students from discovering their ultimate potential for science proficiency, which may in turn make them less likely to pursue higher education or careers in science.
However, the findings of this study must be interpreted with caution, in view of certain inherent limitations. Firstly, the participants are from only three separate senior secondary schools in Jiangsu, China; even though each of these schools is located in a different area, the findings might not be generalizable because of the non-random nature of the sampling. Future studies should select their student sampling in a more random manner, and should consider a wider socioeconomic range, in order to increase the statistical power of the data. Secondly, this study was a cross-sectional test, not a longitudinal study. As a result, certain random effects relating to sampling could impact the results. Thirdly, the statistical analysis does not suggest any mechanism (i.e., interaction effect); the complicated mechanism of progressions by grade across the genders almost certainly requires further study. Finally, given the fact that in China, chemistry is not a compulsory course in Grade 12, the students who take chemistry in Grade 12 are usually interested in chemistry, or in any case, have confidence in their ability to learn chemistry. As that is the case, in China, the students who chose to take chemistry in Grade 12 cannot be seen as representative of regular senior secondary school students; note that this is particularly true of female twelfth graders who have chosen to take the course.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper is the result of work on a project (17JJD880007) funded by the MOE Key Research Institute of Humanities and Social Sciences and the Peak Discipline Construction Project of Education from East China Normal University.References
- Anderson L. W. and Krathwohl D. R. (ed.), (2001), A taxonomy for learning, teaching and assessing: a revision of Bloom's taxonomy of educational objectives, New York: Longman.
- Andreou E., Vlachos F. and Andreou G., (2006), Approaches to studying among Greek university students: the impact of gender, age, academic discipline and handedness, Educ. Res., 48, 301–311.
- Bleidorn W., Arslan R. C., Denissen J. J., Rentfrow P. J., Gebauer J. E., Potter J. and Gosling S. D., (2016), Age and gender differences in self-esteem—A cross-cultural window, J. Pers. Soc. Psychol., 111(3), 396.
- Biggs J. B., (2011), Teaching for quality learning at university: what the student does, McGraw-Hill Education (UK).
- Bloom B. S. (ed.), Engelhart M. D., Furst E. J., Hill W. H. and Krathwohl D. R., (1956), Taxonomy of educational objectives: the classification of educational goals, Handbook 1: cognitive domain, New York: David McKay.
- Boz Y., Yerdelen-Damar S., Aydemir N. and Aydemir M., (2016), Investigating the relationships among students’ self-efficacy beliefs, their perceptions of classroom learning environment, gender, and chemistry achievement through structural equation modeling, Res. Sci. Technol. Educ., 34(3), 307–324.
- Briggs D. C., Alonzo A. C., Schwab C. and Wilson M., (2006), Diagnostic assessment with ordered multiple-choice items, Educ. Assess., 11(1), 33–63.
- Bunce D. M. and Gabel D., (2002), Differential effects on the achievement of males and females of teaching the particulate nature of chemistry, J. Res. Sci. Teach., 39(10), 911–927.
- Cheung D., (2009), Students’ attitudes toward chemistry lessons: the interaction effect between grade level and gender, Res. Sci. Educ., 39(1), 75–91.
- Chi S., Wang Z., Liu X. and Zhu L., (2017), Associations among attitudes, perceived difficulty of learning science, gender, parents’ occupation and students’ scientific competencies, Int. J. Sci. Educ., 39(16), 2171–2188.
- Corcoran T., Mosher F.A. and Rogat A., (2009), Learning progressions in science: an evidence-based approach to reform. Consortium for Policy Research in Education Report #RR-63, Philadelphia, PA: Consortium for Policy Research in Education.
- Devetak I. and Glažar S. A., (2010), The Influence of 16-year-old Students’ Gender, Mental Abilities, and Motivation on their Reading and Drawing Submicrorepresentations Achievements, Int. J. Sci. Educ., 32(12), 1561–1593.
- Devetak I. and Glažar S. A., (2014), Educational Models and Differences between Groups of 16-year-old Students in Gender, Motivation, and Achievements in Chemistry, in Learning with Understanding in the Chemistry Classroom, Springer, Dordrecht, pp. 103–126.
- Duschl R., Maeng S. and Sezen A., (2011), Learning progressions and teaching sequences: a review and analysis, Stud. Sci. Educ., 47(2), 123–182.
- George R., (2006), A cross-domain analysis of change in students’ attitudes toward science and attitudes about the utility of science, Int. J. Sci. Educ., 28(6), 571–589.
- Gilbert J. K. and Treagust D. F. (ed.), (2009), Multiple representations in chemical education, Dordrecht: Springer.
- González-Gómez F., Guardiola J., Rodríguez Ó. M. and Alonso M. Á. M., (2012), Gender differences in e-learning satisfaction, Comput. Educ., 58(1), 283–290.
- Hadenfeldt J. C., Neumann K., Bernholt S., Liu X. and Parchmann I., (2016), Students’ progression in understanding the matter concept, J. Res. Sci. Teach., 53(5), 683–708.
- Jaber L. Z. and BouJaoude S., (2012), A macro–micro–symbolic teaching to promote relational understanding of chemical reactions, Int. J. Sci. Educ., 34(7), 973–998.
- Johnson P. and Tymms P., (2011), The emergence of a learning progression in middle school chemistry, J. Res. Sci. Teach., 48(8), 849–877.
