Diagnosing the quality of high school students’ and pre-service chemistry teachers’ cognitive structures in organic chemistry by using students’ generated systemic synthesis questions
Tamara
Hrin
 *,
Dušica
Milenković
*,
Dušica
Milenković
 and
Mirjana
Segedinac
and
Mirjana
Segedinac
Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovića 3, 21000 Novi Sad, Republic of Serbia. E-mail: tamara.hrin@dh.uns.ac.rs
First published on 15th November 2017
Abstract
The importance of well elaborated cognitive structures in a science knowledge domain has been noted in many studies. Therefore, the main aim of this particular study was to employ a new diagrammatic assessment approach, students’ generated systemic synthesis questions (SSynQs), to evaluate and compare the quality of high school students’ and pre-service chemistry teachers’ cognitive structures in the organic chemistry domain. We used a mixed research sample (N = 83), and SSynQs were constructed following the high school chemical curriculum in the Republic of Serbia. Besides the overall quality of the cognitive structures, the size (extent) and strength (complexity) of the conceptual structures, as external representations of cognitive structures, were also analysed. It was found that both high school students and pre-service chemistry teachers had a substantial size of the conceptual structures, showing relatively good knowledge about IUPAC naming and structures of organic compounds, except for ethers. However, the strength of the conceptual structures, or inter-correlations between organic chemistry concepts, was evaluated as weak within high school students, and medium within pre-service chemistry teachers. This resulted in the identification of three main learning difficulties (LDs), accompanied by a lack of understanding (LU) about the chemical properties and relations of organic compounds. It was surprising to find that all identified LDs and LUs within high school students also appeared within pre-service chemistry teachers. What is more, the most desired and expected cognitive structures (distinguished multidimensional cognitive structures) without LDs and LUs appeared within high school students.
Introduction
Students’ construction and possession of a body of organized knowledge is an important aim of science education (Heyworth, 1999; Lee et al., 2001; Wu and Tsai, 2005), including chemistry (Nash et al., 2000; Taagepera and Noori, 2000; Zhou et al., 2015). Therefore, the significance of cognitive structures or knowledge structures (Taagepera and Noori, 2000; Nakiboglu, 2008) was emphasized as an organization of interrelated concepts in immense long-term memory (Shavelson and Stanton, 1975).Namely, cognitive structures exist in the central, dominant part of human cognition – long-term memory, in the form of cognitive schemas. Cognitive schemas represent “big ideas” that students should construct for themselves in order to reach science understanding (Derry, 1996). Cognitive schemas possess a specific function (Paas and Sweller, 2012) and a degree of complexity (Paas et al., 2004; Van Merriënboer and Sweller, 2005), incorporating multiple concepts into a single element. For example, in organic chemistry one cognitive schema could contain multiple concepts about the preparation of alcohols (e.g. hydration of alkenes, hydrolysis of alkyl-halides, reduction of aldehydes and ketones, and the reaction of aldehydes and ketones with Grignard reagent). Or, another cognitive schema could reflect IUPAC naming for hydrocarbons, including the suffixes of the names for saturated and unsaturated hydrocarbons, the names of the straight chain saturated and unsaturated hydrocarbons (homologous series), the names of the alkyl groups, and the rules for naming branched chain hydrocarbons. However, in order to construct even more complex schema (higher-level schema), a previously learned schema should be activated in working memory, providing context for new, incoming information (Derry, 1996). Hence, incoming information (e.g. opening epoxides for the first example, or identifying and naming the substituents for the second example) could be integrated into previously learned lower-level schema. As a result of this, certain changes occur in long-term memory (Paas et al., 2004). According to Kirschner et al. (2006), “if nothing has changed in long-term memory nothing has been learned” (p. 77).
Such changes in existing cognitive structures could be done by rote and/or in a meaningful way. The new information could be learnt as an isolated element, or a concept with no substantial, or clear connection to existing knowledge. The availability and usage of such rotely acquired concepts is limited (Taber, 2013), as they are subjected to gradual reduction and/or forgetting (Ausubel, 2000). In contrast, meaningfully acquired concepts can be successfully integrated into an existing cognitive structure (Novak, 2002), with minimal modifications (Taber, 2013) and efficient retention (Ausubel, 2000).
The importance of “identifying” students’ cognitive structures has been discussed through several points. Certainly, it can help educators and teachers to better organize instructional materials (Ifenthaler et al., 2011), and enhance students’ learning (Tsai and Huang, 2002), after they find knowledge gaps. Such knowledge gaps could be observed through the students’ learning difficulties, lack of understanding, and alternative conceptions or misconceptions. However, traditional paper-and-pencil tests might not provide proper information about the links between the concepts in a student's mind (Zhou et al., 2015), and hence science educators and researchers have tried to use various methods of assessment.
Researchers of several studies noted the need to observe students’ construction of knowledge and integration of new, incoming concepts into existing cognitive structures in organic chemistry. Taagepera and Noori (2000) applied a knowledge space theory (KST) model on university biology majors. They found that students possessed some misconceptions about the physical and chemical properties of organic compounds, not distinguishing boiling from burning, and not recognizing reaction types (e.g. nucleophilic addition to the carbonyl compounds). Additionally, Rushton et al. (2008) used semi-structured interview with a think aloud protocol and presented fourth year chemistry students’ misunderstandings about reaction mechanisms (e.g. nucleophilic substitution types SN1 and SN2), and breaking bonds and reaction energy (e.g. “molecules with lowest energy are less stable”). Furthermore, McClary and Bretz (2012) developed a multiple-tier multiple-choice inventory to identify misconceptions of acid strength. These authors noted that many chemistry students believed that the functional group (as an isolated entity) determined acid strength (e.g. “acetic acid is more acidic that phenol and 2,4-pentadione because it is a carboxylic acid”). Recently, a three-tiered (answer tier, reason tier, confidence tier) test was administrated on pharmacy students in Serb Republic, revealing several misconceptions about carbohydrates (Milenković et al., 2016a).
Additionally, Zhou et al. (2015) recognized organic chemistry as a significant part of the high school chemical curriculum, and used the flow map method to explore high school students’ cognitive structures. The flow map is a graphical or diagrammatic approach for cognitive structure evaluation in which the sequences of statements are linked by linear and/or recurrent arrows. Ifenthaler et al. (2011) have differed diagrammatic assessment approaches from natural language approaches, to which the mentioned thinking aloud protocol belongs. The analysis of flow maps in the paper by Zhou et al. (2015) indicated that some high school students in China believed that ethanoic acid is called glacial acetic acid at low temperature; that carboxyl groups contain hydroxyl and hydrogen ions; or that in esterification reactions the hydroxyl group gets rid of the alcohol, and the hydrogen gets rid of the carboxylic acid. Additionally, the same authors were able to perceive some variables along with misconceptions: extent, richness, integration, and flexibility of students’ cognitive structures. For example, they found that students with a higher academic achievement had richer and more flexible cognitive structures than those with a lower academic achievement. Similarly, Thompson and Mintzes (2002) found that complexity and scientific validity of constructed concept maps (another diagrammatic approach of evaluation) increased with the age of the students. University students developed well-organized cognitive structures thanks to a greater expertise in the biology domain, than younger students. Namely, novice students’ cognitive structures could often be diffuse, crude, and without integration (Tsai and Huang, 2002; Stains and Talanquer, 2007), often due to a lack of sufficient, prerequisite knowledge. For example, in the Republic of Serbia there is a big leap from primary to secondary chemical education, and high school students are provided with a lot of new chemical concepts. As a result of this, they might have difficulties with selecting the appropriate ones to which they pay attention. Or, with actively processing such concepts in order to generate the new meaning, by linking them with those previously learned (Nakhleh, 1992). This is, according to Ring and Novak (1971), directly related to students’ success in future learning. On the other hand, experts could organize and store the set of concepts in a much more effective way (Niemi, 1997; Wolf, 2001; Tsai and Huang, 2002), as they possess a large number of schemas specific to the domain (Cook, 2006). Hence, their well-elaborated (Tsai and Huang, 2002) and tightly integrated cognitive structures (Eseryel et al., 2014) should display greater complexity and interrelations (Eseryel et al., 2014; Lopez et al., 2014). As a result of this, experts usually perceive more relations among the concepts, recall relevant concepts more quickly, and spend more time in analyzing non routine problems, or tasks (cited in Niemi, 1997).
