The nature of the interplay among components of pedagogical content knowledge in reaction rate and chemical equilibrium topics of novice and experienced chemistry teachers
Fatma Nur
Akın
 * and
Esen
Uzuntiryaki-Kondakci
* and
Esen
Uzuntiryaki-Kondakci

Middle East Technical University, College of Education, Department of Mathematics and Science and Education, 06800, Ankara, Turkey. E-mail: fatmanur.metu@gmail.com
First published on 11th October 2017
Abstract
We examined the interactions among pedagogical content knowledge (PCK) components of novice and experienced chemistry teachers in teaching reaction rate and chemical equilibrium topics in this qualitative multiple-case design study. For this aim, three chemistry teachers who had different levels of teaching experience in chemistry teaching were selected through a process of purposeful sampling. Multiple types of data were gathered through more than two months. In order to collect and triangulate data, a card-sorting activity, a Content Representation (CoRe) tool, semi-structured interviews, observation of instruction, and field notes were utilized. Data were analyzed through three approaches: in-depth analysis of explicit PCK, the enumerative approach, and constant comparative methods. The results revealed eight characteristics of the interactions of the PCK components: (a) the novice teacher's orientations towards science, in contrast to the experienced teachers’, were more broad and non-specific, which impeded the interactions among the components, (b) the interplay of the PCK components was idiosyncratic and topic specific, (c) the novice teacher's PCK maps were fragmented while the experienced teachers’ PCK maps were integrated, (d) the experienced teachers, in contrast to the novice teacher, interacted more than two PCK components in most of their teaching fragments, (e) knowledge of learner, knowledge of curriculum and knowledge of instructional strategies were central in the interplays of all teacher maps, (f) the experienced teachers were more successful than the novice teacher in translating their knowledge into practice in terms of the integration among PCK components, (g) teacher self-efficacy appeared to play a role in their use of PCK components and constructing interactions among them, and (h) all teachers taught the same topics with similar lesson plans and the same instructional materials; however, they differed in terms of how they connect the PCK components. Implications and suggestions for teacher education and science education research are presented.
Introduction
Chemical concepts are very abstract and chemical reasoning includes a macroscopic/submicroscopic relationship; therefore, learners often have difficulties in explaining chemical phenomena with these concepts (Erduran et al., 2007). Many studies have shown that learners have various alternative conceptions regarding chemistry concepts not only because chemistry is a complex subject, but also because of the way the concepts are taught (e.g., Gabel, 1999). Therefore, teachers require a specific domain of knowledge to accommodate students’ various interests, comprehension, abilities, and experiences (Park and Chen, 2012). In this regard, Shulman (1986, 1987) introduced pedagogical content knowledge (PCK) as a central element in the knowledge base of teaching and teachers’ special practical knowledge needed to help students understand particular content. Shulman (1986) described PCK as “…the most useful forms of content representation, the most powerful analogies, illustrations, examples, explanations, and demonstrations—in a word, the ways of representing and formulating the subject that make it comprehensible to others” (p. 9). He defined PCK as topic-specific knowledge for teaching a particular subject. In addition, PCK involves two components: knowledge of representations and knowledge of learning difficulties. After Shulman's introduction of PCK, some researchers have worked on the concept and identified different components of PCK (e.g.Grossman, 1990; Magnusson et al., 1999; Park and Oliver, 2008a). For instance, Magnusson et al. (1999) expanded the concept by identifying five components of PCK for science teaching (e.g. orientations towards science teaching and knowledge of assessment). Afterwards, Park and Oliver (2008b) conceptualized PCK as an integration of those components and noted that coherence among them is critical in developing PCK. In this respect, scholars have admitted that teachers should be able to connect PCK components consistently in order to successfully plan and perform an instruction in a specific context (e.g., Van Driel et al., 2002). Park and Chen (2012) stated that “Strong PCK has all components connected to each other strongly enough to enable the whole structure of PCK to function for scaffolding student learning” (p. 926). Abell (2008) also stated that PCK is more than the sum of its components. Nevertheless, considering the long history of PCK, it can be noted that few studies have been carried out to examine the nature of integration of all PCK components (e.g., Henze et al., 2008; Padilla and Van Driel, 2011; Park and Chen, 2012; Aydin et al., 2015).Moreover, in the field of science education, so far only a few studies have focused on beginning science teachers’ PCK development (Luft et al., 2011) and the comparison between beginning and experienced science teachers’ PCK (Clermont et al., 1994). In addition, those who investigate secondary science teachers are frequently interested in preservice and in-service teachers’ development. Therefore, science teacher scholars have acknowledged that this point of view is limited, because the induction years of novice teachers are a significant element of teacher development (Luft et al., 2011). The absence of studies about beginning science teachers’ PCK and the significance of first years of teaching for teacher development suggests that research on novice science teachers is worthy of investigation (Luft et al., 2011). Furthermore, in the limited number of studies which described various interactions among PCK components, the subjects were mostly preservice or experienced teachers. Consequently, educational scholars pointed out the need for research on how teachers with different levels of teaching experience use PCK components coherently in order to make the topic more understandable to learners and provide significant implications for teacher education (Abell, 2008; Park and Chen, 2012), and how PCK develops over time (De Jong et al., 2005). To the best of our knowledge, how the interplays among all PCK components grow when teachers gain teaching experience has not been examined deeply through observing teachers’ practice. Another point that needs consideration is that there is a need to examine topic-specificity of PCK in different topics within the same discipline (Abell, 2008). With all these in mind, this study intended to investigate the nature of interactions among all PCK components in the reaction rate and chemical equilibrium topics of novice and experienced chemistry teachers as well as the role of teaching experience, if any, on interactions among the components. Therefore, the results of this study will contribute to the PCK literature by showing that the topic-specificity is based not only on which components comprise a teacher's PCK in a specific topic but also on how and to what degree those components are integrated into the PCK (Park and Chen, 2012). Moreover, it is our view that this study helps novice and experienced teachers to comprehend what PCK is and how knowing PCK may assist in improving their teaching. It is possible with the present study to draw a profile of how novice and experienced teachers integrate PCK components into their PCK by using the PCK map approach. This study also helps us designate the components that novice and experienced teachers have difficulty in interacting with other components for two chemistry topics. This study will make a contribution to the field of PCK by examining the role of teaching experience, if any, on the interactions among PCK components. This is a promising study to support that emphasis should be put not only on the development of individual PCK components but also on the development of integrations among the components into the PCK. Consequently, having such information, better teacher education and professional development programs may be developed.
Literature review
This study is basically grounded on the PCK framework. Therefore, before explaining the study in detail, we present how PCK has been defined and conceptualized, and what kind of interactions among components have been reported in the related literature in the next sections.Conceptualization of PCK
Discussions about subject matter knowledge and pedagogy have been going on since last century. The focus of these discussions has been on seeking the answer to the question of how content knowledge and general pedagogical knowledge are related. Shulman (1986) firstly portrayed PCK as a kind of teachers’ special practical knowledge as well as a special blend of content and pedagogy. PCK consists of understanding how specific topics, problems, or issues are organized, presented, and adapted to the diverse interests and abilities of learners, and how they are presented for instruction (Shulman, 1987). Since the inception of PCK, various scholars have explored and expanded upon the PCK construct, resulting in different PCK models (e.g.Grossman, 1990; Magnusson et al., 1999; Hashweh, 2005; Park and Oliver, 2008b; Friedrichsen et al., 2009). For instance, Grossman (1990) identified PCK as the transformation of three main knowledge domains: general pedagogical knowledge, subject matter knowledge, and knowledge of context. PCK consists of four components (knowledge and beliefs about the purposes for teaching subject matter, curricular knowledge, knowledge of students’ understanding, and knowledge of instructional strategies) (Grossman, 1990). Then, Magnusson and his colleagues (1999) proposed a PCK model for science teaching and changed Grossman's “purposes” to “orientations” and added a knowledge of assessment component. Eventually, their PCK model included five different types of knowledge: (a) Science Teaching Orientations: teachers' knowledge and beliefs about the goals and purposes of teaching science at a specific grade level, (b) Knowledge of Learner: teachers’ knowledge of requirements for learning and areas of student difficulty including misconceptions, (c) Knowledge of Instructional Strategies: teachers’ knowledge about science-specific strategies (e.g. learning cycle) and strategies for specific science topics (e.g., illustrations and analogies), (d) Knowledge of Curriculum: teachers’ knowledge of goals and objectives related to their subjects, as well as the vertical curriculum (vertical relations of the topic to the earlier and later grades) and horizontal curriculum (horizontal relations of the topic to other topics in the same grade) in their subjects, and knowledge of the programs and materials, and (e) Knowledge of Assessment: teachers’ knowledge of dimensions of science learning to assess (e.g., science process skills) and knowledge of methods of assessment (e.g., through portfolios or written tests). They highlighted that effective teachers should develop all components of PCK while teaching a specific topic. The interrelations among the components are very significant, and lack of coherence among them can cause problems in developing and employing PCK (Magnusson et al., 1999). Although they emphasized that these components may connect in highly complex ways, they put the five components in a linear way in their model. The only explicit interplay in this model is between science teaching orientation and each of the other four components. Park and Oliver (2008b), in contrast to Magnusson et al.'s linear model, put the same five components in a pentagonal form to show the interactions among them (Fig. 1). Their model was formed based on the work of Grossman (1990), Tamir (1988), and Magnusson et al. (1999). The present study was conceptually and analytically grounded in the pentagon model, which identifies PCK as an interplay of the five PCK components (Park and Oliver, 2008b).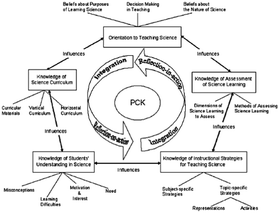 | ||
| Fig. 1 Pentagon model of PCK for science teaching (Park and Oliver, 2008b, p. 815). | ||
The nature of PCK
PCK is a significant element of teacher knowledge and is identified as a special mixture of pedagogy and content in the minds of teachers for teaching a particular topic. Therefore, PCK is different from both subject matter and pedagogical knowledge. This knowledge distinguishes teachers from subject matter specialists (Shulman, 1986). Moreover, PCK is described as the transformation of three areas of knowledge (subject matter knowledge, general pedagogical knowledge and knowledge of context) (Grossman, 1990). It should be emphasized that adequate subject matter knowledge is a prerequisite for PCK development (Van Driel et al., 1998; Abell, 2007). PCK is also subject-specific, topic-specific, person-specific, and context-specific (Kind, 2009).Finally, PCK is not just the total of its components. Rather, the interplay among them is vital. Teachers not only have all PCK components but also incorporate them while planning and performing an instruction (Abell, 2008). In this respect, the degree of the interactions among the components as well as the existence of each component designates the level of a teacher's PCK (Park and Oliver, 2008a; Friedrichsen et al., 2009).
Sources of PCK
PCK demonstrates a dynamic nature. Therefore, teachers can develop their PCK over time as they learn from teacher preparation programs, classroom observations both as a student and during preservice teacher education, professional development opportunities, and teaching experience (Grossman, 1990; Van Driel et al., 1998; Abell, 2008). Many scholars state teaching experience as the main source of PCK growth (e.g., Grossman, 1990; Van Driel et al., 1998; Magnusson et al., 1999), while others underline that teaching experience alone is not sufficient for constructing knowledge for teaching (Leite et al., 2005; Friedrichsen et al., 2009). While this debate has been going on, it should be noted, unfortunately, that there has been a limited number of studies on how PCK improves over time.Research on the interplay among the PCK components
Although given the importance of the conceptualization of PCK, few scholars have studied how the components incorporate during planning and enacting of teaching a specific topic (e.g.Grossman, 1990; Fernandez-Balboa and Stiehl, 1995). Grossman (1990), the first scholar who considered the interactions among PCK components, stated that “…these components are less distinct in practice than in theory” (p. 9). Then, Cochran et al. (1991) conceptualized PCK as the transformation of four areas of knowledge (knowledge of students, content, pedagogy, and environmental context), and emphasized that these areas should be interconnected. Similarly, Fernandez-Balboa and Stiehl (1995) argued that “…it is not the separate existence, but rather the intersection and rightful integration of all these PCK components that comprises good teaching” (p. 294). Magnusson et al. (1999) also reported that it is essential to understand how PCK components interact and how their interactions influence teaching.Although admitting that the different components of PCK might connect in highly complex ways, until now, few scholars have empirically studied the interplay of PCK components and suggested some models that show the relationships among them. For example, Henze et al. (2008) examined PCK growth of nine experienced science teachers in terms of interactions among knowledge of goals and objectives in the curriculum, instructional strategy, learner, and assessment components while teaching the Models of the Solar System and the universe topic. The analysis of interviews conducted in three subsequent academic years put forward two qualitatively different types of PCK (Type A and Type B) with different types of relations. The findings showed that all participants showed an improvement in their initial PCK over time and the growth of two types of PCK was qualitatively different in terms of the interplays of the components. In type A, the development of teachers’ knowledge about instructional strategies was consistent with their knowledge of goals and objectives in the curriculum and was also interrelated to knowledge of learner. Furthermore, teachers’ developing knowledge of learner was linked with their knowledge of assessment. Knowledge of assessment was also related to knowledge of instructional strategies. The authors concluded that some PCK components (especially knowledge of instructional strategies) enhanced much more significantly; however, the interactions among them was rather stable. In type B, the development of the teachers’ instructional strategies knowledge was consistent with their learner, and goals and objectives knowledge. In addition, teachers’ knowledge of assessment usually developed when their knowledge of learner and instructional strategies improved. Similarly, Park and Oliver (2008a) concluded that teachers’ knowledge of students’ misconceptions significantly affected their knowledge of assessment and instructional strategies.
In a quantitative research study, Kaya (2009) studied inter-relationships and intra-relationships among PCK components of preservice science teachers within the topic of ozone layer depletion. The participants were classified as high, average, and low-ability groups based on the level of their subject matter knowledge in the survey. Findings revealed that there was a significant inter-relationship between preservice teachers’ subject matter knowledge and all components of pedagogical knowledge (knowledge of learner, curriculum, assessment, and instructional strategy) (r = 0.77, p < 0.0001). There were also significant intra-relationships among the components of pedagogical knowledge except for the interactions between knowledge of assessment and other components (p > 0.05). Similarly, Padilla and Van Driel (2011) investigated the interactions of PCK components of university professors in teaching quantum chemistry. After analyzing the interview data qualitatively, they analyzed the relationships between different subcomponents for each professor with PRINCALS methodology, a quantitative technique. Interactions were found between science teaching orientations and instructional strategies, and between curriculum and learner components. Similar to the study of Kaya (2009), the assessment component is much less taken into consideration, compared to the curriculum, instructional strategies and learner components.