- Karatjas A. G. and Webb J. A., (2015), The Role of Gender in Grade Perception in Chemistry Courses, J. Coll. Sci. Teach., 45(2), 30–35.
- Kermen I. and Méheut M., (2009), Different models used to interpret chemical changes: analysis of a curriculum and its impact on French students' reasoning, Chem. Educ. Res. Pract., 10(1), 24–34.
- Linacre J. M., (2011), A user's guide to WINSTEPS/MINISTEP: Rasch-model computer programs, Chicago, IL: Winsteps.com.
- Linacre J. M. and Wright B. D., (2000), Winsteps, Chicago, IL: MESA Press.
- Linn M. C. and Petersen A. C., (1985), Emergence and Characterization of Sex Differences in Spatial Ability: A Meta-Analysis, Child Dev., 56 (6), 1479.
- Liu X. and Boone W. J., (2006), Applications of Rasch measurement in science education, JAM Press.
- Liu X. and Lesniak K. M., (2005), Students' progression of understanding the matter concept from elementary to high school, Sci. Educ., 89(3), 433–450.
- Mohan L. and Anderson C.W., (2009), Teaching experiments and the carbon cycle learning progression. Paper presented at the Learning Progressions in Science (LeaPS) Conference, June 2009, Iowa City, IA.
- Mohan L., Chen J. and Anderson C. W., (2009), Developing a multi-year learning progression for carbon cycling in socio-ecological systems, J. Res. Sci. Teach., 46(6), 675–698.
- Morell L., Collier T., Black P. and Wilson M., (2017), A construct-modeling approach to develop a learning progression of how students understand the structure of matter, J. Res. Sci. Teach., 54(8), 1024–1048.
- Musli S., (2008), Die chemische Formelsprache im Spannungsfeld von Schu lerleistung und Lehrererwartungen [The chemical formula language in the tension zone between student achievement and teacher expectations], Munster: Schuling.
- National Research Council, (2007), in Duschl R.A., Schweingruber H.A. and Shouse A.W. (ed.), Taking science to school: learning and teaching science in grades K-8. Committee on Science Learning, Kindergarten through eighth grade, Washington DC: The National Academies Press.
- Nosek B. A., Smyth F. L., Sriram N., Lindner N. M., Devos T., Ayala A., Kesebir S., et al., (2009), National differences in gender-science stereotypes predict national sex differences in science and math achievement, Proc. Natl. Acad. Sci., 106(26), 10593–10597.
- Obrentz S. B., (2012), Predictors of science success: the impact of motivation and learning strategies on college chemistry performance, Georgia State University.
- Özdem Y., Çava P. and Çava B., (2010), An Investigation of Elementary Students’ Scientific literacy levels, J. Baltic Sci. Educ., 9(1), 1648–3898.
- Pazicni S. and Bauer C. F., (2014), Characterizing illusions of competence in introductory chemistry students, Chem. Educ. Res. Pract., 15, 35–46.
- Rubin M., Scevak J., Southgate E., Macqueen S., Williams P. and Douglas H., (2018), Older women, deeper learning, and greater satisfaction at university: age and gender predict university students’ learning approach and degree satisfaction, J. Divers. High. Educ., 11(1), 82–96, DOI:10.1037/dhe000004.
- Sanger M. J., (2005), Evaluating students' conceptual understanding of balanced equations and stoichiometric ratios using a particulate drawing, J. Chem. Educ., 82(1), 131.
- Schmidt H. J., (2000), In the maze of chemical nomenclature-how students name oxo salts, Int. J. Sci. Educ., 22(3), 253–264.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Smith C., Wiser M., Anderson C. and Krajcik J., (2006), Implications of research on children's learning for standards and assessment: a proposed learning progression for matter and atomic-molecular theory, Measurement, 14 (1&2), 1–98.
- Stevens S. Y., Delgado C. and Krajcik J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach., 47(6), 687–715.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Taskin V. and Bernholt S., (2014), Students' Understanding of Chemical Formulae: a review of empirical research, Int. J. Sci. Educ., 36(1), 157–185.
- Treagust D., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Veloo A., Hong L. H. and Lee S. C., (2015), Gender and ethnicity differences manifested in chemistry achievement and self-regulated learning, Int. Educ. Stud., 8(8), 1.
- Vincent-Ruz P., Binning K., Schunn C. D. and Grabowski J., (2018), The effect of math SAT on women's chemistry competency beliefs, Chem. Educ. Res. Pract., 19, 342–351.
- Wang Z., Chi S., Luo M., Yang Y. and Huang M., (2017), Development of an instrument to evaluate high school students' chemical symbol representation abilities, Chem. Educ. Res. Pract., 18(4), 875–892.
- Wood C. and Breyfogle B., (2006), Interactive demonstrations for mole ratios and limiting reagents, J. Chem. Educ., 83(5), 741.
- Wu H. K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88(3), 465–492.
- Wu H. K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Yan F. and Talanquer V., (2015), Students’ ideas about how and why chemical reactions happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37(18), 3066–3092.
- Yang S., Hsu W. C. and Chen H. C., (2016), Age and gender's interactive effects on learning satisfaction among senior university students, Educ. Gerontol., 42(12), 835–844.
- Ziegler A. and Stoeger H., (2004), Evaluation of an Attributional Retraining (Modeling Technique) to Reduce Gender Differences in Chemistry Instruction, High Abil. Stud., 15(1), 63–83.
| This journal is © The Royal Society of Chemistry 2018 |