Therefore, the main aim of this study was to employ the new diagrammatic assessment approach, students’ generated systemic synthesis questions (SSynQs), to evaluate and compare the quality of cognitive structures of participants with different levels of expertise in the organic chemistry domain: high school students and pre-service chemistry teachers.
Relying on Ausubel constructivist theory of learning and current views of brain function (Lagowski, 2009), Fahmy and Lagowski introduced several types of systemic assessment questions (SAQs) (see Fahmy and Lagowski, 2012, 2014), including SSynQs. Vachliotis et al. (2011) defined SAQs as “two-dimensional spatial arrangement and representation of concepts under study, and their interrelations” (p. 338). Hence, observing their design, it can be said that SAQs have two main structure elements: “nodes” which represent proper concepts, and arrows which show how the node pairs (concepts) are related (Hrin et al., 2016a, 2016b, 2017). As all concepts are directly or indirectly related, SAQs were presented as a modified, cyclic concept mapping technique (Vachliotis et al., 2014). Namely, the crucial difference between SAQs and concept maps is in the arrangement of concepts: concept maps are hierarchical networks (Novak, 2002), in which more specific concepts are set under more general ones, while SAQs are closed, interacting conceptual systems – concept clusters (Fahmy and Lagowski, 2003). This constellation of concepts follows triangular, quadrilateral, pentagonal, or hexagonal geometric shapes, depending of the number of concepts included in the SAQs.
In order to solve SAQs students should be able to: (1) organize concepts, (2) define or perceive relations between concepts, (3) synthesize these components into subsystems and further into coherent systems, and (4) analyse such systems to the fundamental components (Vachliotis et al., 2014). In line with this, SAQs and their more specific type (SSynQs) were used to enhance, or assess students’ meaningful learning (Vachliotis et al., 2011; Hrin et al., 2016a, 2016b), as well as higher order thinking skills in organic chemistry, such as systems thinking (Vachliotis et al., 2014; Hrin et al., 2016c, 2017).
It should be mentioned that SSynQs used in our previous studies had a more constrained format: a fill-in-the-blank format. Namely, a diagrammatic form of the task was provided to the students and they should have recognized defined relations, or some initial concepts in order to identify elements that were missing (i.e. filling empty fields in the provided diagrammatic form) (see Hrin et al., 2016a, 2016b, 2016c, 2017). However, when considering a diagrammatic task format in general, three types should be distinguished (cited in Stoddart et al., 2000; Vachliotis et al., 2011):
(1) previously mentioned constrained tasks;
(2) intermediate tasks that specify a list of concepts and/or linking words to be used, but do not give any other restrictions on how the diagram should be drawn;
(3) open-ended tasks that only provide prompt concepts that should be used in combination with some others (unprovided), in order to draw the diagram.
According to Vachliotis et al. (2011) student-generated SSynQs (2nd and/or 3rd type) are worth investigating, and our intention in this study was to provide a real insight into students’ thoughts and beliefs about organic chemistry compounds by using these tools. Concrete examples of applied SSynQs with an intermediate format are provided in the “Methodology” and “Results and discussion” sections.
Methodology
Aim of the study
Our evaluation with student generated SSynQs has a quantitative and qualitative aspect. Hence, for the further purpose of this study, it is important to differ, and also to link the terms “cognitive structure” and “conceptual structure”. According to Shavelson and Stanton (1975), the “structure” can be objective (real) and/or internal (subjective). Taber (2008) used the internal/subjective dimension in order to describe the cognitive structures as neural circuits in the brain, which cannot be measured directly (Ifenthaler et al., 2011). Therefore, the conceptual structure was described as external representation of the individual's cognitive structure (Hoz et al., 1992). According to this, we believe that it is appropriate to analyse and/or measure the individual's conceptual structure (quantitative aspect of the evaluation), in order to provide a real quality of the individual's cognitive structure (qualitative aspect of the evaluation).Therefore, in the present study unexamined student generated SSynQs have been used to accomplish the following research tasks:
(1) Analyse and compare the size (extent) and the strength (complexity) of high school students’ and pre-service chemistry teachers’ conceptual structures in organic chemistry.
(2) Examine the possible learning difficulties (LDs) and the lack of understanding (LU) in high school students’ and pre-service chemistry teachers’ written responses to SSynQs.
(3) Give insights into the overall quality of high school students’ and pre-service chemistry teachers’ cognitive structures in organic chemistry.
Namely, we believed that answers to the presented research tasks might provide some information about changes in participants’ cognitive structures with the development of expertise in the organic chemistry domain.
Context and participants
This study was carried out at two places. One was at a public general type grammar school in Novi Sad, and the other was at the Faculty of Sciences, the University of Novi Sad, Republic of Serbia.N = 71 high school students (48% males and 52% females) were selected to participate in our study. All participants were taught by the same licensed chemistry teacher who majored in chemistry and holds a Master's degree. She has been teaching high school students for more than ten years. According to her evaluation, at the end of the first semester, 37% of students had excellent achievement in organic chemistry, 28% had very good achievement, 22% had good achievement, and 13% had satisfactory achievement.
To be eligible to participate in our study, pre-service chemistry teachers were required to have representative background knowledge in organic chemistry. Therefore, among the pre-service chemistry teachers, 100% passed the course Organic chemistry I (average grade 8.50 from 10.00), 92% passed the course Organic chemistry II (average grade 8.73), and again 100% passed the course Organic chemistry III (average grade 7.08). All pre-service chemistry teachers agreed to voluntarily participate in our study.
Procedure and instrument
Before the testing was conducted, all the participants were informed about the nature and methods of the study. The researcher informed the high school students that the aim was to examine what they had learned about the nomenclature, structures and chemical reactions of different organic compounds, placing the alcohols and ethers in the limelight. On the other hand, pre-service chemistry teachers were informed that the contents were selected in accordance with the high school organic chemistry curriculum. After participants were informed about the aim, nature and methods of the study, they were asked whether they want to participate in the research.In addition, the researcher instructed both groups of participants in solving SSynQs, by drawing one common example on the blackboard. Namely, they were instructed to visually represent the closed framework of provided concepts (organic compounds), making proper relations between them using labelled lines with reagents and/or conditions (light, catalyst, temperature). The test was administrated after the students’ satisfactory degree of familiarity with SSynQs had been observed.