Recently, a different approach—the PCK Map—was used by Park and Chen (2012), in order to investigate the nature of the interactions among PCK components in the photosynthesis and heredity topics of four biology teachers. In-depth analysis of explicit PCK, enumerative and constant comparative methods resulted in some patterns which were: the interplays among the components were idiosyncratic and topic-specific; the knowledge of learner and instructional strategies components were central in the integration of the components; knowledge of curriculum had the most limited interactions with other components; knowledge of assessment was more frequently linked with learner and instructional strategies components than with the other components; and teachers’ didactic science teaching orientation influenced their instructional strategies by preventing its interaction with other components. Likewise, Aydin and Boz (2013) utilized Park and Chen's PCK map approach to identify the relationships among PCK components in the electrochemical cells and redox reactions topics of two experienced chemistry teachers. Different from Park and Chen (2012), they formed a scoring rubric to classify the interplays based on their quality and usefulness for students’ learning. In addition to patterns proposed by Park and Chen (2012), new features of the relations were asserted by the authors of the study such as nature of the integrations differed based on their complexity, and integrations could have diverse parts (understand, decision-making, enactment, and reflection). Another effort to examine the relations of all PCK components (Aydin et al., 2015) concentrated on how interactions among PCK components of preservice teachers’ developed throughout a CoRe-based mentoring enriched practicum course. They observed three preservice chemistry teachers’ teaching of rate of reaction. Analyses of pre- and post-PCK maps indicated that the development of interactions was idiosyncratic; the interplays of the components changed from fragmented to a more integrated one at the end of the course; the most significant growth was observed between curriculum and other components; and the assessment component was not linked with instructional strategies in any map.
It seems that science teacher educators have reached a consensus that for an effective teaching and a well-developed PCK, teachers should have all PCK components incorporated together. The preceding review opened a fruitful door to realize how PCK components interrelate with each other. Still, it brings out the need for research on the diagnosing of PCK components that teachers mostly lack or have, and the difficulty of linking with other components for a specific topic (Park and Chen, 2012). In addition, there is much to be learned about how PCK is constructed for teaching different topics within the same discipline (Abell 2008), and how PCK and the integrations of the components are different for novice versus experienced teachers (Park and Chen, 2012). Taking these gaps into consideration, we aimed to investigate the nature of interactions among PCK components of novice and experienced chemistry teachers’ teaching of two different chemistry topics within the same grade level. Then, we concentrated on the following research questions: What is the nature of interactions among PCK components of novice and experienced chemistry teachers in teaching the reaction rate and chemical equilibrium topics? What is the role of teaching experience, if any, on the interactions among PCK components in teaching the reaction rate and chemical equilibrium topics?
Methodology
Research design
Qualitative research methodology was used in order to examine the nature of interactions among PCK components of novice and experienced chemistry teachers to teach the reaction rate and chemical equilibrium topics. Case study, one of the qualitative research methods, guided this study in terms of designing, gathering, and analyzing the data (Patton, 2002). In particular, this study was a multiple case study in which multiple cases (novice and experienced chemistry teachers’ teaching) were compared and contrasted to show the different views on the issue (the nature of interplays among their PCK components) within the context of teaching reaction rate and chemical equilibrium topics.Participants
In this study, three chemistry teachers were chosen through a process of purposeful sampling based on certain criteria to reach a full understanding of the phenomenon as much as possible. The first criterion was related to the context of the study. PCK is influenced by the context in which the teachers work (Van Driel et al., 1998); therefore, the participants were selected from the same context, which was a private high school. In this context, they had similar instructional materials and equipment (e.g., smart boards, computers, benches, chemicals) and taught reaction rate and chemical equilibrium topics from the 11th grade chemistry curriculum. The average class size was 20, consisting of approximately 10 girls and 10 boys. The students’ ages ranged between 16 and 18. The second criterion was that teachers had different levels of teaching experience. They were three chemistry teachers who were suited to the objective of the study. At the period of the present study, Ela was a novice teacher with three-years of teaching experience and she taught both reaction rate and chemical equilibrium topics for the first time in a classroom environment. Sema and Bora had 12 and 20 years of teaching experience, respectively, at the time of the study. They had been teaching both topics for many years. All teachers graduated from the same chemistry education program in the same university. They had a similar background in terms of coursework (chemistry, pedagogical and subject-specific pedagogical courses). However, they might have different content knowledge and different dispositions. The third criterion was to choose easily accessible participants to obtain deep information on PCK in a specific topic. Finally, the appropriate schedules of the teachers without any overlap helped us choose these teachers from the same context. They voluntarily accepted to participate in this study. Pseudonyms are used for confidentiality.The topic selection
The reaction rate and chemical equilibrium topics were chosen for several reasons. First, although two of the observed participants had been teaching these two topics for years, the novice teacher taught them for the first time at the 11th grade level. Therefore, we decided to select chemistry topics from the 11th grade level. The topics taught in the 11th grade curriculum are enthalpy, electrochemistry, reaction rate, and chemical equilibrium at the time of study. Among these topics, we selected the reaction rate and chemical equilibrium topics. Second, the scarcity of research on teachers’ PCK and on interactions among PCK components for teaching reaction rate and chemical equilibrium made us concentrate on these topics. Third, the two topics we selected had to be at the same grade level because orientation to science teaching is grade specific (Magnusson et al., 1999). Finally, the time for observing these two topics was at the researchers’ convenience.Data collection sources
The complexity of teachers’ knowledge cannot be captured by a single instrument (Kagan, 1990). Therefore, in order to gain in-depth information about the interactions of PCK components of the teachers, multiple sources of data, namely a card-sorting activity, pre-interview in the form of content representation (CoRe), observation of the instruction, field-notes, and post-interviews about the instruction were used. Fig. 2 displays the data collection stages in order. Before starting observation of the teachers’ instruction, a card-sorting task was conducted. Additionally, pre-interviews in the form of CoRe were carried out at the beginning of each topic. Then, all teachers’ instruction was observed during the teaching reaction rate and chemical equilibrium topics. At the end of each week, post-interviews about the instruction were conducted. Data were collected over a two-month period. In the following parts, all these data sources will be explained in detail. Additionally, this study was carried out in Turkish and we translated the data into English. Then, back translation was made to check the accuracy of the translation. An expert in English edited the manuscript.Card-sorting activity
In order to identify the teachers’ orientations for teaching chemistry, a card-sorting activity (Friedrichsen and Dana, 2003) was carried out before their instruction. The purpose of this activity was to elicit the teachers’ purposes and goals for teaching chemistry. First, 12 cards included scenarios related to teaching reaction rate and chemical equilibrium topics were designed by the researchers. The scenarios reflected science teaching orientations proposed by Magnusson et al. (1999) (e.g., didactic, discovery, etc.), curriculum goals stated in the chemistry curriculum of Ministry of National Education (MoNE) (2011) (history of science, terminology, and science-technology-society), and a high stakes university entrance exam (see PCK coding table in Appendix B for all science teaching orientations). These scenarios depicted an instructional strategy, laboratory activity, planning technique or assessment strategy. One of the sample scenarios is “As a chemistry teacher, you have decided that the best way to teach how the rate of a reaction changes over time is to let students discover the relation between time and reaction rate on their own.” After the scenarios were prepared, two experts in chemistry education checked their grammar, wording, and whether they were consistent with the orientation categorization of Magnusson et al.'s (1999) and the national chemistry curriculum goals. Then, the scenarios were piloted with two chemistry preservice teachers in order to check whether the card-sorting task worked in the way we intended. Before observing the teaching sessions, the teachers were requested to sort the cards into three categories: representative (i.e. scenarios that best represent their teaching), not representative (i.e. scenarios that do not represent their teaching), and unsure (i.e. scenarios where the teacher is not sure whether s/he teaches in that way). During card-sorting task, we wanted the teachers to think aloud because what the teacher said during the card-sorting task provided more insight into their science teaching orientations than how the teacher sorted the specific cards (Friedrichsen and Dana, 2003). After sorting the cards into the categories, the teachers were requested to describe how the scenarios in the representative categories reflected their purposes and goals for teaching chemistry. They were asked which aspects of their teachings were similar to or different from those defined in the cards. Classroom observations and post-interviews about the instruction were also a source for eliciting their purposes and goals for teaching chemistry.Pre-interview in the form of content representation (CoRe)
The CoRe (see Appendix A) is a tool developed for attaining science teachers’ understanding of the content as well as a way of representing this knowledge (Loughran et al., 2004). It is a matrix involving big ideas/concepts about the topic (e.g. Le Chatelier's principle, factors affecting chemical equilibrium) in the horizontal axis. There are factors that affect teachers’ decisions on such issues as learners’ difficulties and ways of assessing students’ understanding of concepts in the vertical axis (Loughran et al., 2004). Similar to Loughran et al. (2004), we used the CoRe as a pre-interview tool with all teachers to capture and portray their PCK and the interactions among the components before they started to teach both the reaction rate and chemical equilibrium topics. In these pre-interviews in the form of CoRe, we asked about the items in the CoRe to deeply comprehend the teachers’ topic-specific nature of PCK regarding the two topics and how they will design their teachings. Sample questions are given below: Which instructional strategies are you going to use to teach the chemical equilibrium topic? What are the particular reasons for using them? Which assessment techniques are you going to use to assess learners’ understanding of the reaction rate topic?Observation of instruction
After conducting CoRe, the teachers’ instruction was observed to obtain a better understanding of their actual teaching practices and the context in which they teach. For each participant, one of their 11th grade classes was chosen and observed from the beginning to the end of teaching each topic. Each participant's 17-class-sessions for reaction rate and then 18-class-sessions for chemical equilibrium were observed with field notes. Each of the class sessions was 40 minutes. This helped us gain a more complete picture of what happens in their classrooms as well as formulate our post-interview questions. All class sessions of the two topics were recorded on audiotape and then transcribed verbatim.Weekly post-interviews about instruction
After observing the teaching sessions, weekly semi-structured post-interviews were conducted to reveal the teachers’ PCK and the sources of their knowledge. We asked questions to gain an in-depth understanding of the teachers’ PCK and its components and their use of different instructional and assessment strategies/techniques. Additionally, we asked the reasons why they used them, how they decided to use them, and whether these strategies/techniques helped the learners understand the topics. By this means, the teachers had opportunities to revisit their lesson and to articulate the reasons for their instructional decisions. Sample interview questions are as follows: Why did you use an animation while teaching proper and improper orientations at the sub-microscopic level? How do you think this animation helped students learn about this topic? What knowledge about students did you use while doing the experiment about reaction rate? Each interview took approximately 30 minutes. All the interviews were audio-taped and transcribed verbatim.Data analysis
This study included different techniques used for data analysis for science teaching orientations and the interplays of PCK components. First of all, for data analysis, we used some integration of predetermined and emerging codes during the coding process (see Appendix B for Coding Table). Magnusson et al.'s (1999) PCK model with its components and sub-components formed the predetermined codes and sub-codes in this study. For the science teaching orientation component, we also added some extra sub-codes related to chemistry curriculum goals stated in the Turkish chemistry curriculum (e.g., history of science) (MoNE, 2011), and the goal related to preparation for high stakes university entrance exam. Additionally, we developed a sub-code on the basis of the emerging information collected from the participants. While observing teaching sessions and conducting interviews, we realized that some of the participants had a goal to relate chemistry to daily life. We named this goal as everyday application. After coding the teachers’ science teaching orientations according to the integration of predetermined and emerging codes, we analyzed their orientations based on two categories: central and peripheral goals (Friedrichsen and Dana, 2005).After analyzing their orientations for science teaching, in order to investigate the possible interactions among PCK components, we used Park and Chen's (2012) approach based on the pentagon model (Park and Oliver, 2008b). The analysis of data was performed following a stepwise procedure: (a) In-depth analysis of explicit PCK: After coding the data, we started to generate categories inductively to analyze the possible interactions of PCK components. For this, we first identified “teaching fragments” from the teachers’ instruction, which include an integration of two or more PCK components in the pentagon model. In other words, a teaching fragment represented the existence of two or more PCK components. A teaching fragment reflected what the teacher and students did, their roles, and which components of PCK were interacted and evidence of the presence of the portrayed components. This delineation was derived mainly from observations of instruction, but completed through interviews and instructional documents used in teaching fragments. For example, in a teaching fragment, teachers’ use of a specific assessment technique to assess a specific curriculum objective designates an interaction between knowledge of assessment and curriculum. These teaching fragments constituted the unit of analysis for this study. Finally, we generated 10 categories inductively reflecting all possible two-way interactions of PCK components based on the interview transcripts and observations of the instruction. Table 1 shows all these interactions of the components (i.e., categories) for analyzing the data and their explanations.
| Categories | Explanation |
|---|---|
| STO-KoIS interplay | Utilizing a specific instructional strategy to attain goals and purposes for science teaching |
| STO-KoC interplay | Taking a particular curriculum emphasis in class (i.e., nature of science objectives) into consideration according to teacher's goals and purposes for science teaching |
| STO-KoL interplay | Taking students’ difficulties, misconceptions or pre-requisite knowledge into consideration according to teacher's goals and purposes for science teaching |
| STO-KoA interplay | Assessing a particular knowledge or skill for determining whether students reached teacher's goals and purposes for science teaching |
| KoL-KoIS interplay | Utilizing a specific instructional strategy to handle a difficulty, misconception or pre-requisite knowledge |
| KoL-KoC interplay | Taking a difficulty, misconception, or pre-requisite knowledge into consideration while examining the curriculum regarding what students have learned so far and will learn about those topics |
| KoL-KoA interplay | Utilizing different assessment methods to specify students’ difficulties, misconceptions or pre-requisite knowledge |
| KoC-KoIS interplay | Utilizing a specific instructional strategy to address a particular curriculum objective |
| KoC-KoA interplay | Utilizing different assessment methods to define learners’ achievement regarding the goals and objectives related to the subjects, or to bring out what students know about the topic within a grade and across grades |
| KoA-KoIS interplay | Reviewing the instructional strategies according to the feedback taken from assessments |
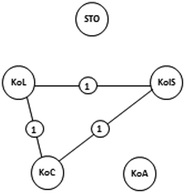
(b) Enumerative approach: After employing in-depth analysis of explicit PCK and identifying teaching fragments, an enumerative approach was used to represent the interactions among the components in a clear and explicit way (LeCompte and Preissle, 1993). We constructed PCK maps for all participants’ teaching of the reaction rate and chemical equilibrium topics, as an analytic device (Park and Chen, 2012) which indicated the interactions among the components using the pentagon model. In the PCK Map, each circle represents each PCK component. When we identified an interaction between any of the two components in the data, we showed that interaction on the map with a link between the related components. Assume that one interaction was identified among knowledge of curriculum, instructional strategies, and learner as shown in Fig. 3. Even though the strength of one link may be different from another, we presumed the same strength of 1 for each link for analytic convenience similar to what Park and Chen (2012) did.