First of all, it should be highlighted that the test was carried out in Serbian, which is the language used in both high school and university. A paper-and-pencil test was conducted a week after the instruction on ethers in April 2016, presented as a systemic synthesis questions test with four SSynQs arranged by growing complexity. Namely, SSynQ1 contained 3 concepts and 3 relations, SSynQ2 had 4 concepts and 4 relations, SSynQ3 contained 5 concepts and 5 relations, while the most complex SSynQ4 possessed 6 concepts and 5 relations. Compounds were considered as key ideas, or the focal concepts pertaining to the selected organic chemistry domain, and/or organic chemistry reactions sub-domain (according to Vachliotis et al., 2011). The provided groups of concepts (organic compounds) could be interconnected in chemical transformations (reactions) forming closed systems of concepts. Therefore, the total number of focal concepts (organic compounds) was 18, even though some concepts were repeated in two questions (e.g. ethanol), while the total number of relations was 17. The requirements of each SSynQ will be presented subsequently.
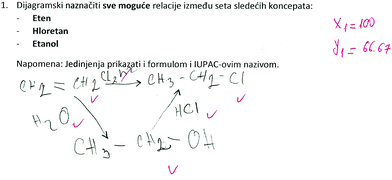
SSynQ1: “Construct the closed diagram by highlighting all possible relations between the following set of concepts: ethene, chloroethane, ethanol. Note: all concepts should be presented in the diagram by writing the appropriate structural formulas.”
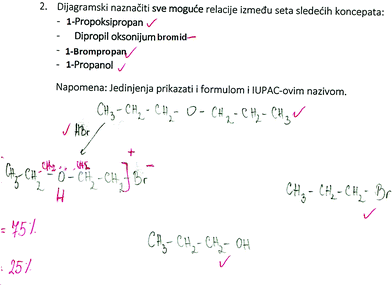
SSynQ2: “Construct the closed diagram by highlighting all possible relations between the following set of concepts: 1-propoxypropane, dipropyl oxonium bromide, bromopropane, propanol. Note: all concepts should be presented in the diagram by writing the appropriate structural formulas.”
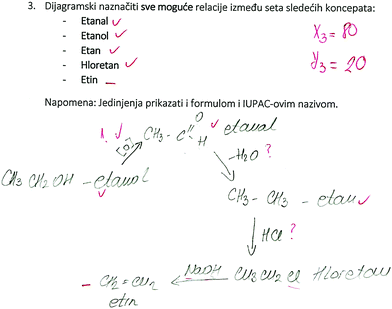
SSynQ3: “Construct the closed diagram by highlighting all possible relations between the following set of concepts: ethanal, ethanol, ethane, chloroethane, ethyne. Note: all concepts should be presented in the diagram by writing appropriate structural formulas.”
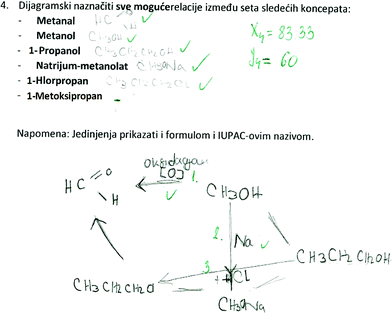
SSynQ4: “Construct the closed diagram by highlighting all possible relations between the following set of concepts: methanal, methanol, 1-propanol, sodium-methanolate, 1-chloropropane, 1-metoxypropane. Note: all concepts should be presented in the diagram by writing the appropriate structural formulas.”
The time for solving the SSynQs was limited to 45 minutes (one school class) for both groups of participants.
Scoring rubric
The responses for each SSynQ were evaluated using a scoring rubric developed by Lomask et al. (1993). By analysing concept maps in biology, these authors distinguished two main variables of interest: (x) size (extent) of the conceptual structure and (y) strength (complexity) of the conceptual structure. The variable x was defined as a ratio of the number of concepts determined by a student and the total number of concepts (highlighted in the task), multiplied by 100%. In addition, the variable y was specified as a ratio of the number of valid relations made by the student and the total number of relations, multiplied by 100%. The strength of the conceptual structure is conceptually connected to the size of the structure, because the size could determine the number of expected relations (Lomask et al., 1993). For example, if the student includes three concepts (e.g. methanal, methanol, and sodium-methanolate) for a SSynQ that contains six concepts, a maximum of two relations might be expected (i.e. relations methanal – methanol and methanol – sodium-methanolate). According to variable x (Table 1), the conceptual structure could be characterized as complete, substantial, partial, small, and insignificant. Additionally, observing variable y, the strength of the conceptual structure could be strong, medium, or weak.| Size/strength | Strong (66% < y ≤ 100%) | Medium (33% < y ≤ 66%) | Weak (0% < y ≤ 33%) |
|---|---|---|---|
| Complete (80% < x ≤ 100%) | 5 | 4 | 3 |
| Substantial (60% < x ≤ 80%) | 4 | 3 | 2 |
| Partial (40% < x ≤ 60%) | 3 | 2 | 1 |
| Small (20% < x ≤ 40%) | 2 | 1 | 1 |
| Insignificant (0% < x ≤ 20%) | 1 | 1 | 1 |
Following the combinations of variable x and variable y presented in Table 1, the cognitive structure could be determined as nominal (score 1), when the student possesses an insignificant size of the conceptual structure with a weak, medium, or even strong strength (complexity) of the relations between the recognized concepts. Additionally, the same level is attributed to the student who possesses a small conceptual structure with a medium or weak strength of the relations between the set of concepts. Even those students with a partial size of conceptual structure and a weak strength of the conceptual structure might have a nominal cognitive structure.
Furthermore, students who have a small size of conceptual structures, but strong strength of the relations between determined concepts (score 2) are still in the process of learning. The same might be said for those with a partial size of the conceptual structure with a medium strength, or a substantial structure and a weak strength. This group of participants is marked with a functional cognitive structure.
Students with a structural cognitive structure are scored with 3 (Table 1), and could have a partial conceptual structure with a strong strength, or a substantial conceptual structure with a medium strength, or a complete size of the conceptual structure with a weak strength of the conceptual structure.
At the end, the group of students with a substantial conceptual structure and a strong strength, and/or with a complete conceptual structure with a medium strength of relations possess a multidimensional cognitive structure (score 4). Distinguished multidimensional cognitive structure (score 5) characterizes only those with a complete conceptual structure and strong strength (complexity) of relations between the concepts.
332 SSynQs collected from 71 high school students and 12 pre-service chemistry teachers were evaluated and scored by three raters, who were subject-matter experts: a full professor, an assistant professor, and a teaching assistant in the field of Chemistry teaching. The experts worked together and each disagreement was solved through discussion. At the end, the reliability of the applied scoring method was calculated, separately for high school students and pre-service chemistry teachers, and separately for two scales: (i) the size of the conceptual structure and (ii) the strength of the conceptual structure. The reliability of internal consistency was calculated for four items per scale (scale 1 with items SSynQ1(x), SSynQ2(x), SSynQ3(x), SSynQ4(x); scale 2 with items SSynQ1(y), SSynQ2(y), SSynQ3(y), SSynQ4(y)), using Cronbach's alpha coefficient. Additionally, the split-half method considered several different coefficients: Cronbach's alpha for part 1, Cronbach's alpha for part 2, correlation between parts, Spearman–Brown coefficient for equal parts, Spearman–Brown for unequal parts, and Guttman split-half coefficient. The obtained coefficients showed good, or even excellent reliability of the applied instrument and scoring method in most of the cases (Table 2). However, several values were below the typically acceptable value of 0.70, and as noted Wren and Barbera (2014), instruments that measure aspects of students’ performances that are typically not coherent (e.g. students’ conceptions, or misunderstandings) might have lower reliability coefficient values.
| Reliability coefficients | Group of participants | |||
|---|---|---|---|---|
| High school students | Pre-service chemistry teachers | |||
| Scale 1 | Scale 2 | Scale 1 | Scale 2 | |
| Cronbach's alpha | 0.90 | 0.79 | 0.60 | 0.65 |
| Cronbach's alpha for part 1 | 0.71 | 0.54 | 0.12 | 0.19 |
| Cronbach's alpha for part 2 | 0.90 | 0.81 | 0.08 | 0.55 |
| Correlation between parts | 0.84 | 0.68 | 0.72 | 0.62 |
| Spearman–Brown coefficient for equal parts | 0.91 | 0.81 | 0.84 | 0.77 |
| Spearman–Brown for unequal parts | 0.91 | 0.81 | 0.84 | 0.77 |
| Guttman split-half coefficient | 0.89 | 0.80 | 0.84 | 0.77 |
Data analysis
This study is based on a mixed method research design, using quantitative and qualitative procedures for data analysis, finding integration, and drawing inferences (according to Teddlie and Tashakkori, 2009). After collection, the quantitative data were analysed using software packages IBM SPSS Statistics 19 and Microsoft Office Excel. The results are presented and discussed below, following the sequence of the defined research tasks.Results and discussion
To determine whether there was a difference between independent groups (high school students and pre-service chemistry teachers), observing two dependent variables: the size and strength of the conceptual structure, a Welch's t-test was used. We decided to use the Welch's t-test because of the unequal sample size in the two groups and the small sample size in the group of pre-service chemistry teachers (according to De Winter, 2013). For the normality assumption, skewness and kurtosis values were obtained (Table 3), which fall within the acceptable range of ±2. Moreover, the Shapiro–Wilk test of normality confirmed these results, according to which we were able to conduct the Welch's t-test.| Groups | Variables | M (%) | SD | Min. (%) | Max. (%) | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|
| High school students | x | 62.00 | 27.04 | 0.00 | 83.33 | −1.32 | 0.52 |
| y | 19.54 | 20.47 | 0.00 | 52.50 | 0.82 | −0.24 | |
| SSynQ1x | 86.74 | 30.69 | 0.00 | 100.00 | −2.29 | 3.88 | |
| SSynQ1y | 42.25 | 38.20 | 0.00 | 100.00 | 0.15 | −1.47 | |
| SSynQ2x | 37.32 | 23.48 | 0.00 | 100.00 | −0.51 | −0.38 | |
| SSynQ2y | 8.80 | 16.95 | 0.00 | 100.00 | 2.82 | 11.26 | |
| SSynQ3x | 71.36 | 37.43 | 0.00 | 100.00 | −1.09 | −0.36 | |
| SSynQ3y | 16.90 | 25.67 | 0.00 | 100.00 | 1.59 | 2.04 | |
| SSynQ4x | 52.56 | 29.94 | 0.00 | 100.00 | −0.65 | −0.78 | |
| SSynQ4y | 10.19 | 17.72 | 0.00 | 83.30 | 2.13 | 4.78 | |
| Pre-service chemistry teachers | x | 68.16 | 18.24 | 41.67 | 95.00 | −0.03 | −1.38 |
| y | 44.69 | 20.16 | 23.33 | 83.75 | 1.19 | 0.15 | |
| SSynQ1x | 97.22 | 9.62 | 66.67 | 100.00 | −3.46 | 12.00 | |
| SSynQ1y | 91.67 | 20.72 | 33.33 | 100.00 | −2.55 | 6.24 | |
| SSynQ2x | 41.25 | 37.67 | 0.00 | 100.00 | 0.13 | −1.77 | |
| SSynQ2y | 27.08 | 37.63 | 0.00 | 100.00 | 0.98 | −0.66 | |
| SSynQ3x | 84.17 | 14.43 | 50.00 | 100.00 | −0.89 | 1.83 | |
| SSynQ3y | 33.33 | 21.46 | 0.00 | 80.00 | 0.80 | 0.90 | |
| SSynQ4x | 50.00 | 34.81 | 0.00 | 100.00 | 0.00 | −1.05 | |
| SSynQ4y | 26.67 | 27.41 | 0.00 | 80.00 | 0.80 | −0.51 | |
Analysis of high school students’ and pre-service chemistry teachers’ size of the conceptual structures
In order to evaluate our participants’ size of the conceptual structures in organic chemistry, we observed their knowledge of structural formulas. The results of the Welch's t-test showed that there was no significant difference between high school students’ and pre-service chemistry teachers’ achievement observing variable x (t = −1.000, p = 0.329). Both groups of participants managed to recognize the provided IUPAC-names of organic compounds and correctly presented them by drawing structural formulas in SSynQs (M(high school students) = 62.00%; M(pre-service chemistry teachers) = 68.16%) to be characterized with substantial conceptual structures (Tables 1 and 3). This could be explained by the fact that in order to master IUPAC-names and/or symbolic two-dimensional representation of organic compounds with oxygen, students are usually instructed to bring the IUPAC-names and/or formulas of hydrocarbons from long-term memory into working memory, in order to be processed and linked. For example, while naming and constructing the structural formula of ethanol, students rely on the IUPAC name and the structural formula of ethane. Therefore, the procedure of IUPAC naming and drawing formulas of organic compounds might be called meaningful per se.After considering participants’ average achievement on variable x, we were interested in their achievement on each particular concept included in SSynQs. By that, more detailed analysis and closer relation between participants’ achievement on variables x (size of the conceptual structure) and y (strength of the conceptual structure) might be provided. Looking at Fig. 1 it could be said that participants had a good achievement in drawing structures of hydrocarbons and their halogen derivatives, alcohols and aldehydes, with a relatively small difference between high school students and pre-service chemistry teachers, except for aldehydes and alkyl halides. Pre-service chemistry teachers’ higher scores on aldehydes could be considered reasonable, as according to curriculum regulations of the Republic of Serbia, high school students had not been introduced to this class of organic compounds in primary school. They were introduced to aldehydes in secondary school for the first time. On the other hand, high-school students had higher achievement than pre-service chemistry teachers on alkyl halides. Namely, it was noted that some pre-service chemistry teachers left out drawing structures if they have difficulties with providing relations. This was observed in the most complex SSynQ4, in which the structure of 1-chloropropane should have been provided, and in SSynQ2 in which the structure of 1-bromopropane should have been provided.
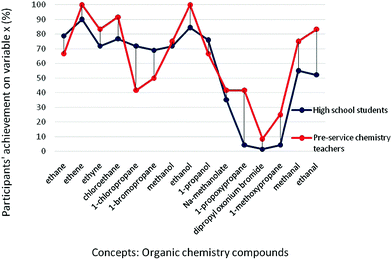 | ||
| Fig. 1 High school students’ and pre-service chemistry teachers’ achievement on variable x for each concept. | ||
Observing participants’ achievements on variable x, the lowest values were observed in SSynQ2 (high school students: M = 37.32%; pre-service chemistry teachers: M = 41.25%) and SSynQ4 (high school students: M = 52.56%; pre-service chemistry teachers: M = 50.00%) (Table 3). As both questions include ethers, participants’ poor knowledge about this class of organic compounds should not be neglected (Fig. 1). This was especially noticeable for high school students, who had low achievement on all three concepts from this class: 1-propoxypropane (4.22%), dipropyl oxonium bromide (1.41%), and 1-methoxypropane (4.22%). The complexity of the mentioned concepts might be highlighted, as they have two alkyl or aryl substituents (groups) bonded to an oxygen atom (Moreira, 2013). Additionally, as more than one IUPAC name can be provided for the compound (e.g. 1-propoxypropane and dipropyl ether), we might assume that high school students were less familiar with the listed names. In line with this assumption is the written response of one female student who firstly translated the IUPAC name of 1-methoxypropane into methyl propyl ether, in order to draw the formula of this compound.