After conducting the same procedure for all teaching fragments, the frequency of each link between any of the two PCK components was summed up across all teaching fragments and it was shown on the PCK map. Therefore, the numbers on the line represent the frequency of the interactions between any of the two PCK components. For example, Fig. 4 shows Ela's PCK map for the chemical equilibrium topic. This map shows that there are four teaching fragments identified, and in these fragments knowledge of learner and assessment are interacted. The more interplays among PCK components a teacher had, the more interplays there are on the PCK map. Additionally, the numbers in each circle show how many times the PCK components were interacted with the other components on the map. To illustrate, Ela's PCK map indicated that knowledge of curriculum was interacted 13 times with the other PCK components.
(c) Constant-comparative method: In this method, the data analysis concentrated on specifying common themes and patterns that emerged from the data, particularly interviews and observations, regarding the nature of the interactions among the PCK components without using any prior categories or framework (Glaser and Strauss, 1967). The identification of these themes and patterns indicated eight characteristics for the nature of interactions of PCK components. This method of data analysis is primarily inductive and comparative (Merriam, 2009).
For credibility, data triangulation (CoRe tool, interview transcripts, and observation of instruction), investigator triangulation (observations of all participants’ teaching by another researcher), methods triangulation (in-depth analysis, enumerative approach, and constant-comparative methods), long-term observation, and rich and thick description of the data were employed. In addition, after checking all transcripts, inter-coder agreement was conducted to determine the level of consistency of the coding. For this, the first author and an external coder who has experience in chemistry education, qualitative research, and PCK coded the interview data by using the coding table (see Appendix B) and Table 1. We coded one of the interview datasets independently for possible interactions among PCK components in teaching fragments and then compared our coding. We mainly agreed on the coding; however, some discrepancies between codes were seen. At this point, we calculated inter-rater reliability as 88% (Miles and Huberman, 1994). After resolving inconsistencies between the coders, we coded another interview dataset independently and reached about 95% agreement.
Ethical considerations
Before conducting this study, ethical standards were taken into consideration and the necessary permission for carrying out this study was obtained from the Institutional Review Board. All the participants voluntarily accepted to attend the study by signing a consent form and pseudonyms were used for all participants. With this consent form, they were fully aware of the purpose of the study and they were informed about their rights. If they felt disturbance, they could quit participating in the study. Moreover, all participants were informed about the use of an audio recorder and data collection sources. In this way, possible psychological harm might be prevented. In addition, nobody except the researchers and other coder had access to the data collected for the study. Therefore, all the issues pertaining to ethics in research (deception of the participants, confidentiality, and protection of the participants from harm) were ensured.Results
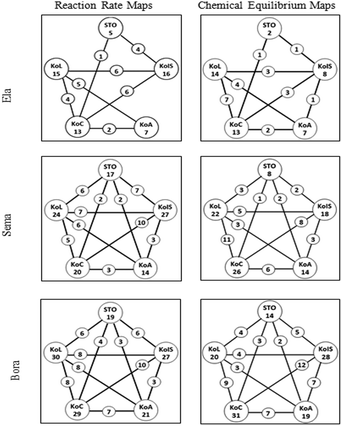
The analysis of the teachers’ PCK maps (see Fig. 5) and the patterns that appeared by the constant comparative method showed eight characteristics of the interplays of PCK components. The findings are presented according to those characteristics: (a) the novice teacher's orientations towards science, in contrast to the experienced teachers’, were more broad and non-specific, which impeded the interactions among the components, (b) the interplay of the PCK components was idiosyncratic and topic-specific, (c) the novice teacher's PCK maps were fragmented while the experienced teachers’ PCK maps were integrated, (d) the experienced teachers, in contrast to the novice teacher, interacted more than two PCK components in most of their teaching fragments, (e) knowledge of learner, knowledge of curriculum and knowledge of instructional strategies were central in the interplays of all teacher maps, (f) the experienced teachers were more successful than the novice teacher in translating their knowledge into practice in terms of the integration among PCK components, (g) teacher self-efficacy appeared to play a role in their use of PCK components and constructing interactions among them, and (h) all teachers taught the same topics with similar lesson plans and the same instructional materials; however, they differed in terms of how they connect the PCK components. In the following parts, each characteristic of the interplay among the PCK components is explained in detail with examples from CoRes and interviews.The novice teacher's orientations towards science, in contrast to the experienced teachers’, were more broad and non-specific, which impeded the interactions among the components
To gain a better understanding of the interactions, this study involved an analysis of the participants’ science teaching orientations because it is known that this is an overarching component of PCK and influences the other PCK components (Grossman, 1990). First, the results of a card-sorting task directed before the observations of the instruction are presented in Table 2. This table describes the participants’ science teaching orientations based on three categories: representative, not representative and unsure.| Teacher | Scenarios | ||
|---|---|---|---|
| Bora | Sema | Ela | |
| Representative |
Activity-driven
Discovery Conceptual Change Process Academic-rigor |
Activity-driven
Discovery Conceptual Change Guided-inquiry Process |
Activity-driven
Discovery Guided-inquiry Project-based science Curriculum goal: terminology |
| Not representative |
Didactic
Curriculum goal: History of science High stakes university entrance exam |
Didactic
Academic-rigor Curriculum goal: History of science High stakes university entrance exam |
Not been selected |
| Unsure |
Guided-inquiry
Curriculum goal: terminology Curriculum goal: STS Project-based science |
Curriculum goal: terminology
Curriculum goal: STS Project-based science |
Didactic
Conceptual Change Academic-rigor Process Curriculum goal: History of science High stakes university entrance exam Curriculum goal: STS |
On the other hand, during classroom observations and post-interviews about the instruction, we realized several conflicts between teachers’ science teaching orientations elicited during the card-sorting task (called as ideal orientations) and real classroom practice. In other words, the information elicited from the card-sorting task was not consistent with the observations of the teachers’ instruction and post-interviews about their instruction. Therefore, we also tried to diagnose their observed orientations based on the long-observation period and post-interviews about the instruction as shown in Table 3. This table describes the teachers’ central (e.g., didactic) and peripheral goals (e.g., activity-driven).
| Teachers | Central goals | Peripheral goals |
|---|---|---|
| Bora |
To relate chemistry to daily life (Everyday application)
To provide necessary knowledge to learners (Didactic) To develop conceptual understanding of chemistry (Conceptual change) To develop science-process skills (Process) |
To prepare learners to high stakes university entrance exam
To make learners active with materials and hands-on experiences (Activity-driven) To represent chemistry with difficult problems and activities (Academic-rigor) |
| Sema |
To provide necessary knowledge to learners (Didactic)
To develop conceptual understanding of chemistry (Conceptual change) |
To relate chemistry to daily life (Everyday application)
To prepare learners to high stakes university entrance exam To make learners active with materials and hands-on experiences (Activity-driven) To develop science-process skills (Process) |
| Ela | To provide necessary knowledge to learners (Didactic) |
To make learners active with materials and hands-on experiences (Activity-driven)
To develop science-process skills (Process) |
A close analysis of Tables 2 and 3 revealed that there were much more differences between the novice teacher's ideal and observed orientations than that of the experienced teachers. According to the card-sorting activity conducted before the teaching sessions, the novice teacher, Ela, chose the scenarios reflecting discovery, activity-driven, guided-inquiry, project-based science and curriculum goal: terminology for representative categories. In the pre-interview, she also stated that everyday application was also her essential science teaching orientation. However, she could not reflect her decisions in her teaching sessions that we observed. Ela selected the scenario reflecting didactic orientation for the unsure category; however, it was observed that her teaching was primarily based on lecturing. During post-interviews in combination with classroom observations, we realized that she only held didactic orientation as a central goal and activity-driven and process orientations as peripheral goals. She presented information to students didactically in most of her instruction. Her science teaching orientations were dominated by the view that teaching is telling and learning is listening. All these discrepancies imply that the novice teacher's orientations towards science were vague and non-specific. During the post-interviews, we asked about the reasons for these discrepancies. She stated that the loaded chemistry curriculum and her lack of teaching experience influenced her instructional decisions and impeded her ideal orientations. This view is reflected in the weekly post-interview excerpt below:
The first reason is that the chemistry curriculum is too loaded; therefore, I have to focus only on covering all the topics on time. The second reason is teaching experience. Actually, I would prefer to teach my lessons based on discovery and inquiry; however, I could not. Giving students responsibility to state hypotheses, define variables, develop procedures, and justify explanations as well as guiding students requires teaching experience. (Ela, weekly post-interview, reaction rate).
This vagueness related to her orientations also affected her interactions between science teaching orientations and the other PCK components. When we analyzed Ela's PCK maps for both topics, we could easily observe that it was the orientation component with which she constructed the least of links. In her maps, the orientation component did not have any link with assessment and learner components. Therefore, we could infer that her science teaching orientations did not inform her learner and assessment components. In order to address her students’ misconceptions and difficulties, she usually warned them and re-explained the confusing parts without utilizing an additional instructional strategies or materials. Moreover, she did not check the students’ understanding after her explanations. In the weekly post-interview, Ela answered our questions as follows:
Researcher: Which difficulties did your students have while learning reaction rate?
Ela: They had difficulties in expressing reaction rate both in terms of rate of decomposition and formation, and equating these two rates.
Researcher: Before the instruction, were you aware that the students might have these difficulties or did you realize them while teaching the topic?
Ela: I had predicted.
Researcher: How did you address these difficulties?
Ela: Because I thought that they might have these difficulties on expressing reaction rates, I warned them about possible difficulties that they might face.
In terms of peripheral goals, one of the goals of the novice teacher (Ela) was activity-driven. In order to achieve this goal, she sometimes used laboratory studies. For instance, after teaching factors affecting the reaction rate, she let her students to do cook-book experiments related to the factors. She provided a laboratory procedure step-by-step that the students had to follow. She also told students what data to gather and what results to expect. It was observed that laboratory activities were solely used for verification, since she always had students complete the cook-book lab. As a result, her instruction was mainly traditional, and her science teaching orientation was non-specific and didactic in nature which shaped her instructional decisions and how to implement them. In other words, her non-specific and didactic orientation filtered her instructional decisions.
On the other hand, although there were some differences between the experienced teachers’ ideal and observed orientations, they could reflect most of their decisions in their teaching sessions. In addition, they could construct interactions between science teaching orientations and the remaining PCK components intensively. During the card-sorting task, Bora (experienced teacher) stated that discovery was one of his central goals, and Sema (experienced teacher) stated that discovery and guided-inquiry were among her central goals. However, they could not reflect these decisions in their teaching sessions that we observed. Although Bora and Sema selected the scenario reflecting didactic orientation for not the representative category, it was observed that their teaching was primarily based on lecturing. During the post-interviews, we asked about the reasons for these differences. The experienced teachers stated that the loaded chemistry curriculum and high stakes testing influenced their instructional decisions and impeded their ideal orientations. The experienced teachers primarily focused on transmitting new terms and concepts to students; however, they also gained much more additional goals than did the novice teacher. For instance, one of the central goals of these experienced teachers was to develop conceptual understanding of chemistry concepts (conceptual change science teaching orientation). Additionally, relating chemistry to daily life (everyday application science teaching orientation) and developing science process skills (process science teaching orientation) were central goals for Bora, while they were peripheral goals for Sema. In addition, Bora held peripheral goals such as activity-driven, academic-rigor and preparing learners for a high stakes university entrance exam. Sema also held peripheral goals such as activity-driven and preparing learners to a high stakes university entrance exam. Different from the novice teacher, the experienced teachers utilized these distinctive science teaching orientations and the remaining PCK components intensively. They enriched their instruction with analogies, demonstrations, animations, experiments, daily life examples and simulations more than the novice teacher did. Particularly Bora, the most experienced teacher, used a variety of instructional strategies and materials in order to eliminate his students’ difficulties and misconceptions. He frequently stated that in light of his teaching experience, he was familiar with common students’ difficulties and misconceptions in each topic; therefore, he handled them with an additional instructional strategy effectively. As an example, Bora was aware about students’ difficulties in understanding the difference between average and instantaneous reaction rate. With a didactic science teaching orientation view, he taught the difference between these two concepts whereby lectures supported an analogy and questions in order to help his students. He was able to connect his knowledge of instructional strategy and knowledge of learner in light of his science teaching orientation:
Assume that you are travelling from city A to city B. The distance between these two cities is 450 km and it takes approximately 5 hours. What can you say about your average velocity? [Students answered] The answer was 90 km h−1. Then, do you drive with the same velocity, 90 km h−1? [Students answered] No, it changes. So, are the average and instantaneous velocity the same or not? [Students answered] No. (Bora, field notes, reaction rate).
In another example, Bora's process science teaching orientation shaped the way he addressed his students’ misconceptions. In other words, his science teaching orientation influenced his knowledge of learner and knowledge of instructional strategies. In the following weekly post-interview excerpt, Bora stated that
I was aware of my students’ misconception that for an endothermic reaction, as the temperature is raised, only the rate of the forward reaction increases. When I asked the reason, the students stated that according to the Le Chatelier's principle, for an endothermic reaction, an increase in temperature increases only the rate of the forward reaction. Drawing a graph is important at this point. To address their misconception, I wanted them to draw graphs (concentration vs. reaction path and reaction rate vs. reaction path) and interpret them. They tried to draw these graphs. Then, I checked their drawings and wanted them to explain their graphs (Bora, weekly post-interview, chemical equilibrium).