On the other hand, pre-service chemistry teachers’ difficulties with this group of organic compound were mostly noted among mixed (unsymmetrical) ethers (1-methoxypropane), as only 25% of them were able to correctly present this concept. In addition, considerable difficulties were found in drawing the charged intermediate product for acidic ether cleavage, which will be discussed in more detail later in this paper.
Analysis of high school students’ and pre-service chemistry teachers’ strength of the conceptual structures
Furthermore, we were interested in high school students’ and pre-service chemistry teachers’ skills to correlate concepts – organic chemistry compounds. Namely, one aspect of participants’ conceptual understanding was observed: seeking connections among diverse pieces of provided information (cited in Nieswandt, 2007).Firstly, it should be highlighted that high school students’ and pre-service chemistry teachers’ achievements on variable y were significantly lower than on variable x, especially for high school students, who managed to correlate approximately 20% of the presented concepts. Such strength of relations in conceptual structure was evaluated as weak (Tables 1 and 3). On the other hand, the pre-service chemistry teachers were statistically better than high school students observing variable y (Welch's t-test: t = −3.989, p < 0.05), revealing around 45% of required relations (medium strength of relations). However, it should be highlighted that our SSynQs were constructed according to the Curriculum regulations of the secondary school level, and we expected to find better strength of relations within pre-service chemistry teachers’ conceptual structures.
Finding the low (high school students) and medium (pre-service chemistry teachers) strength of relations in the conceptual structures, in further analysis of our participants’ written responses on SSynQs, we expected to see learning difficulties (LDs), but also to identify some lack of understanding (LU) (Table 4). We observed LDs as more general, or basic problems in acquiring knowledge, while LU as a more specific result of the identified LDs. However, 19 high school students were separated from their group, as they did not provide any relation in SSynQs (they only draw the structures), and it was not possible to talk about their LDs and/or LU. Henceforth, the number of analysed responses was 52 for high school students and 12 for pre-service chemistry teachers.
| Codes | Description | Frequency | |
|---|---|---|---|
| High school students (N = 52) | Pre-service chemistry teachers (N = 12) | ||
| LU1 | Chlorination of alkenes should be initiated with light | 6 | 1 |
| LU2 | Alkanes can be reduced to an alkene, and/or alkyne | 2 | 2 |
| LU3 | Alkanes can occur in the reaction with hydrogen chloride to produce alkyl halide | 8 | 4 |
| LU4 | Ethers undergo cleavage using water | 4 | 1 |
| LU5 | Alcohols can be directly converted into alkanes | 14 | 3 |
| LU6 | Alcohols can be produced by hydration of an alkyne | 2 | 2 |
| LU7 | Alcohols can undergo a neutralization reaction with NaOH to obtain an alcoholate and water | 7 | 2 |
At the beginning, we identified three main LDs for both analysed groups of participants:
(1) Providing equal atom numbers in the chemical equations (LD1).
(2) Bond breaking and formation; reaction mechanisms (LD2).
(3) Extending the carbon chain in the chemical reactions (LD3).
Firstly, it was surprising to find that 20 high school students and 4 pre-service chemistry teachers had LD1. For example, one high school student believed that a molecule of monocholoroethane is produced when a molecule of ethene reacts with a molecule of chlorine (Fig. 2). This particular response revealed the student's inability to provide equal numbers of chlorine and hydrogen atoms in the chemical equation (LD1). It was noted in the literature that writing chemical equations and providing equal numbers of all atoms on both sides of the equation is much less meaningful to novices than for more experienced students, or experts (Hesse and Anderson, 1992). Beginner students have not mastered the required chemical concepts completely: facts and theories associated with the described change, and/or conservation of matter (Hesse and Anderson, 1992). However, some pre-service chemistry teachers in our study also had serious difficulties with chemical changes and providing equal atom numbers in chemical equations included in SSynQs.
It was interesting to note that the mentioned high school student (Fig. 2) was successful in providing two other relations included in SSynQ1. She managed to link alcohols with alkenes or even alkyl halides, however, the difficulties occurred when alkenes should have been linked with alkyl halides in order to close the structure (diagram). Apparently, she memorized this material when they were needed for the exam (first semester of the school year), and forget it soon after the exam, and/or made some serious misunderstandings. She believed that chlorination of ethene should be initiated with light (LU1), perhaps observing alkenes as a quite inert class of organic compounds. Previously, Zoller (1990) stated that students might have misconceptions about relative chemical reactivity and/or stability of single, double, and triple bonds. Students often believe that alkenes are less reactive than alkanes, as a double bond is stronger than a single bond. However, they should know that the reactivity of alkenes is associated with the cleavage of the π bond only. In addition to the mentioned high school student, five other high school students and one pre-service chemistry teacher showed the same LU1 (Table 4).
Apart from LD1 with chemical equations, 2 high school students and 2 pre-service chemistry teachers possessed misunderstandings about oxidation and reduction reactions in organic chemistry. Namely, they believed that alkanes (e.g. ethane) are more oxidized than unsaturated hydrocarbons, and can be reduced with reductants such as H2, Pd/C, or Zn, into alkenes (e.g. ethene) and alkynes (e.g. ethyne) (LU2). In future research, it would be important to include high school students’ and pre-service chemistry teachers’ calculation of the oxidation state of each carbon atom involved in the reaction. By that we might identify high school students’ and/or pre-service chemistry teachers’ inability to provide proper oxidation states, which was previously highlighted in the literature (e.g.Garnett and Treagust, 1992).
A lot of LU revealed among participants was the result of their bad knowledge of bond breaking and formation, and fundamental reaction mechanisms (LD2). For example, a molecule of ethane reacted with a molecule of hydrogen chloride to obtain monochloroethane (Fig. 3). In that case, 4 pre-service chemistry teachers and 8 high school students had LU3, believing that the initiation step of the mechanism of chlorination of an alkane involves heterolytic cleavage of a polar covalent H–Cl bond instead of homolytic cleavage of a nonpolar Cl–Cl bond. These participants were not able to see that a reaction mechanism requires free radicals that could not be obtained by heterolytic cleavage of the polar covalent H–Cl bond. In the previous studies, it has been noted that reaction mechanisms, or more precisely “curved arrows” are often meaningless even to graduate students (Bhattachatyya and Bodner, 2005). According to this, Rushton et al. (2008) found misconceptions while students were evaluating the stability of a final product instead of the feasibility of the reaction mechanism required to obtain the product.
Participants’ difficulties with the reaction mechanism (LD2) were especially noted in SSynQ2 (Fig. 4), as only one high school student and one pre-service chemistry teacher could correctly draw the structure of a charged intermediate product for acidic ether cleavage, and provide proper relations between the ether, the intermediate product (oxonium salt) and the main products. On the other hand, 3 pre-service chemistry teachers correctly noted that cleavage of ethers could be done with a strong acid in the presence of a nucleophile, however, they made mistakes while drawing the structure of the intermediate (Fig. 4). In addition, one pre-service chemistry teacher and 4 high school students noted that ethers undergo cleavage with water, believing that the hydroxyl group is a good enough nucleophile for this reaction (LU4).
In addition, LU5 and LU6 might also be associated with LD2. Namely, 14 high school students and 3 pre-service chemistry teachers possessed a misunderstanding regarding the direct conversion of an alcohol into an alkane (LU5, Table 4). Having bad knowledge about reaction mechanisms and intermediate products, they might not know that –OH is a bad leaving group, but it can be easily converted into another superior leaving group –OH2+, that allows the reaction to proceed by forming a carbocation intermediate prepared for a nucleophile attack. These results are in line with what Zoller (1990) found about students’ misunderstandings of nucleophilic capability and good leaving groups.
Furthermore, 2 high school students and 2 pre-service chemistry teachers noted that alcohols could be produced by hydration of an alkyne (LU6), confusing it with hydration of an alkene which also require a strong acid (usually sulfuric acid).
Observing the most complex SSynQ4 (Fig. 5), it could be said that both high school students and pre-service chemistry teachers struggled to include all the concepts in the closed structure. A closer look at their written responses provided the information that 1-propanol was the most critical concept. Namely, they knew the relation between 1-propanol and 1-chloropropane, however, they were not sure how to link 1-propanol with some other concept (Fig. 5). For example, no-one from the high school students’ group could remember to add a Grignard reagent to methanal to produce alcohol (1-propanol), extending the carbon chain by two carbon atoms (LD3). Additionally, only 2 pre-service chemistry teachers were able to provide an equation for this reaction. This result was not in accordance with what Rushton et al. (2008) found about the Grignard reaction, as the overall performance of their fourth grade chemistry students was about 50%.
In the same task (SSynQ4) some participants (7 high school students and 2 pre-service chemistry teachers) revealed LU7. They believed that alcohol could react with sodium hydroxide to give alcoholate and water. They were not able to perceive that the acidity of an alcohol is about the same as that of water (pKa(H2O) = 15.7 and pKa(CH3OH) = 15.5), and alcohol cannot be converted completely into its alcoholate (conjugated base) in an aqueous sodium hydroxide solution. Even though Zhou et al. (2015) noted that most of their students could state the acidity of ethanoic acid without misconceptions, they had difficulties in comparing the acidity between ethanoic acid and inorganic acids (sulfuric, hydrochloric, nitric, carbonic acid).
Looking at our participants’ size and strength of the conceptual structure, which revealed some LDs and LU, we were further interested in the overall quality of their cognitive structures.
High school students’ and pre-service chemistry teachers’ quality of cognitive structures in organic chemistry
Before presenting the distribution of high school students and pre-service chemistry teachers through the defined levels of cognitive structures, the correlation analysis using Pearson's correlation coefficient was conducted on the following variables (Table 5):| Variables | Groups | |
|---|---|---|
| High school students (r) | Pre-service chemistry teachers (r) | |
| 1 and 2 | 0.56 | 0.76 |
| 1 and 3 | 0.62 | 0.85 |
| 1 and 4 | 0.29 | 0.36 |
| 2 and 3 | 0.43 | 0.82 |
| 2 and 4 | 0.43 | 0.04 |
| 3 and 4 | 0.32 | 0.15 |
(1) size of the conceptual structure (variable x);
(2) strength of the conceptual structure (variable y);
(3a) high school students’ organic chemistry average grades achieved at the end of the first semester of the school year (M = 3.89/5.00, SD = 1.05);
(3b) pre-service chemistry teachers’ average grade achieved in organic chemistry courses (M = 8.15/10.00, SD = 0.88);
(4) number of identified LUs per participant (high school students: M = 0.61/7, SD = 0.93; pre-service chemistry teachers: M = 1.25/7, SD = 1.06).
Firstly, it should be highlighted that significant correlation was found between the size (variable x) and the strength of the conceptual structure (variable y), within both groups of participants (Table 5). According to Dunn (2001), correlation was evaluated as strong (r = 0.76) within pre-service chemistry teachers, and as moderate (r = 0.56) within high school students. The results also indicated that the participants’ grades are closely related to their achievement on variable x (size of the conceptual structure). The correlation was evaluated as strong (r = 0.62) within high school students, and as very strong within pre-service chemistry teachers (r = 0.85). Participants with higher organic chemistry grades not only tend to be higher achievers on variable x, but also on variable y (high school students: r = 0.43; pre-service chemistry teachers: r = 0.82). Additionally, the correlation between variable x and the number of identified LUs was evaluated as weak, for both high school students (r = 0.29) and pre-service chemistry teachers (r = 0.36). Finally, a correlation of moderate strength was found between variable y and the number of identified LUs within high school students (r = 0.43), while a correlation of statistical significance did not appear within the group of pre-service chemistry teachers (r = 0.04). The same might be said for the correlation between participants’ grades and the number of identified LUs. While significant correlation did not appear within pre-service chemistry teachers (r = 0.15), a weak correlation was found within high school students (r = 0.32). According to the positive values of the correlation coefficient, it could be concluded that participants (especially pre-service chemistry teachers) with quantitatively more extensive and complex conceptual structures and higher previous knowledge (i.e. higher organic chemistry grades) actually might have more LUs than those with quantitatively poorer conceptual structures. Due to this fact, in further analysis attention was paid to finding the sub-group in which LUs appeared the most.
At the beginning, the highest level of cognitive structures – distinguished multidimensional cognitive structures, was assigned to three high school students (5.77%) and three pre-service chemistry teachers (25%, Table 6). We were very pleased to find high school students with such a quality of cognitive structures, as they are beginner students in this field, and they might be expected to have some LDs and LUs. However, no LU has been identified within our high school students with distinguished multidimensional cognitive structures. Clearly, high school students benefited from prior knowledge (e.g. chemical equations, oxidation states, and types of organic reactions, etc.), constructing well organized cognitive structures with no LU. Although three pre-service chemistry teachers were as good as the mentioned three high school students observing the quality of cognitive structures, LUs appeared in their written responses. It should be highlighted that one third of all identified LUs within the pre-service chemistry teachers group belonged to those with the richest cognitive structures and the highest grades in organic chemistry courses. They showed LUs of fundamental reaction mechanisms (LU3, LU4, LU5, LU6), as well as the acidity of alcohols (LU7).
| Cognitive structure | Participants’ distribution | Identified LU | ||||
|---|---|---|---|---|---|---|
| High school students (N = 52) | Pre-service teachers (N = 12) | High school students | Pre-service teachers | |||
| Distinguished multidimensional (5) | 3 | 3 | — | LU3 | 1 | |
| LU4 | 1 | |||||
| LU5 | 1 | |||||
| LU6 | 1 | |||||
| LU7 | 1 | |||||
| Multidimensional (4) | 11 | 0 | LU2 | 1 | — | |
| LU3 | 2 | |||||
| LU4 | 2 | |||||
| LU5 | 4 | |||||
| LU7 | 3 | |||||
| Structural (3) | 17 | 3 | LU1 | 4 | LU1 | 1 |
| LU2 | 1 | |||||
| LU3 | 3 | LU3 | 2 | |||
| LU5 | 7 | |||||
| LU6 | 2 | LU5 | 1 | |||
| LU7 | 3 | |||||
| Functional (2) | 13 | 3 | LU1 | 2 | LU2 | 2 |
| LU3 | 3 | |||||
| LU4 | 1 | |||||
| LU5 | 3 | LU3 | 1 | |||
| LU6 | 1 | |||||
| LU7 | 1 | |||||
| Nominal (1) | 8 | 3 | LU4 | 1 | LU5 | 1 |
| LU6 | 1 | |||||
| LU7 | 1 | |||||
Additionally, no pre-service chemistry teachers with a multidimensional cognitive structure were found, while 11 high school students (21.15%, Table 6) possessed such a level of constructed cognitive structures. Within this sub-group different LUs appeared (five LUs with the frequency of 12), most of which were assigned to pre-service chemistry teachers with distinguished multidimensional cognitive structures. The most common was LU5 (with the frequency of 4) in the direct conversion of an alcohol to an alkane, and LU7 (with the frequency of 3) regarding the acidity of alcohols.