Similarly, in light of her process science teaching orientation, Sema encouraged her students to draw graphs and solve questions by using graphs for some concepts and interpreted them in order to help students develop science process skills. For instance, in the weekly post-interview about the instruction, she stated that
The students can solve verbal questions. For instance, they can calculate the enthalpy of reaction by using the formula (i.e., the energy difference between forward and reverse reaction) when the data are given verbally. However, when I write the same data on a graph, they cannot solve it [knowledge of areas of students’ difficulties]. In addition, I expect them to draw the graph of enthalpy of reaction vs. reaction path, and the graph of number of particles vs. kinetic energy. Therefore, in order for students to visualize the data, I want students to draw graphs and interpret them [knowledge of topic-specific representations]. I believe that they understand better (Sema, weekly post-interview, reaction rate).
The excerpt above indicated that Sema's process science teaching orientation informed her knowledge of learner and instructional strategies components.
The interplay of the PCK components was idiosyncratic and topic specific
Although the teachers taught the same topics with the same instructional materials and similar lesson plans, their PCK Maps differed from each other. In addition, each teacher's PCK Map showed variances for the two topics. These findings might indicate the idiosyncratic nature and topic-specificity of the interactions among the PCK components as exemplified below. First of all, the experienced teachers (Sema and Bora) demonstrated more coherently structured PCK Maps for both topics than the novice teacher (Ela). For instance, in the reaction rate topic, Bora and Sema interacted the PCK components 63 and 51 times in total, whereas Ela interacted them only 28 times. In the equilibrium topic, Bora and Sema connected the components 56 and 44 times in total, respectively, whereas Ela interacted them only 22 times. For the frequency of total interactions among PCK components for the reaction rate and chemical equilibrium topics, see Table 4.| Teacher | Topic | |
|---|---|---|
| Reaction rate | Chemical equilibrium | |
| Bora | 63 | 56 |
| Sema | 51 | 44 |
| Ela | 28 | 22 |
In addition, all participants had more interactions among the PCK components in the reaction rate topic than in the equilibrium topic. On the PCK maps (Fig. 5), the numbers in each circle indicate how many times the teachers interacted the components with other PCK components. For instance, in the reaction rate topic, the most experienced teacher, Bora connected knowledge of learner, instructional strategies, curriculum, assessment and orientation components 30, 27, 29, 21 and 19 times, respectively. In the equilibrium topic, he connected the same components, 20, 28, 31, 19, and 14 times, respectively. On the other hand, the least experienced teacher, Ela, interacted knowledge of learner, instructional strategies, curriculum, assessment and orientation components 15, 16, 13, seven, and five times, respectively, in reaction rate. She integrated the same components 14, eight, 13, seven, and two times, respectively, in the chemical equilibrium topic. Throughout the interviews, Ela often declared that teaching chemical equilibrium was more challenging than reaction rate. She stated:
I taught the “concentrations vs. time and rate vs. time graphs when a change is made to a system at equilibrium” for the first time in the chemical equilibrium topic. Actually, it was difficult for me. I was not sure whether I had a misunderstanding or not. Therefore, before the instruction, I observed both Bora and Sema's teaching of these graphs. In addition, in order to learn the graphs, I tried to draw these graphs many times before the instruction (Ela, weekly post-interview, equilibrium).
Moreover, Ela expressed that because her students had many difficulties in understanding the concepts in chemical equilibrium, teaching this topic was difficult for her as she described below:
As a matter of fact, [in chemical equilibrium topic], I knew what to teach them step by step. However, because students had difficulties in understanding of heterogeneous and homogeneous equilibrium, and the reasons for omitting concentration terms for solids and liquids while writing heterogeneous equilibrium, teaching chemical equilibrium was difficult for me (Ela, weekly post-interview, chemical equilibrium).
Finally, the most and the least frequent interactions among PCK components showed differences between the topics for the same teacher which indicated the idiosyncratic nature and topic-specific nature of the interplays (see Table 5). For instance, Sema's reaction rate PCK map showed that the most frequent interaction was between curriculum and instructional strategies components (10 times). The less frequent interactions were between science teaching orientations and curriculum components (two times) and between science teaching orientations and assessment components (two times). For teaching chemical equilibrium, Sema made more interactions between knowledge of learner and curriculum components (11 times), and less interaction between science teaching orientations and curriculum components (one time).
| Teacher | The most frequent interactions | The least frequent interactions | ||
|---|---|---|---|---|
| Topic | ||||
| Reaction rate | Chemical equilibrium | Reaction rate | Chemical equilibrium | |
| STO: science teaching orientations, KoC: knowledge of curriculum, KoL: knowledge of learner, KoIS: knowledge of instructional strategies, KoA: knowledge of assessment. | ||||
| Bora | KoC-KoIS (10) | KoC-KoIS (12) |
STO-KoA (3)
KoA-KoIS (3) |
STO-KoA (2) |
| Sema | KoC-KoIS (10) | KoL-KoC (11) |
STO-KoC (2)
STO-KoA (2) |
STO-KoC (1) |
| Ela |
KoC-KoIS (6)
KoL-KoIS (6) |
KoL-KoC (7) |
STO-KoL (0)
STO-KoA (0) KoA-KoIS (0) |
STO-KoL (0)
STO-KoA (0) |
The novice teacher's PCK maps were fragmented while the experienced teachers’ PCK maps were integrated
The experienced teachers were able to use and interact all PCK components while the novice teacher did not make all interactions. Therefore, it can be concluded that whereas the novice teacher's PCK maps were fragmented, the experienced teachers’ maps were integrated. The PCK maps indicated that the novice teacher rarely integrated science teaching orientations and knowledge of assessment into her PCK. As an example, in Ela's teaching of the chemical equilibrium and reaction rate topics, interaction was neither detected between orientations and learner components nor between orientations and assessment components. In addition, her limited topic-specific knowledge about learner, assessment, and instructional strategies might also prevent the interactions of the components. During the interviews, she frequently reflected that she did not have enough experience for teaching these two topics; therefore she did not have enough knowledge, for instance, about difficulties and misconceptions that students had. She was aware that students might have difficulties and misconceptions; however, she was not familiar with students’ common difficulties and misconceptions in the reaction rate and chemical equilibrium topics. This view is reflected in the interview excerpt below:Researcher: You will teach reaction rate topic for the first time. Do you think teaching this topic is difficult for you or not?
Ela: It may be difficult for me in terms of students’ misconceptions because I only know a few student misconceptions in reaction rate from the articles that I read. However, I do not know what difficulties students have and what kinds of questions they can ask about the topic while teaching (Ela, pre-interview in the form of CoRe, reaction rate).
Moreover, she stated that when she faced a difficulty in the classroom, she did not know the way to address it. She only warned her students and re-explained the points that the students had difficulties with. She did not endeavor to help them better understand it. An excerpt from weekly post-interview reflects this situation:
The students had difficulties in understanding the difference between equilibrium constant (Kc) and reaction quotient (Qc). They frequently asked why we wrote reaction quotient as equilibrium constant, and the difference between them. In addition, the relations between the molar concentrations of the reactants and products when the mixture goes to equilibrium are another difficult point for them. In order to address their difficulties, I stressed that point again and again (Ela, weekly post-interview, chemical equilibrium).
On the other hand, the experienced teachers could utilize all PCK components and integrate them coherently. It could be observed in their teaching sessions that all PCK components informed each other many times; this was not the case for the novice teacher. For instance, Sema, in contrast to the novice teacher, had enough knowledge about instructional strategies and learner; therefore, she could integrate them. When students had a difficulty, she could easily address it. The interaction between these two components is shown in the following quote from a weekly post-interview:
Researcher: You used an illustration of quicklime (calcium oxide). Why did you prefer to use this illustration?
Sema: I used it for heterogeneous equilibrium. The students had difficulties in understanding why the concentration of [pure] solids or liquids is constant [knowledge of learner]. When I explained that the pressure of the carbon dioxide does not depend on the amount of CaCO3 and CaO, it does not revive in the students’ mind. In order to show that equilibrium and the pressure of the carbon dioxide is not affected by the amount of these substances, I used that illustration [knowledge of instructional strategies] and explained it in detail (Sema, weekly post-interview, chemical equilibrium).
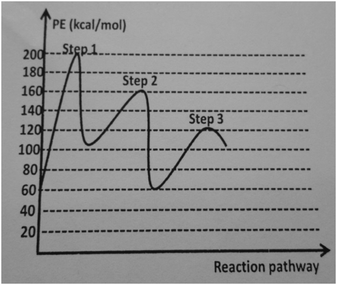
Similarly, Bora identified his students’ difficulties or misconceptions and attempted to tailor his instructional strategies to address their difficulties or misconceptions in both topics. For instance, during the instruction, he drew the following graph on the board (see Fig. 6) and asked the students:
Bora: Which step is the rate determining step?
Student 1: We cannot know this.
Student 2: The middle or the left one.
Bora: Please, come to the board and calculate the activation energies.
Student 2: I do not know how to calculate it [activation energy]. (Bora, observation of the instruction, reaction rate)
During the instruction, some students did not analyze the energy and reaction pathway graph, and which step the rate-determining step in the reaction mechanism is. In particular, they did not understand the relationship between the slow step and activation energy. Then, Bora used an analogy that was:
There are two barriers, one of them is low and the other one is high. Passing over the high barrier needs much more energy than passing over the low barrier. The number of people passing over the low barrier per unit time is much more than that of the high barrier. Therefore, for the same number of people, passing over the high barrier will be slower than the low barrier (Bora, observation of the instruction, reaction rate).
Then, he made a connection between this analogy and the relationship between the slow step and activation energy in a reaction mechanism in order to address the students’ difficulties. In the weekly post-interview, we asked the reason for using this analogy. He stated that “The students could not understand the relation between activation and rate-determining step [knowledge of learner]. Therefore, I used that analogy [knowledge of instructional strategies] in order to make it [the relation between activation and rate-determining step] much more understandable” (Bora, weekly post-interview, reaction rate).
The experienced teachers, in contrast to the novice teacher, interacted more than two PCK components in most of their teaching fragments
During the in-depth analysis of explicit PCK, we first identified teaching fragments from the teachers’ instruction, which include an interaction of two or more PCK components. In these teaching fragments, we analyzed that some of the interactions were simple in that one PCK component linked to another one while some others were complicated including more than two different PCK components. We realized that the novice teacher (Ela) interacted only two PCK components in most of her teaching fragments. For example, her knowledge of curriculum informed her knowledge of instructional strategies in the reaction rate topic. In the weekly post-interview, she stated that it was instructive to draw a potential-energy diagram [knowledge of instructional strategies] in order to teach the activation energy associating with the energy of the reactants and products, which is stated in the national chemistry curriculum [knowledge of curriculum].On the other hand, the experienced teachers interacted more than two PCK components in most of their teaching fragments. For instance, in the pre-interview, Bora stated
There was an objective in the 11th grade chemistry curriculum which read “to explain the effect of temperature on chemical equilibrium” [knowledge of curriculum]. In general, the students had difficulties in understanding the effect of temperature on chemical equilibrium for endothermic and exothermic reactions [knowledge of areas of students’ difficulties]. In order to help them and make an evaluation, I will design an instruction in which students perform an experiment, collect and interpret data, and then draw a conclusion [knowledge of instructional strategies and knowledge of assessment] (Bora, pre-interview in the form of CoRe, chemical equilibrium).
Then, we observed that he made an instruction in which students made an experiment to observe the effect of temperature on chemical equilibrium. Then, in the weekly post-interview about the instruction, he stated that the learners realized the effect of temperature on chemical equilibrium for endothermic and exothermic reactions during the experiment. He added that they also answered the questions correctly in the experiment report. It appeared that in light of his process science teaching orientation, his knowledge of curriculum and students’ difficulties informed his knowledge of instructional strategies as well as his knowledge of assessment.
Similarly, Sema interacted more than two PCK components in most of her teaching fragments. To illustrate, during teaching heterogeneous equilibrium, she tried to teach when a system in heterogeneous equilibrium is disturbed by a change (e.g., concentration) how the system shifts in equilibrium composition in a way tending to counteract the change. She wrote the following reaction on the board:
| CaCO3(s) ↔ CaO(s) + CO2(g) ΔH > 0. |
She asked her students:
Sema: How does the system shift when we add CaCO3(s)?
Student 1: The system shifts right.
Sema: Any other ideas?
Student 2: [The system] shifts right.
Sema: Please, remember how we wrote equilibrium constant expression for a heterogeneous equilibrium.
Student 3: The concentration of the solids and liquids are not included in the [equilibrium constant] expression.
Sema: In a heterogeneous equilibrium, reactants and products can be in different phases. The concentration change in the solids and liquids is always constant. As we did for homogeneous equilibrium, let's draw a concentration vs. time graph when we add CaCO3(s). (Sema, observation of instruction, chemical equilibrium).
Then, she encouraged all students to draw this graph. She gave time to the students. When some of the students had difficulties in drawing the graph, she helped them to draw the graph and made necessary explanations. When we asked her the reason why she encouraged the students to draw the graph, she stated:
While teaching heterogeneous equilibrium [knowledge of curriculum], as I expected, my students had difficulties in understanding when a system in heterogeneous equilibrium is disturbed by a change [of a concentration, temperature, or pressure, how the system shifts in equilibrium composition in a way inclining to respond the change] [knowledge of learner]. In order to address their difficulties, I oriented them to draw a concentration vs. time graph when a change of concentration, pressure, or temperature occurs. In my opinion, when they learn drawing this graph, they can understand this [heterogeneous equilibrium]. [Her process orientation informed her knowledge of instructional strategies] (Sema, weekly post interview, chemical equilibrium).
In this example, she realized her students’ difficulties while teaching heterogeneous equilibrium. To eliminate their difficulties, with the influence of her process science teaching orientation she used an instructional strategy, i.e., make them draw graphs.