Furthermore, a lot of LU was revealed within the largest sub-group of participants with structural cognitive structures (17 high school students, 32.69% and 3 pre-service chemistry teachers, 25%). Namely, six LU issues with a frequency of 20 appeared within high school students, while three LUs with a frequency of 4 appeared within pre-service chemistry teachers. While LU1, LU3 and LU5 were common for both groups, LU2, LU6 and LU7 were only found among high school students. For example, LU in oxidation–reduction reactions in organic chemistry (LU2) was only evident among high school students, perhaps showing their lack of knowledge in general chemistry. However, LU6 in the hydration of alkyne and keto–enol tautomerization appeared for the first time amongst high school students who had structural cognitive structures. On the other hand, among the pre-service chemistry teachers, the same LU6 appeared amid those with the richest cognitive structures.
A very similar situation was found among participants with functional cognitive structures. Namely, 13 high school students (25%) showed six LU issues with a frequency of 18, and 3 pre-service chemistry teachers (25%) showed two LU issues with a frequency of 3. However, only LU3 was common for both groups. In contrast, LU2 appeared only among pre-service chemistry teachers with a functional cognitive structure. The same LU2 appeared earlier amongst high school students with multidimensional and structural cognitive structures.
It should be noted that most of the high school students who were separated from their group had a nominal cognitive structure (N = 12). As previously noted, many high school students with nominal cognitive structures were not able to provide relations in SSynQs and we were not even able to talk about their LU. Hence, 8 high school students (15.38%) and 3 pre-service chemistry teachers (25%) had nominal cognitive structures, and the least LU was found in this sub-group of participants. While only one LU appeared within high school students, LUs noted within pre-service chemistry teachers with nominal cognitive structures also appeared within those with structural, or even distinguished multidimensional cognitive structures.
Conclusions and implications
Science (chemical) educators encourage the application of different methods and tools in order to tap into students’ cognitive structures (Zhou et al., 2015). In this particular study, the usage of new students’ generated SSynQs provided a vast amount of valuable information in light of high school students’ and pre-service chemistry teachers’ cognitive structure outcomes. Observing the written responses of our participants, we were able to perceive that pre-service chemistry teachers and high school students had relatively good familiarity with IUPAC naming and chemical structures of a wide range of organic chemistry compounds (except for ethers). Accordingly, the substantial size (extent) of conceptual structures in organic chemistry was noted, within both groups of participants.Additionally, we were able to see that our participants, especially high school students, had difficulties with the interconnection of several different organic compounds, in order to close the system – the conceptual structure. Hence, students’ conceptual structure networks in organic chemistry often remain weak and non-interactive for future learning situations. In the Republic of Serbia, this might be especially true for high school students. Namely, they need to move from one specific view which Davidowitz and Rollnick (2011) called the “product oriented” view of organic reactions (primary school students’ view), to another which takes into account the mechanisms. Hence, it might be concluded that some changes occur in students’ cognitive structures in organic chemistry with the development of expertise (on the way from secondary school through to a bachelor diploma stage), which reflect on the students’ greater ability to correlate different concepts.
In accordance with the previous study about ethanoic acid (Zhou et al., 2015), the results of the correlation analysis in our study confirmed that poorer (smaller) and badly interconnected conceptual structures are mostly related to those students with lower academic grades. In contrast, participants with higher grades tended to have larger (richer) and more interconnected (complex) conceptual structures.
However, further and deeper insights into our participants’ conceptual structures, surprised us by revealing that even complex and rich enough conceptual structures might “hide” misunderstandings, mostly those emerging from organic reaction mechanisms. This was especially expressed among pre-service chemistry teachers, who were based on quantitatively rich and complex conceptual structures assigned with the most desired, distinguished multidimensional cognitive structures. This was different from what Zhou et al. (2015) found about students’ misconceptions of ethanoic acid. However, we agree with Nakhleh (1992, cited in Derman and Eilks, 2016), who noted that when students develop incorrect concepts and incorporate them into cognitive structures, they result in persistent misconceptions. Namely, students bring misunderstandings with them from the prior level of education into the university level, which are very stable over long periods despite learning, resistant to change, and readily evoked even years later (Taber and Tan, 2011). According to the results of our study, we might add that among pre-service chemistry teachers who have several years of learning organic chemistry, the quantum of knowledge in organic chemistry increased (e.g. knowledge about a larger number of representatives of classes of organic compounds, and/or reaction types), but LUs were retained.
On the other hand, we managed to find several high school students with the same complexity and richness of the conceptual structures, but without LUs. Certainly, a lot should be done in the training of pre-service chemistry teachers. Perhaps, organic chemistry lectures should be organized differently. Namely, the main ideas might be reaction types: i.e. addition reactions, substitution reactions, oxidation and reduction, etc., instead of classes of organic compounds. In some countries, organic chemistry lectures are already organized through big and well-articulated ideas (see Davidowitz and Rollnick, 2011), in order to effectively guide university students in their transition from novices to experts. Additionally, we agree with Taber and Tan (2011) and Vladušić et al. (2016), who noted that university teachers should be familiar with such students’ misunderstandings while preparing their own teaching. By that, the reinforcement of misunderstanding in specific subject domains could be prevented. Also, during instruction teachers might prepare conceptual change texts and ask conceptual problems (questions) and require university students’ explanations in order to “activate” their misunderstandings and facilitate conceptual changes (Azizoğlu et al., 2006; Taştan et al., 2010).
Certainly, one of the main directions for future research would be to apply students’ generated SSynQs to first year chemistry students, before they start their first organic chemistry course at university. After that, we might be able to track the construction and development of their cognitive structures over time (according to the study of Ifenthaler et al., 2011), as well as the persistence and/or changes of the identified misconceptions. In order to verify if some of the revealed LUs are indeed misconceptions (presented among a larger number of students), we are highly motivated to combine our tools, SSynQs, with other existing techniques. For example, multiple-tiered multiple choice tests (see McClary and Bretz 2012; Milenković et al., 2016a, 2016b) seem to be useful. Additionally, we will also concentrate on finding a software alternative to provide and analyse students’ responses to SSynQs to increase the validity and reliability of the applied instrument and the obtained results.
Limitations of the present study
It should be noted that the nomenclature, structure and reactions are important parts of the organic chemistry domain, however, there are many other parts that are not included in this study: molecular geometry, stereochemistry, physical properties, the importance of organic compounds and their applications, etc. Additionally, students’ generated SSynQs could be used to obtain a deep and distinct insight into cognitive structures of students with different expertise in organic chemistry, however, there are some limitations related to their application. Observing the group of high school students, it was noted that a certain number of students were not able to draw and construct a closed diagrammatic form, even in the case of a smaller number of concepts. As these students belonged to the sub-group with nominal cognitive structures, we have not managed to get a complete picture of their LDs and LUs. This limitation could be overcome by providing students with longer-lasting instruction about SSynQs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia under Grant No. 179010.References
- Ausubel D. P., (2000), The facilitation of meaningful verbal learning in the classroom, Educ. Psychol., 12(2), 162–178.
- Azizoğlu N., Alkan M. and Geban Ö., (2006), Undergraduate pre-service teachers’ understandings and misconceptions of phase equilibrium, J. Chem. Educ., 83(6), 947–953.
- Bhattachatyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90(6), 1073–1091.
- Davidowitz G. and Rollnick M., (2011), What lies at the heart of good undergraduate teaching? A case study in organic chemistry, Chem. Educ. Res. Pract., 12(3), 355–366.