Knowledge of learner, knowledge of curriculum and knowledge of instructional strategies were central in the interplays of all teacher maps
The PCK maps indicated that the most central components in all teachers’ teaching were the knowledge of learner, curriculum and instructional strategies components. All teachers frequently integrated knowledge of learner, curriculum and instructional strategies components into their PCK. Furthermore, the interactions among these three components often appeared in the teachers’ instruction. In other words, the interactions among these three components were the most frequent ones among all interactions in the PCK maps. For instance, in both Bora and Sema's teaching of chemical equilibrium, a frequent interaction was observed between knowledge of curriculum and knowledge of instructional strategies (Bora: 12; Sema: 8). The close analysis among sub-components of PCK showed that the experienced teachers’ knowledge about goals and objectives to learn the reaction rate and chemical equilibrium topics [knowledge of curriculum] most frequently informed their knowledge of topic-specific representations [knowledge of instructional strategies]. For instance, Sema drew concentration vs. time and rate vs. time graphs [knowledge of instructional strategies] in order to teach Le Chatelier's principle [knowledge of curriculum] (observations of instruction, chemical equilibrium). Similarly, Bora frequently linked these two sub-components (i.e., knowledge about goals and objectives, and knowledge of topic specific representations). For example, he used the following analogy in order to explain dynamic equilibrium, which includes a forward reaction, in which reactants are converted to products, and a reverse reaction, in which products are converted to the original reactants, and the rate of these two reactions are equal (observation of instruction). When it was asked during the weekly post-interview; he stated:In order to explain dynamic equilibrium [knowledge about goals and objectives], I used an analogy which was: “assume that there are 12 students in this class. Among these students, 11 of them are sitting and one of them is standing. When the student who is standing sits, at the same time, one of the students who is sitting will stand. This process is on going. Anyone who is looking at this class from the outside thinks that there is no change in the number of sitting students [knowledge of topic specific representations] (Bora, weekly post-interview, chemical equilibrium).
Then, he connected this analogy to the chemical dynamic equilibrium with the decomposition of dinitrogen tetroxide (N2O4).
Conversely, Ela integrated mostly knowledge of learner with knowledge of curriculum seven times in her teaching of chemical equilibrium. When we analyzed the interactions among sub-components of these two components, we realized that her knowledge of goals and objectives [knowledge of curriculum] most frequently informed her knowledge of areas of student difficulty [knowledge of learner]. This interaction was reflected in the interview excerpt below:
I predicted that the student might have difficulties in writing equilibrium-constant expressions for a heterogeneous equilibrium and the reason for omitting the concentration terms for solids and liquids [knowledge of learner]. Therefore, while explaining heterogeneous equilibrium [knowledge of curriculum], I tried to focus on their difficulties. Indeed, I can say that understanding which concentration terms are omitted or not was difficult for them (Ela, weekly post-interview, chemical equilibrium).
Moreover, among all teachers, Bora connected reaction rate and chemical equilibrium much more frequently both to the topics taught in previous years (e.g. gases, energy and bonding, etc.) and to topics taught within the same grade (e.g. enthalpy, endothermic reaction, etc.) while planning and enacting his teachings than the other two teachers. On the other hand, none of the participants brought their knowledge of subject-specific strategies (e.g. learning cycle, inquiry, etc.) into play while teaching both topics. Their teaching was generally based on lecturing; however, they enriched their instruction with topic specific activities and representations. Bora, the most experienced teacher, used topic-specific activities (e.g., demonstrations, experiments) and topic-specific representations (e.g., drawing graphs, daily life examples, analogies) much more frequently in his instruction than the other teachers (observations of instruction). For instance, he provided a lot of daily life examples such as comparing the rate of burning of wood and oxidation of iron (observation of instruction, reaction rate).
The experienced teachers were more successful than the novice teacher in translating their knowledge into practice in terms of the integration among PCK components
The in-depth analysis of explicit PCK from teaching fragments and interviews showed that some of the interplays were only observed at the knowledge level, while others were translated into practice. For example, during the card-sorting task before the instruction, Sema and Ela stated that some of their instruction would be based on guided-inquiry; however, we did not observe any lesson based on guided-inquiry. It can be also stressed that we did not observe any subject-specific instructional strategies (e.g., inquiry, learning cycle) in all teachers’ instruction. Therefore, it can be concluded that they were not able to translate their knowledge about subject-specific instructional strategies from knowledge into practice. On the other hand, they planned to use many topic-specific instructional strategies (e.g., experiments, animations), and then they used them during their instruction. Accordingly, they could translate their knowledge about topic-specific instructional strategies into practice.When we compared the teachers, the experienced teachers were more successful than the novice teacher in translating their knowledge into practice in terms of the integration among PCK components. Particularly, Bora could mostly translate the interactions among PCK components from knowledge into practice. To illustrate, he emphasized that the students’ difficulties and misconceptions would influence his instruction and lesson plan. Accordingly, in most of his teaching fragments, he used a rich repertoire of topic-specific instructional strategies in order to address his students’ misunderstandings and difficulties. To illustrate, in the pre-interview in the form of CoRe, Bora stated that
Students usually have difficulties in understanding dynamic equilibrium. In order to help them, I usually prefer to teach this concept by drawing concentration vs. time and reaction rate vs. time graphs when we add reactants or products, and change volume or temperature. I believe that they will learn better when they understand these graphs. (Bora, pre-interview in the form of CoRe, chemical equilibrium)
During the instruction, we observed that he drew concentration vs. time and reaction rate vs. time graphs for different reactions as he mentioned in the pre-interview in order to increase his students’ understanding. For instance, he wanted his students to draw concentration vs. time and rate vs. time graphs for the equilibrium reaction, H2(g) + I2(g) ⇌ 2HI(g), if (a) H2 is added, and (b) the volume is decreased to half of the original volume. Then, he gave time to all students for drawing these graphs. He checked their drawings by rounding in the class. Then, he drew the graphs on the blackboard and explained them in detail (Bora, observation of instruction, chemical equilibrium). It can be inferred that the interplay of knowledge of learner and instructional strategies was translated from knowledge into practice.
Similarly, Sema usually was able to translate the interactions among PCK components from knowledge into practice. For instance, in the pre-interview conducted before reaction rate, she stated that
We need to increase the rate of some reactions in our environment while we decrease the rate of some others. In order to achieve this, we should know the factors that affect reaction rate. I will give some examples from daily life. For instance, the recommended duration is 6 months for ‘minced meat’ in the freezer while it is 8 months for ‘meat’. I will ask them what can be the reason of this difference. In addition, I asked them why we put our food in the freezer. (Sema, pre-interview in the form of CoRe, reaction rate)
Then, during the instruction, we observed that she provided daily life examples to teach factors effecting reaction rate. She initiated a discussion to elicit the students’ ideas by using these examples from daily-life as she planned (observation of instruction). This example showed that in light of her everyday application science teaching orientation, her knowledge of curriculum informed her knowledge of topic-specific representations. Therefore, it can be concluded that Sema was able to translate this interaction from knowledge into practice.
On the other hand, in the pre-interviews, Ela stated that the students’ difficulties and misconceptions would influence her instruction and lesson plans. She also stated that “When I realize a misconception, I try to find different examples to address their misconception” (Ela, pre-interview in the form of CoRe, reaction rate). However, in most of her teaching segments, when the students failed to understand the initial explanations, she usually repeated her explanations didactically without using an additional instructional strategy such as analogy, illustrations, demonstrations, etc. (observations of instruction). Moreover, she stated that she wanted to relate chemistry to daily-life and so she planned to give some daily life examples [everyday application science teaching orientation], while teaching the reaction rate topic. However, she did not provide any daily-life examples in this topic during her instruction as we observed. She did not reflect her decisions on her teaching sessions. Therefore, for these examples, it can be inferred that she could not translate her PCK from knowledge into practice. On the other hand, for some teaching segments, Ela could translate her knowledge into practice in terms of interaction among the components. For example, in the pre-interview in the form of CoRe, she stated that while teaching the reaction rate topic, she would use graphs (e.g., number of molecules vs. time graph and number of particles vs. collision energy graph) to help students’ understanding. In order to teach the concepts in the reaction rate topic [knowledge of curriculum], she drew graphs (e.g., number of molecules vs. time graph) and sometimes she wanted her students to draw graphs and make interpretations [knowledge of instructional strategies] (observations of instruction). In this example, it is shown that the interaction between these components was translated from knowledge into practice.
Teacher self-efficacy appeared to play a role in their use of PCK components and constructing interactions among them
During the in-depth analysis of explicit PCK from teaching fragments and interviews, we observed the role of teachers’ self-efficacy in the teachers’ use of PCK components as well as their construction of interplays among the components. The role of self-efficacy in the teachers’ use of PCK components was not foreseen based on the conceptual framework of this study. However, some teaching fragments and interviews provided representative examples of how teacher efficacy played a role in the enactment of PCK. Based on social cognitive theory (Bandura, 1986), teacher efficacy is defined as “the teacher's belief in his or her capability to organize and execute courses of action required to successfully accomplish a specific teaching task in a particular context” (Tschannen-Moran et al., 1998, p. 233). Teachers develop efficacy beliefs through evaluation of information coming from four sources: mastery experiences, vicarious experiences, social persuasion, and physiological and emotional states (Bandura, 1997). Teacher efficacy influences teaching and learning (Woolfolk Hoy and Spero, 2005). Moreover, it is sensible to view teacher efficacy as a part of teachers’ knowledge, because it has an important role in identifying problems and deciding teaching strategies to solve the problems (Park and Oliver, 2008a). Particularly, Ela's teaching fragments and the interviews provided representative examples of how teacher efficacy plays a role in the enactment of PCK. For example, in the pre-interview, we asked herResearcher: How well can you elicit your students’ difficulties and misconceptions?
Ela: I feel deficient myself about that point. For example, I have been teaching for different classes, and in these classes different things come to students’ mind that I did not consider before. Students can be confused. Therefore, I feel inadequate myself about that point [difficulties and misconceptions]. I always attribute it to teaching experience. However, it will be better in time.
Researcher: How well can you provide alternative instructional strategies when your students have difficulties or misconceptions during teaching?
Ela: I cannot say that I feel sufficient myself. Therefore, I am studying the topic from different sources to find different examples for supporting students with alternative examples (Ela, pre-interview in the form of CoRe, reaction rate).
The interview excerpt above indicated that she partially believed in her capability to identify students’ difficulties and misconceptions, and to provide alternative strategies or explanations to deal with them. Moreover, for knowledge of instructional strategies, she answered the question:
Researcher: How well can you use instructional strategies?
Ela: I do not feel completely sufficient in using different instructional strategies, because I have not applied strategies such as experiments in this topic before. I do not have any idea about the possible problems and difficulties while conducting them [experiments] (Ela, pre-interview in the form of CoRe, reaction rate).
Moreover, she added that the use of analogies and daily life examples was easy but she was not comfortable with using them in class, because she might cause misconceptions in the students’ mind (Ela, pre-interview in the form of CoRe, chemical equilibrium). Along this line, she could not enact her PCK effectively in an actual classroom. During the teaching of reaction rate and chemical equilibrium, when her students had difficulty in understanding the concepts, she mostly warned them and re-explained those concepts again (observation of the instruction). For example, during the instruction, she stated that “You are still confusing the effect of catalysts on chemical equilibrium. Catalysts accelerate the reaction rate; however, they do not have an influence on equilibrium position” (observation of the instruction). After the instruction, she stated that “I realized that the students did not understand the effect of catalysts on chemical equilibrium. Therefore, I warned them and explained it again” (Ela, weekly post-interview, chemical equilibrium). Actually, she could have asked the students why they thought like that and explained the reason of this situation in detail with an additional instructional strategy (e.g., animation, simulation, and graph). However, she did not use alternative instructional strategies to address her students’ difficulties since she felt uncomfortable with using them. The novice teacher, Ela attributed her low self-efficacy regarding knowledge of learner and instructional strategies to her lack of teaching experience, because she was teaching the reaction rate and chemical equilibrium topics for the first time in a classroom environment. During pre- interview, she stated:
I do not have enough teaching experience; therefore, it may be difficult for me to identify students’ difficulties and misconceptions about the concepts in the reaction rate and equilibrium topics. In addition, when students have difficulties and misconceptions during teaching, I do not consider myself fully capable of providing alternative instructional strategies to address their difficulties. However, I believe that after a few years, when I gain enough experience, it will be better. After gaining experience about learners and their difficulties, next year it will be better for me (Ela, pre-interview in the form of CoRe, reaction rate).
On the other hand, Ela believed in her knowledge of curriculum. The interview data in combination with classroom observations showed that she frequently integrated her knowledge about goals and objectives whereas she could less frequently integrate horizontal and vertical curriculum knowledge into her PCK when needed. During pre-interview, she stated
Researcher: How well could you connect the reaction rate and chemical equilibrium topics to the other topics within the 11th grade and across grades?
Ela: I do not have any experience about teaching these topics. I sometimes feel insufficient myself to connect the topics to the others. I think that I cannot meet the students’ needs completely. (Ela, pre-interview in the form of CoRe)
On the other hand, the experienced teachers believed in their capability to enact their PCK effectively; therefore, they could frequently integrate and enact the components of PCK into their actual classrooms. In particular, Bora believed in his capability to use different instructional strategies in order to address the students’ difficulties and misconceptions. When we asked him:
Researcher: How well can you provide alternative instructional strategies when your students have difficulties or misconceptions?
Bora: If I know the students one to one, it is more efficient, because I can provide different examples [instructional strategies] related to the students’ own experiences. There is no limit for me in providing different examples. This happens in time with getting experience and reading a lot. If you read much more, you can provide much more different examples (Bora, pre-interview in the form of CoRe, reaction rate).
The interview excerpt above provided evidence that he believed in his capability to provide different instructional strategies when it was needed. Moreover, he attributed his high self-efficacy in the enactment of PCK components to his teaching experience and reading a lot. We also observed that when he identified the students’ difficulties, he could provide additional instructional strategies to address their difficulties (e.g., daily life examples, analogies, etc.). For example, before the instruction, Bora was aware of the students’ possible difficulties and described how he would help them address their difficulties. He said:
Some of the students have difficulties in understanding the reaction rate at the sub-microscopic level, and why the rate of reaction is fast at the beginning of the reaction. Therefore, I decided to use an illustration to show the reaction in which A is converted to B (A → B) at the sub-microscopic level. They can see the changes in the number of particles in terms of A and B in each time interval. In this topic, they will also learn activation energy and collisions of molecules. When they understand what happens at the sub-microscopic level now, this will help them comprehend activation energy and collisions of molecules (Bora, pre-interview in the form of CoRe, reaction rate).
During the instruction, he initiated a discussion about the changes in the concentration of molecules A and B over time at the sub-microscopic level, and wanted them to explain the illustration below (see Fig. 7). He sensed that some students had difficulties in understanding concentration change in each time interval and why the rate of reaction is fast at the beginning of the reaction. He tried to elicit all students’ ideas and their reasoning. This discussion led the students to comprehend those changes in the concentration of molecules A and B in each time interval and why the rate of reaction is fast at the beginning of the reaction. In the weekly post-interview, Bora said that “Explaining concepts at the sub-microscopic level is always a difficulty for them. I am aware of it. With that illustration, we discussed it. I was able to help them comprehend it.” This example showed that Bora's beliefs about his capability to make students comprehend explanations at the sub-microscopic level appeared to play a role in his use of knowledge of learner and instructional strategies and constructing the interaction between them.
 | ||
| Fig. 7 The progress of reaction A → B (Chang and Overby, 2011, p. 467). | ||
Similarly, Sema's teaching fragments and interviews data indicated the role of teacher self-efficacy in her use of PCK components as well as the construction of interplays between the components. For instance, she believed in her knowledge of curriculum. She also could translate her curriculum knowledge into her teachings when needed (observation of instruction). She also stated that “At the beginning of my teaching profession, I did not have enough knowledge about the chemistry curriculum. Now, I have knowledge about objectives, what we have to teach and what we do not have to teach.” (Sema, pre-interview in the form of CoRe, reaction rate). Furthermore, Sema believed her capability to use different instructional strategies in order to address the students’ difficulties and misconceptions. When we asked her
Researcher: How well can you elicit your students’ difficulties and misconceptions?
Sema: Actually, I can predict in which points the students have problems. As time passes, I have learnt the things that the students can learn easily or have difficulties with. For instance, now I am teaching three different classes, and the students in these classes usually have difficulties at similar points or ask similar questions. Consequently, over the years I have learnt in which points the students have problems. If the students do not ask any question related to these points, I usually draw their attention to these points and ask questions to elicit their ideas. Therefore, teaching experience is very important.
Researcher: Which misconceptions may students have related to the reaction rate topic?
Sema: The students do not comprehend the difference between average and instantaneous reaction rate. They have difficulty in understanding activation energy. They do not think that activation energy is a requirement for all reactions. This is a misconception.
Researcher: How well can you provide alternative instructional strategies when your students have difficulties or misconceptions during teaching?
Sema: I can provide many different examples [instructional strategies] and solve different questions when I realize their difficulties. I point out to them the points where they may have difficulties and warn them. (Sema, pre-interview in the form of CoRe, reaction rate)
The interview excerpt above provided evidence that she believed in her capability to provide different instructional strategies in order to address her students’ difficulties. Moreover, she attributed her high self-efficacy in the enactment of PCK components to her teaching experience. During her teachings, we observed that when she realized the students’ difficulties, she could provide additional instructional strategies to address their difficulties (e.g., daily life examples, analogies, etc.). For example, as she predicted, some students thought that the activation energy is a not a requirement for exothermic reactions. During the instruction, one of the students said
Student: Exothermic reactions give energy. Why do they need activation energy?
Teacher: Please, think about burning a piece of paper or burning of natural gas. Both of them are exothermic reaction. What do we use to burn it?
Student: A match or spark.
Teacher: Yes, because, we need energy to initiate a reaction. The activation energy is the minimum amount of energy required to initiate a chemical reaction. If the reactant molecules do not have the minimum activation energy, they do not produce the products.
She sensed that some students had a misunderstanding that was activation energy is a not a requirement for exothermic reaction. In order to address their misconception, she asked questions related to daily life. This discussion led the students to comprehend that the activation energy is necessary for all reactions to produce reactants. In the weekly post-interview, Sema said that “They usually have this misconception. They do not think that exothermic reactions needs activation energy in order to initiate a reaction. Therefore, I gave daily life examples. I think they understood it.”
This example showed that Sema's teacher efficacy appeared to play a role in her use of knowledge of learner and instructional strategies and constructing the interaction between them.
All teachers taught the same topics with similar lesson plans and same instructional materials; however, they differed in terms of how they connect the PCK components
All teachers taught the reaction rate and chemical equilibrium topics with the same instructional materials and similar lesson plans. However, during the in-depth analysis of explicit PCK from teaching fragments and interviews, we realized that they differed in terms of how they interact the PCK components in their teaching segments even if they used the same instructional materials. For example, all teachers planned to use the same demonstration to show the effect of surface area on reaction rate; however, the use of this topic-specific activity showed differences among teachers. During the instruction, Ela first explained the effect of surface area on the reaction rate as “If we make the pieces of the reactants smaller, we increase the number of particles on the surface which can react. This makes the reaction faster. As we increase the surface area, the reaction rate increases” (Ela, observation of the instruction, reaction rate). After this explanation, she started to make the demonstration. She put some flour on a plate and tried to burn it. Only the surface of the flour burned. After a few seconds, it went out. She stated that the contact surface area is small. Then, she stated let's increase the surface area. In order to increase the surface area, she used a straw and blew the flour into the flame of a Bunsen burner. They observed a very big flame. She stated that she increased the surface area and it burned. Then, she explained the reason for these observations without taking students’ ideas. She stated that as we increase the number of particles on the surface which can react, reaction rate increases (Ela, observation of the instruction, reaction rate). However, she could have asked what students expect and take their reasons. After the observation of the demonstration, she could have made a whole class discussion. This topic-specific activity [knowledge of instructional strategy] was used only for verification to show the relationship between reaction rate and surface area [knowledge of curriculum]. In the post interview about the instruction, she provided evidence for this interaction and stated that “This demonstration will remain in the students’ mind and also attracts their attention. It was important that the students observed the effect of surface area on reaction rate” (Ela, weekly post-interview, reaction rate).Similarly, Sema first explained the effect of surface area on reaction rate. During the instruction, she stated:
When we decrease particles size, we increase the surface area. As we increase the surface area, we increase the number of particles which collide with each other at the same time. Thus, we increase the reaction rate. When we decrease particles size, we increase the surface area of the substances. By the way, we increase the rate of reaction. You are always confusing the meaning of decreasing the particle size and increasing the surface area. Actually, they have the same meaning. In the exams, you are writing them wrong. Please, be careful (Sema, observation of instruction, reaction rate).
Based on her previous teaching experience, she warned the students about the same meaning of decreasing the particle size and increasing the surface area. However, before warning them, she did not take her students’ ideas. She preferred to directly warn them without checking whether they had a misunderstanding or not. Actually, she could have taken the students’ ideas. Then, she asked the students which one dissolves faster, granulated sugar or lump of sugar. The students answered as granulated sugar and could explain the reason why granulated sugar dissolves faster. In this point, she used her knowledge about students’ pre-requisite knowledge. Then, she started to prepare the same demonstration as Ela made. She explained what she would do and what they would observe at the each steps of the demonstration. She put some flour on a plate and tried to burn it. She said
You are sensing a soft smell and only the particles on the surface of the flour burned, because the surface area is small. Only the particles on the surface of the flour gave a reaction. When we use a straw and blow the flour into the flame of the Bunsen burner, you will observe a big difference (Sema, observation of instruction, reaction rate).
Then, she blew the flour to the flame of the Bunsen burner with the straw. The students observed the same things that their teacher told them before. However, she could have asked what students expect and take their reasons. She did not let her students make a bridge between the surface area and reaction rate. This topic-specific activity [knowledge of instructional strategy] was only used for verification to show the relationship between reaction rate and surface area [knowledge of curriculum]. In the weekly post-interview, we asked:
Researcher: Why did you prefer to use the burning of flour demonstration?
Sema: It is a funny demonstration. I wanted to show what happens when we increase surface area. This demonstration allows them to observe the things [the effect of surface on rate of reaction] that I explained to them verbally (Sema, weekly-post interview, reaction rate).
Finally, Bora implemented the same demonstration using a more student-centered approach. Before explaining the relationship between surface area and reaction rate, first he asked his students:
Bora: We mentioned about dissolving sugar at 9th grade level. Which one dissolves faster, granulated sugar or lump of sugar? And why?
Student: Because of the increase in the surface area, granulated sugar dissolves faster.
Bora: Contact surface area of the particles increases. Let's try to burn flour in different ways. Observe it. [He put some flour on a plate and tried to burn it. Then, he blew the flour to the flame of Bunsen burner.] What is the difference between these two states?
Student: We increase the surface area when you blew the flour.
(Then, Bora initiated a whole class discussion).
Bora: The difference is that we increase the surface area of flour that is in contact with the flame. (Bora, observation of the instruction, reaction rate)
In light of his academic-rigor science teaching orientation, he provided a class environment in which students were challenged to make a relationship between surface area and reaction rate by using the demonstration. He integrated his knowledge of learner, curriculum and topic-specific activities. In the weekly post-interview, when we asked the reason for using this demonstration, he stated “I expected them to comprehend that concept [effect of surface area on reaction rate] and to make a relationship between the concept and their observations. With this demonstration, I expected them to make inference based on their observations” (Bora, weekly-post interview, reaction rate).
Discussion and conclusion
This following discussion will contemplate the eight characteristics of the interplay of the PCK components of novice and experienced chemistry teachers’ teaching of the reaction rate and chemical equilibrium topics, and what role teaching experience may play in the interactions among the components. Accordingly, the results of this study showed that there were much more differences between the novice teacher's ideal and observed orientations than those of the experienced teachers. These findings served as evidence for the fact that the novice teacher's orientations towards science were more broad and non-specific (Friedrichsen and Dana, 2003); and this may inhibit the interactions between orientations and the other components (Friedrichsen et al., 2009; Park and Chen, 2012; Demirdöğen, 2016). Accordingly, lack of teaching experience might impede the novice teacher's ideal orientation transfer into her teaching practice and the interaction between the orientations and other components. Unlike the novice teacher, the experienced teachers could reflect most of their decisions on their teaching sessions and construct the relationships between science teaching orientations and the other components. All these findings infer that teaching experience may have a role in the teachers’ science teaching orientations. The more teaching experience they gained, the more distinctive orientations they had.Analysis of PCK maps also indicated that the interplay of the components was idiosyncratic and topic-specific. While the idiosyncratic nature and topic-specificity of PCK have been empirically supported by many scholars (Grossman, 1990; Van Driel et al., 1998; Loughran et al., 2008), our results, however, showed that those characteristics not only stem from different PCK components involved in a teaching fragment but also different interactions of the components and sub-components included in a teaching fragment. In this study, idiosyncratic and topic-specific nature of the interactions of PCK components was observed since all participants’ PCK maps showed differences for the teachings on reaction rate and chemical equilibrium. In addition, each teacher's PCK maps for each topic displayed differences. These findings confirm the suggestion of Park and Chen (2012) that the topic-specificity can be linked to not only which components compose a teacher's PCK for a specific topic but also how and to what degree those components interplay with each other. Moreover, the findings showed that the experienced teachers had much more coherently structured PCK maps for both topics than the novice teacher. For all participants in this study, the context, the topics, and the lesson plans were the same. Additionally, their educational backgrounds were similar. Therefore, the participants’ level of teaching experience, an important source of PCK, might shape the idiosyncrasy of the teachers’ PCK integration. In addition to teaching experience, science teaching orientations, personal characteristics and characteristics of students might shape the idiosyncrasy of teachers’ PCKs (Park and Oliver, 2008a). The findings also showed that novice and experienced teachers interacted the components into their PCK much more in reaction rate than chemical equilibrium. The novice teacher, Ela provided evidence that her students experienced many difficulties in understanding terms and explanations in chemical equilibrium; therefore, teaching this topic was challenging for her. Sema, the experienced teacher, also expressed that relating chemistry to daily life in the chemical equilibrium topic was more difficult for her than the reaction rate topic. These findings are consistent with the view that chemical equilibrium is one of the most complex topics in chemistry; therefore, most teachers and students themselves struggle with some concepts of chemical equilibrium (e.g., Van Driel et al., 1998; Tyson et al., 1999; Voska and Heikkinen, 2000; Cakmakci, 2010). In addition, its content is very abstract and it requires a high degree of connections with other topics in chemistry as well as content and terminology of specific explanations (Tyson et al., 1999). It can be inferred that in order to learn chemical equilibrium, learners need to have far more prerequisite knowledge. In a similar vein, teachers are required to have much more knowledge about the vertical (e.g., gases, bonds, etc.) and horizontal curriculum (e.g., reaction rate, chemical reactions and energy, etc.) to fulfill learners’ needs. As a result, the nature of the topic is an indicator of how and to what extent components interact with each other, which is also supported by Park and Chen (2012).
The findings also indicated that the novice teacher's PCK maps, in contrast to the experienced teachers’, were fragmented since there were some missing interactions among the components. In addition to the existence of each PCK component, the degree of the interactions and coherence among the components showed the level of a teacher's PCK (Park and Oliver, 2008a; Friedrichsen et al., 2009). The appropriate interplay among the components might be the most critical factor for the teachers’ successful teaching (Fernandez-Balboa and Stiehl, 1995). From this point of view, although the novice teacher had separate PCK components, she could not utilize them in harmony in her teaching of reaction rate and chemical equilibrium. This finding supported the view that the novice teachers had a limited PCK level in spite of their science backgrounds (Lee et al., 2007). Therefore, the poor PCK of the novice teacher's may cause fragmented integration of PCK components. On the other hand, the experienced teachers’ PCK maps were integrative, because they could utilize all PCK components coherently. This result can be anticipated because the experienced teachers’ knowledge bases were more extensive than those of the novice teacher. Based on their teaching experiences, the experienced teachers, in contrast to the novice teacher, easily attempted to tailor their instructional strategies to meet the students’ learning needs in reaction rate and chemical equilibrium. This finding is similar to other studies (e.g., Clermont et al., 1994) revealing that experienced teachers recognized the students’ difficulties and sources of these difficulties and knew the ways of addressing them much more frequently than did the novice teachers. This finding is also similar to the studies (Brown et al., 2008; Brown et al., 2013) advocating that as preservice teachers gain more teaching experience, the interactions among PCK components develop. Teaching experience reinforces the growth, choice and utilization of PCK (Gess-Newsome, 1999).
The findings of this study also revealed that the experienced teachers, in contrast to the novice teacher, interacted more than two PCK components in most of their teaching fragments. This finding might result from the nature of PCK which is that the interplays among PCK components do not occur as a linear process; instead, multiple variations may appear concurrently (Fernandez-Balboa and Stiehl, 1995). Although research has revealed that PCK components interact with each other in a highly complex way (Magnusson et al., 1999; Park and Oliver, 2008a), very few empirical studies have investigated the level of complexity of the integration among the components (Aydin and Boz, 2013). Aydin and Boz (2013) examined the nature of integrations of the experienced teachers’ PCK components, and asserted that some of the interactions were so simple that one PCK component informed the other one. On the other hand, some of the interactions were so complicated at least two PCK components interacted with each other. Different from the results of this study, we concluded that the experienced teachers’ instruction revealed much more complex interactions consisting of more than two PCK components than the novice teacher's. This could be linked to their teaching experience, since teaching experience promoted the integration of the components (Brown et al., 2008; Friedrichsen et al., 2009; Brown et al., 2013). Although this study put effort into understanding the complex nature of integration among PCK components, still there is a need for research on the complex nature of interactions among the components (Magnusson et al., 1999; Park and Chen, 2012).
Furthermore, the results also pointed out that knowledge of learner, curriculum and instructional strategies components played an influential role in shaping the three teachers’ PCK maps, because they frequently integrated them into their PCK. Shulman conceptualized PCK as knowledge of learner and instructional strategies which were the key components of PCK and most scholars have agreed with him although their descriptions of PCK differ (Magnusson et al., 1999; Park and Oliver, 2008a). In this regard, the frequent interaction between knowledge of learner and instructional strategies can be anticipated considering that teachers should know students’ prerequisite knowledge and difficulties in a specific topic in order to compose instructional strategies (Magnusson et al., 1999). Therefore, the present study empirically supported that knowledge of learner and instructional strategies were the key components of PCK, and they were influential in shaping the teachers’ PCK maps (Park and Chen, 2012; Aydın and Boz, 2013). Additionally, knowledge of curriculum was another most frequent component in the present study. This finding is inconsistent with the research stating that the curriculum component is less frequently connected with other PCK components (Park and Chen, 2012; Aydin and Boz, 2013). However, research also has provided evidence that curriculum knowledge is probably the tool with the highest potential for improving teacher knowledge (Arzi and White, 2007). Arzi and White (2007) reported that “the required school curriculum is the single most powerful factor affecting teacher content knowledge, serving as both knowledge organizer and source” (p. 230). Of the three teachers, Bora showed greater awareness than the other teachers in the sequence of the curriculum within a grade and across grades while planning and enacting his teachings. This evident difference may stem from the teachers’ different levels of teaching experience, because teaching experience reinforces the development and use of PCK as also claimed by Gess-Newsome (1999). Moreover, Sickel (2012) concluded that teaching experience helped the teachers develop their knowledge of the horizontal curriculum. On the other hand, none of the participants brought their knowledge of subject-specific strategies (e.g., learning cycle) into play while teaching both topics. This implies that teaching experience alone did not make any difference in implementing subject-specific strategies regarding teaching these two topics. This finding aligns with the research reporting that both preservice teachers and teachers had limited knowledge of subject-specific instructional strategies (Friedrichsen et al., 2009), and that the experienced teachers did not use any subject-specific instructional strategies during their instruction (Aydin et al., 2014). This finding could be linked to several factors. First, because of their science teaching orientations which directly shape their instructional decisions, they might prefer to use didactic teaching (Magnusson et al., 1999; Friedrichsen et al., 2009). Their strong didactic science teaching orientation might filter the teachers’ instructional decisions (Friedrichsen et al., 2009), which resulted in less room for the influence of other orientations on their subject-specific instructional decisions. Second, teachers might have difficulties while implementing subject-specific strategies (Settlage, 2000; Brown et al., 2013). For example, Brown et al. (2013) stated that during the teacher preparation program, prospective secondary science teachers learned about, experienced, and designed 5E instructional sequences in science methods courses; however, they did not use 5Es in their internship classrooms. They were unable to implement the 5Es in their teaching. Third, the choice of instructional strategies can also be influenced by the national curriculum and high stakes testing (Haney and McArthur, 2002). During the interviews, the teachers stated that the need to cover the entire chemistry curriculum and high stakes testing influenced their instructional decisions. In conclusion, all these factors appeared to be obstacles to the implementation of the subject-specific instructional decisions.
Moreover, the findings indicated that in some cases, even if the teachers had the knowledge, they could not translate their knowledge into practice. Considering the nature of PCK which is that “…PCK is both an external and internal construct, as it is constituted by what a teacher knows, what a teacher does, and the reasons for the teacher's actions” (Baxter and Lederman, 1999, p. 158), this finding does not seem to be surprising. The translation of teachers’ knowledge into classroom practice is an important feature of PCK and this forces us to observe actual teaching fragments (Baxter and Lederman, 1999). Additionally, when the PCK definition, which is based on teachers’ understanding and their enactment (Park and Oliver, 2008a), is taken into account, it seems plausible to observe some of the interactions at the knowledge level, while others were translated from knowledge into practice. PCK growth incorporated knowledge acquisition and knowledge use. They were interrelated during instructional practices rather than following the sequence acquisition and enactment (Park and Oliver, 2008a). Moreover, comparison of interactions among the components put forward that the experienced teachers were more successful than the novice teacher in translating the integration of PCK components from knowledge into their teaching practice. For effective teaching, teachers should integrate the components and enact them within a context (Park and Oliver, 2008a). The enactment of PCK in a topic requires a teacher to interplay various PCK components (Park and Oliver, 2008a). Accordingly, the novice teacher's fragmented PCK might prevent her from translating the interactions of PCK components from knowledge into practice. It can be inferred that teaching experience might help teachers achieve translation of the integration of PCK components from knowledge into practice. Park and Oliver (2008a) supported that teachers should produce knowledge for teaching based on their own experiences, and the strongest changes in teacher knowledge stem from experiences.
The findings also showed that teachers’ self-efficacy appeared to play a role in the teachers’ use of PCK components and constructing interactions among them. The experienced teachers relied on their own capability in enacting their PCK effectively. Therefore, they could frequently integrate and enact the PCK components into their actual classrooms. Conversely, the novice teacher partially believed in her own capability to enact her PCK in an actual classroom. Therefore, she sometimes could not display her PCK effectively in an actual classroom. These findings also align with the research providing evidence that teacher efficacy has a strong effect on teaching effectiveness and PCK (Knoblauch and Woolfolk Hoy, 2008; Park and Oliver, 2008a). Actually, there is a bidirectional relationship between PCK and teacher efficacy (Park and Oliver, 2008a). Moreover, Park and Oliver (2008a) advocated that PCK consists of two dimensions: understanding and enactment. At this point, teacher efficacy served as a conduit to transfer PCK from understanding into enactment. High teacher efficacy enables teachers to enact their understanding. “When the enactment was successfully performed, teacher efficacy was in turn increased” (Park and Oliver, 2008a, p. 278). Furthermore, when it was asked, the experienced teachers (Sema and Bora) attributed their high teacher efficacy in the enactment of PCK components to their teaching experience. The novice teacher attributed her low teacher efficacy regarding PCK components to her inadequate teaching experience. It seems reasonable, because self-efficacy beliefs are developed through enactive mastery experience (Bandura, 1977). The mastery experiences acquired in the form of successful teaching is an important source of teacher efficacy (Mulholland and Wallace, 2001; Park and Oliver, 2008a). Moreover, this assertion also supports the idea that it seems plausible to view teacher efficacy as a component of PCK (Park and Oliver, 2008a; Gess-Newsome, 2015), because it plays a critical role in defining problems and determining teaching strategies to solve the problems (Park and Oliver, 2008a). Finally, this finding of the present study can also be explained by a recent model proposed by Gess-Newsome (2015). According to this model, teacher professional knowledge and skill (TPK and S) is relatively different from the one originally introduced by Magnusson et al. (1999). In the model of TPK and S, teacher orientations and beliefs such as teacher efficacy, motivation, and dissatisfaction are removed from the PCK construct and viewed as amplifiers or filters for classroom practice. Based on this point of view, the role of teacher efficacy beliefs regarding the use of and establishing interactions among PCK components appears to be plausible.
Finally, the findings showed that all teachers taught the same topics with the same instructional materials and similar lesson plans; however, they differed in terms of how they integrate the PCK components into their teaching segments. This result may stem from the teachers’ different levels of teaching experience. Teaching experience may influence the integration of the PCK components, because PCK can be improved through teaching experience (Lee et al., 2007). It can be inferred that as they gain experience, they might enhance robust PCK and enactment of their PCK in an actual classroom.
Implications and suggestions for practice and limitations
This study provides valuable information on the nature, dynamics and complexities of the interplays among components and sub-components of PCK in chemistry teaching. All these findings may inform science teacher educators and science education researchers. For science teacher educators, the findings of this study imply a need to focus on developing the teachers’ PCK and interactions among the components in teaching specific topics within the same discipline. Accordingly, if preservice teachers are supported to comprehend PCK as knowledge during teacher education programs, this may positively contribute to their professional development as novice science teachers. Therefore, during preservice teacher education courses, PCK construction, integration of the components, and its nature should be explicitly introduced to preservice teachers as a professional knowledge base for science teaching. For instance, in the courses related to methods of science teaching, preservice teachers should learn about PCK, its components and its nature as well as the integration among PCK components. In practice teaching courses, preservice teachers should develop their PCK, and design and experience instruction by using that knowledge much more effectively. In these courses, it may be helpful to present CoRe as a lesson planning tool. Moreover, preservice teachers should be encouraged to reflect on their own PCK, to realize and discuss both weak and strong parts in their PCK components and the integrations of them. They also might be expected to draw their own PCK maps in order to visualize their own PCK integration. Furthermore, considering the relation between teacher efficacy and teaching experience, teacher education programs should provide preservice teachers with an extensive practicum experience in cooperating schools so that they gain successful mastery experiences to increase their teacher efficacy, which in turn will develop their PCK.Furthermore, the findings of this study imply several implications for in-service teacher education programs. The findings showed that teaching experience plays an influential role in obtaining distinctive science teaching orientations. Still, the novice and experienced teachers had strong didactic orientation. Therefore, this study implies that both novice and experienced teachers should be guided in translating their ideal orientations into their instructional practices. The findings also showed that both novice and experienced teachers need support for implementing subject-specific instructional strategies, which is consistent with their ideal orientations. Consequently, in professional development programs, in-service teachers should be dissatisfied with their simplistic views of teaching, and provided opportunities to make their teachings much more student-centered and reform-based. Teacher educators should provide guidance and mentoring regarding how to transfer their ideal orientations into their real practices. For instance, during professional development activities, teachers may design an instruction by utilizing a subject-specific instructional strategy (e.g., inquiry, learning cycle), which is more student-centered and reform-based. In this way, they can find an opportunity to translate their ideal orientations (e.g., discovery and inquiry) into their instructional practices as well as integrate their orientations with their subject-specific instructional strategies. Additionally, this study implies that in-service teachers, especially novice teachers need support to translate the integration of PCK components from knowledge into their teaching practice. Thus, professional development activities should focus on making PCK much more explicit in different science topics in order to enable them to notice the process of the interactions of the components. For instance, in-service teachers may be introduced to CoRe as a lesson planning tool which helps teachers develop their topic-specific PCK and integration of the components. They may prepare CoRes for different topics in groups. After preparing the CoRes, groups may make reflections on the CoRes by focusing on PCK components and the integrations among them. Then, they may implement these CoRes in their actual classrooms and take video-records of their own instruction. After that, during professional development programs, by watching these videos, they may make reflections on their performance focusing on how they apply their CoRes, and how they translate the integration of PCK components from knowledge into their teaching practice. These critical and detailed analyses of their performance allow teachers to see the strong and weak parts of their performance. Then, they may initiate a discussion on how they develop their teaching as well as the integration of PCK components. As a conclusion, teaching performance and specific performance feedback from a colleague/expert may provide them mastery experiences and verbal persuasion, which enhance their teacher efficacy. Particularly for novice teachers, in addition to mastery experiences, verbal persuasion in the form of encouragement and advice is a powerful source of efficacy (Mulholland and Wallace, 2001). Moreover, during professional development activities, sample cases may be analyzed. These cases may draw a profile of how experienced teachers integrate their PCK components into their teachings. After watching experienced teachers’ teaching, they can discuss the use of their PCK and the integration of the components. Experienced teachers’ rich repertoire of teaching specific topics and how they connect PCK components during instruction serve as a guide/model for novice teachers in order to develop their own teaching. This may provide vicarious experiences, one of the sources of efficacy, for the in-service teachers (Bandura, 1977). When in-service teachers observe that a credible model teaches well, the efficacy of the observer teachers may be improved.
In spite of the strong points of this study, there may be two limitations of the present study. First, this study involves a small number of participants and the generalizability of the results of this study may be limited. However, the purpose of this study is to broaden the theory of PCK especially in terms of the interactions of PCK components within a specific discipline and not to make a statistical generalization. The expectation is that the findings from this research may be replicated and developed upon. Moreover, by studying both novice and experienced teachers, researchers may examine and compare how these teachers construct their topic-specific PCK and the relationships between the components for teaching different topics. More empirical studies are needed to understand the role of teaching experience in the interactions among PCK components. Science education researchers, in further studies, may employ longitudinal studies to examine whether the interactions among teachers’ PCK components grow and improve significantly over time for different topics within the same discipline. The second limitation of this study was that we assumed the same strength for each link among PCK components while conducting the PCK map approach. This might lead to a risk of oversimplifying the complex construct of PCK (Park and Chen, 2012). Still, it is a valuable effort, because the PCK map approach helped us to identify, quantify, and visualize the interplays among PCK components. With further studies, the PCK map approach should be developed to be able investigate the strength and quality of the interactions among PCK components across different topics. In addition, with further research efforts, the PCK map approach can be used to investigate how PCK relates to student outcomes, which is an unanswered question in the PCK literature. Overall, the findings of this study can make contributions to the relevant literature by portraying novice and experienced teachers’ PCK components in relation to each other.
Authors' note
This study is a part of the first author's doctoral dissertation and parts of the study were presented at the European Conference on Educational Research – ECER, Budapest, 2015.Conflicts of interest
There are no conflicts to declare.Appendix
A Content representation (CoRe)
| Important science ideas/concepts | |||
|---|---|---|---|
| Big Idea 1 | Big Idea 2 | Etc. | |
| 1. What you intend the students to learn about this idea | |||
| 2. Why it is important for students to know this | |||
| 3. What else you know about this idea (that you do not intend students to know yet) | |||
| 4. Difficulties/limitations connected with teaching this idea | |||
| 5. Knowledge about students' thinking which influences your teaching of this idea | |||
| 6. Other factors that influence your teaching of this idea | |||
| 7. Teaching procedures (and particular reasons for using these to engage with this idea) | |||
| 8. Specific ways of ascertaining students' understanding or confusion around this idea (include likely range of responses) | |||
B PCK coding table
PCK coding table used in this Study (Magnusson et al., 1999, MoNE, 2011).| Codes | Sub-codes | Definition |
|---|---|---|
| Science teaching orientations | Didactic | Transfer the facts of science |
| Activity-driven | Make learners active with materials and hands-on experiences | |
| Discovery | Supply opportunities for learners to discover aimed science concepts on their own | |
| Conceptual change | Ease the improvement of scientific knowledge by contradicting learners with contexts to clarify that challenge their naive conceptions | |
| Academic-rigor | Present a specific body of knowledge | |
| Guided-inquiry | Found a community of students whose members share responsibility for comprehension of the physical world, especially with respect to utilizing the tools of science | |
| Project-based science | Include learners in examining solutions to authentic problems | |
| Process | Help learners improve science process skills | |
| History of science | Develop an understanding of the historical improvement of basic concepts of the matter | |
| Science-technology-society | Develop an understanding of the effects of concepts on individuals, society, the economy and the technological world | |
| Terminology | Develop skills for utilizing chemical terminology for explaining those concepts or models | |
| High Stakes University Entrance Exam | Prepare learners for a high stakes university entrance exam | |
| Everyday application | Use science to understand everyday objects and events | |
| Knowledge of curriculum | Knowledge of goals and objectives | Teachers’ knowledge of goals and objectives related to their subjects for students |
| Knowledge of horizontal curriculum | Teachers’ knowledge of the curriculum about relations to other topics in the same grade in their subjects | |
| Knowledge of vertical curriculum | Teachers’ knowledge of the curriculum about vertical relations of the topic to the earlier and later grades | |
| Knowledge of learner | Knowledge of requirements for learning | Teachers’ knowledge about prerequisite knowledge needed for learners in order to learn particular scientific topics |
| Knowledge of areas of student difficulty | Teachers’ knowledge about science concepts or topics that learners find difficult to learn. | |
| Knowledge of areas of student misconception | Teachers’ knowledge about learners’ ideas different from scientifically accepted description | |
| Knowledge of assessment | Knowledge of dimensions of science learning to assess (What to assess) | Teachers’ knowledge about assessment of students’ learning as related to stated goals |
| Knowledge of methods of assessment (How to assess) | Teachers’ knowledge of how to assess student learning as related to stated goals | |
| Knowledge of instructional strategies | Knowledge of subject-specific strategies for science teaching | Teachers’ knowledge of strategies used for teaching science which are more general and particular to only teaching science (e.g., learning cycle, inquiry) |
| Knowledge of topic-specific strategies for science teaching | Teachers’ knowledge of topic-specific representations (e.g., analogies, models, examples) and topic-specific activities (e.g., experiments, demonstrations, simulations) for teaching specific topics in science | |
References
- Abell S., (2007), Research on science teacher knowledge, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education, Mahwah, NJ: Lawrence, pp. 1105–1149.
- Abell S. K., (2008), Twenty years later: Does pedagogical content knowledge remain a useful idea? Int. J. Sci. Educ., 30(10), 1405–1416.
- Arzi H. and White R., (2007), Change in teachers’ knowledge of subject matter: a 17-year longitudinal study, Sci. Educ., 92(2), 221–251.
- Aydin S. and Boz Y., (2013), The nature of integration among PCK components: a case study of two experienced chemistry teachers, Chem. Educ. Res. Pract., 14, 615–624.
- Aydin S., Friedrichsen P. M., Boz Y. and Hanuscin D. L., (2014), Examination of the topic-specific nature of pedagogical content knowledge in teaching electrochemical cells and nuclear reactions, Chem. Educ. Res. Pract., 15, 658–674.
- Aydin S., Demirdogen B., Akin F. N., Uzuntiryaki-Kondakci E. and Tarkin A., (2015), The nature and development of interaction among components of pedagogical content knowledge in practicum, Teach. Teach. Educ., 46, 37–50.
- Bandura A., (1977), Self-efficacy: toward a unifying theory of behavioral change, Psychol. Rev., 84, 191–215.
- Bandura A., (1986), Social foundations of thought and action: a social cognitive theory, Englewood Cliffs, NJ: Prentice Hall.
- Bandura A., (1997), Self-efficacy: the exercise of control, New York: Freeman.
- Baxter J. A. and Lederman N. G., (1999), Assessment and measurement of pedagogical content knowledge, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Dordrecht, Netherlands: Kluwer Academic Publishers, pp. 147–160.
- Brown P., Abell S. and Friedrichsen P., (2008), Investigating teacher knowledge of learners and learning and sequence of science instruction in an alternative certification program, Paper presented at the National Association for Research in Science Teaching, Baltimore, MA.
- Brown P., Friedrichsen P. and Abell S., (2013), The development of prospective secondary biology teachers PCK, J. Sci. Teach. Educ., 24, 133–155.
- Cakmakci G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey, J. Chem. Educ., 87(4), 449–455.
- Chang R. and Overby J., (2011), General chemistry: the essential concepts, 6th edn, Mc Graw Hill.
- Clermont C. P., Borko H. and Krajcik J. S., (1994), Comparative study of the pedagogical content knowledge of experienced and novice chemical demonstrators, J. Res. Sci. Teach., 31, 419–441.
- Cochran K. F., King R. A. and De Ruiter J. A., (1991), Pedagogical content knowledge: a tentative model for teacher preparation, Paper presented at the Annual Meeting of the American Educational Research Association, April, 1991, Chicago.
- De Jong O., van Driel J. and Verloop N., (2005), Preservice teachers’ pedagogical content knowledge of using particle models in teaching chemistry, J. Res. Sci. Teach., 42, 947–964.
- Demirdöğen B., (2016), Interaction between science teaching orientation and pedagogical content knowledge components, J. Sci. Teach. Educ., 27(5), 495–532.
- Erduran S., Bravo A. A. and Naaman R. M., (2007), Developing epistemologically empowered teachers: examining the role of philosophy of chemistry in teacher education, Sci. Educ., 16, 975–989.
- Fernandez-Balboa J. M. and Stiehl J., (1995), The generic nature of pedagogical content knowledge among college professors, Teach. Teach. Educ., 11(3), 293–306.
- Friedrichsen P. M. and Dana T. M., (2003), Using a card-sorting task to elicit and clarify science-teaching orientations, J. Sci. Teach. Educ., 14(4), 291–309.
- Friedrichsen P. M. and Dana T. M., (2005), Substantive-level theory of highly regarded secondary biology teachers’ science teaching orientations, J. Res. Sci. Teach., 42(2), 218–244.
- Friedrichsen P. J., Abell K. A., Pareja E. M., Brown P. L., Lankford D. M. and Volkmann M. J., (2009), Does teaching experience matter? Examining biology teachers’ prior knowledge for teaching in an alternative certification program, J. Res. Sci. Teach., 46(4), 357–383.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76(4), 548–554.
- Gess-Newsome J., (1999), Pedagogical content knowledge: an introduction and orientation nature, sources and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Dordrecht, Netherlands: Kluwer Academic Publishers, pp. 3–17.
- Gess-Newsome J., (2015), A model of teacher professional knowledge and skill including PCK, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, Routledge, pp. 28–42.
- Glaser B. G. and Strauss A. L., (1967), The discovery of grounded theory: strategies for qualitative research, Chicago, IL: Aldine Publishing Company.
- Grossman P., (1990), The making of a teacher: teacher knowledge and teacher education, New York: Teachers College Press.
- Haney J. J. and McArthur J., (2002), Four case studies of prospective science teachers’ beliefs concerning constructivist teaching practices, Sci. Educ., 86, 783–802.
- Hashweh M. Z., (2005), Teacher pedagogical constructions: a reconfiguration of pedagogical content knowledge, Teach. Teach.: Theory Pract., 11, 273–292.
- Henze I., Van Driel J. and Verloop N., (2008), Development of experienced science teachers’ pedagogical content knowledge of models of the solar system and the universe, Int. J. Sci. Educ., 30(10), 1321–1342.
- Kagan D. M., (1990), Ways of evaluating teacher cognition: inferences concerning the Goldilocks Principle, Rev. Educ. Res., 60(3), 419–469.
- Kaya O. N., (2009), The nature of relationships among the components of pedagogical content knowledge of pre-service science teachers: ‘Ozone layer depletion’ as an example, Int. J. Sci. Educ., 31(7), 961–988.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204.
- Knoblauch D. and Woolfolk Hoy A., (2008), “Maybe I can teach those kids.” The influence of contextual factors on student teachers’ efficacy beliefs, Teach. Teach. Educ., 24, 166–179.
- LeCompte M. D. and Preissle J. (ed.), (1993), Ethnography and qualitative design in educational research, 2nd edn, San Diego, CA: Academic Press.
- Lee E., Brown M. N., Luft J. A. and Roehrig G. H., (2007), Assessing beginning secondary science teachers' PCK: pilot year results, Sch. Sci. Math., 107(2), 52–60.
- Leite L., Mendoza J. and Borsese A., (2005), Teachers’ and prospective teachers’ explanations of liquid-state phenomena: a comparative study involving three European countries, J. Res. Sci. Teach., 44(2), 349–374.
- Loughran J., Mulhall P. and Berry A., (2004), In search of pedagogical knowledge in science: Developing ways of articulating and documenting professional practice, J. Res. Sci. Teach., 41, 370–391.
- Loughran J., Mulhall P. and Berry A., (2008), Exploring pedagogical content knowledge in science teacher education, Int. J. Sci. Educ., 30(10), 1301–1320.
- Luft J. A., Firestone J. B., Wong S. S., Ortega I., Adams K. and Bang E, (2011), Beginning secondary science teacher induction: a two-year mixed methods study, J. Res. Sci. Teach., 48(10), 1199–1224.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Dordrecht, Netherlands: Kluwer Academic Publishers, pp. 95–132.
- Merriam S. B., (2009), Qualitative research: a guide to design and implementation, San Francisco: Jossey-Bass.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: An expanded sourcebook, 2nd edn, Thousand Oaks: Sage Publications.
- Ministry of National Education, (2011), Secondary 11th Grade Chemistry Curriculum, Ankara, Turkey: Ministry of Education.
- Mulholland J. and Wallace J., (2001), Teacher induction and elementary science teaching: enhancing self-efficacy, Teach. Teach. Educ., 17, 243–261.
- Padilla K. and Van Driel J., (2011), The relationships between PCK components: the case of quantum chemistry professors, Chem. Educ. Res. Pract., 12(3), 367–378.
- Park S. and Chen Y. C., (2012), Mapping out the integration of the components of pedagogical content knowledge (PCK): examples from high school biology classrooms, J. Res. Sci. Teach., 49(7), 922–941.
- Park S. and Oliver J. S., (2008a), Revisiting the Conceptualization of Pedagogical Content Knowledge (PCK): PCK as a Conceptual Tool to Understand Teachers as Professionals, Res. Sci. Educ., 38, 261–284.
- Park S. and Oliver J. S., (2008b), National Board Certification (NBC) as a catalyst for teachers’ learning about teaching: the effects of the NBC process on candidate teachers’ PCK development, J. Res. Sci. Teach., 45(7), 812–834.
- Patton M. Q., (2002), Qualitative Research and evaluation methods, 3rd edn, Thousand Oaks, California: Sage Publications, Inc.
- Sickel A., (2012), Examining beginning biology teachers’ knowledge, beliefs, and practice for teaching natural selection, Unpublished Doctoral Dissertation, Columbia, USA: University of Missouri.
- Settlage J., (2000), Understanding to learning cycle: influences on abilities to embrace the approach by preservice elementary school teachers, Sci. Educ., 84, 43–50.
- Shulman L., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L., (1987), Knowledge and teaching: Foundations of the new reform, Harvard Educ. Rev., 57(1), 1–22.
- Tamir P., (1988), Subject matter and related pedagogical knowledge in teacher education, Teach. Teach. Educ., 4(2), 99–110.
- Tschannen-Moran M., Woolfolk Hoy A. and Hoy W., (1998), Teacher efficacy: Its meaning and measure, Rev. Educ. Res., 68, 202–248.
- Tyson L., Treagust D. F. and Bucat R. B., (1999), The complexity of teaching and learning chemical equilibrium, J. Chem. Educ., 76(4), 554–558.
- Van Driel J., Verloop N. and De Vos W., (1998), Developing science teachers’ pedagogical content knowledge, J. Res. Sci. Teach., 35, 673–695.
- Van Driel J., De Jong O. and Verloop N., (2002), The development of preservice chemistry teachers’ pedagogical content knowledge, Sci. Educ., 86, 572–590.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems, J. Res. Sci. Teach., 37(2), 160–176.
- Woolfolk Hoy A. and Spero R. B., (2005), Changes in teacher efficacy during the early years of teaching: a comparison of four measures, Teach. Teach. Educ., 21, 343–356.
| This journal is © The Royal Society of Chemistry 2018 |