- Derman A. and Eilks I., (2016), Using a word association test for the assessment of high school students’ cognitive structures on dissolution, Chem. Educ. Res. Pract., 17(4), 902–913.
- Derry S. J., (1996), Cognitive schema theory in the constructivist debate, Educ. Psychol., 31(3/4), 163–174.
- De Winter J. C. F., (2013), Using the Student's t-test with extremely small sample sizes, Pract. Assessment Res. Evaluation, 18(10), 1–12.
- Dunn D. S., (2001), Statistics and data analysis for the behavioural sciences, New York, NY: The McGraw-Hill Companies.
- Eseryel D., Law V., Ifenthaler D., Ge X. and Miller R., (2014), An investigation of the interrelationships between motivation, engagement, and complex problem solving in game-based learning, Educ. Technol. Soc., 17(1), 42–53.
- Fahmy A. F. M. and Lagowski J. J., (2003), Systemic reform in chemical education: an international perspective, J. Chem. Educ., 80(9), 1078–1083.
- Fahmy A. F. M. and Lagowski J. J., (2012), Systemic assessment as a new tool for assessing students learning in chemistry using SATL methods: systemic true false [STFQs] and systemic sequencing [SSQs] question types, Afr. J. Chem. Educ., 2(2), 66–78.
- Fahmy A. F. M. and Lagowski J. J., (2014), Systemic assessment as a new tool for assessing students learning in chemistry using SATL methods: systemic matching [SMQS], systemic synthesis [SSYNQS], systemic analysis [SAN Q, S], systemic synthetic – analytic [SSYN-ANQ, S], as systemic questions types, Afr. J. Chem. Educ., 4(4), 35–55.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29(2), 121–142.
- Hesse J. J. and Anderson C. W., (1992), Students’ conceptions of chemical change, J. Res. Sci. Teach., 29(3), 277–299.
- Heyworth R. M., (1999), Procedural and conceptual knowledge of expert and novice students for the solving of a basic problem in chemistry, Int. J. Sci. Educ., 21(2), 195–211.
- Hoz R., Champagne A. B. and Klopfer L. E., (1992), Multitechnique-multimethod matrix methodology for construct validation and its application for the construct of cognitive structure, Percept. Motor Skill, 74(1), 3–34.
- Hrin T. N., Milenković D. D. and Segedinac M. D., (2016a), The effect of systemic synthesis questions [SSynQs] on students’ performance and meaningful learning in secondary organic chemistry teaching, Int. J. Sci. Math. Educ., 14(5), 805–824.
- Hrin T. N., Fahmy A. F. M., Segedinac M. D. and Milenković D. D., (2016b), Systemic synthesis questions [SSynQs] as tools to help students to build their cognitive structures in a systemic manner, Res. Sci. Educ., 46(4), 525–546.
- Hrin T. N., Milenković D. D., Segedinac M. D. and Horvat S., (2016c), Enhancement and assessment of students’ systems thinking skills by application of systemic synthesis questions in the organic chemistry course, J. Serb. Chem. Soc., 81(12), 1455–1471.
- Hrin T. N., Milenković D. D., Segedinac M. D. and Horvat S., (2017), Systems thinking in chemistry classroom: the influence of systemic synthesis questions on its development and assessment, Think. Skills Creat., 23, 175–187.
- Ifenthaler D., Masduki I. and Seel N. M., (2011), The mystery of cognitive structure and how we can detect it: tracking the development of cognitive structures over time, Instr. Sci., 39(1), 41–61.
- Kirschner R. A., Sweller J. and Clark R. E., (2006), Why minimal guidance during instruction does not work: an analysis of the failure of constructivist, discovery, problem-based, experiential, and inquiry-based teaching, Educ. Psychol., 41(2), 75–86.
- Lagowski J. J., (2009), SATL, learning theory, and the physiology of learning, in Gupta-Bhowon M., Jhaumeer-Laulloo S., Li Kam Wah H. and Ramasami P. (ed.), Chemistry education in the ICT age, Dordrecht, Netherlands: Springer Science + Business Media B.V., pp. 65–74.
- Lee K. W. L., Tang W. U., Goh N. K. and Chia L. S., (2001), The predicting role of cognitive variables in problem solving in mole concept, Chem. Educ. Res. Pract. Eur., 2(3), 285–301.
- Lomask M. S., Baron J. B. and Grieg J., (1993), Assessing conceptual understanding in science through the use of two- and three dimensional concept maps, in The Proceedings of the Third International Seminar on Misconceptions and Educational Strategies in Science and Mathematics, Ithaca, NY: Misconceptions Trust, pp. 1–34, available at: http://www.mlrg.org/proc3pdfs/Lomask_3DConceptMaps.pdf, accessed 15 July 2016.
- Lopez E. J., Shavelson R. J., Nandagopal K., Szu E. and Peen J., (2014), Factors contributing to problem-solving performance in first-semester organic chemistry, J. Chem. Educ., 91(7), 976–981.
- McClary L. K. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students’ alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341.
- Milenković D. D., Hrin T. N., Segedinac M. D. and Horvat S., (2016a), Development of a three-tier test as a valid diagnostic tool for identification of misconceptions related to carbohydrates, J. Chem. Educ., 93(9), 1514–1520.
- Milenković D. D., Segedinac M. D., Hrin T. N. and Horvat S., (2016b), The impact of instructional strategy based on the triplet model of content representation on elimination of students’ misconceptions regarding inorganic reactions, J. Serb. Chem. Soc., 81(6), 717–728.
- Moreira R. F., (2013), A game for the early and rapid assimilation of organic nomenclature, J. Chem. Educ., 90(8), 1035–1037.
- Nakiboglu C., (2008), Using word associations for assessing non major science students’ knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9(4), 309–322.
- Nakhleh M. B., (1992), Why some students don't learn chemistry, J. Chem. Educ., 69(3), 191–196.
- Nash J. G., Liotta L. J. and Bravaco R. J., (2000), Measuring conceptual change in organic chemistry, J. Chem. Educ., 77(3), 333–337.
- Niemi D., (1997), Cognitive science, expert-novice research, and performance assessment, Theor. Pract., 36(4), 239–246.
- Nieswandt M., (2007), Student Affect and Conceptual Understanding in Learning Chemistry, J. Res. Sci. Teach., 44(7), 908–937.
- Novak J. D., (2002), Meaningful learning: the essential factor for conceptual change in limited or inappropriate propositional hierarchies leading to empowerment of learners, Sci. Educ., 86(4), 548–571.
- Paas F., Renkl A. and Sweller J., (2004), Cognitive load theory: instructional implications of the interaction between information structures and cognitive architecture, Instr. Sci., 32(1), 1–8.
- Paas F. and Sweller J., (2012), An evolutionary upgrade of cognitive load theory: using the human motor system and collaboration to support the learning of complex cognitive tasks, Educ. Psychol. Rev., 24(1), 27–45.
- Ring D. G. and Novak J. D. (1971), The effects of cognitive structure variables on achievement in college chemistry, J. Res. Sci. Teach., 8(4), 325–333.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9(2), 122–130.
- Shavelson R. J. and Stanton G. C., (1975), Construct validation: methodology and application to three measures of cognitive structure, J. Educ. Meas., 12(2), 67–85.
- Stains M. and Talanquer V., (2007), Classification of chemical substances using particulate representations of matter: an analysis of student thinking, Int. J. Sci. Educ., 29(5), 643–661.
- Stoddart T., Abrams R., Gasper E. and Canaday D., (2000), Concept maps as assessment in science inquiry learning – a report of methodology, Int. J. Sci. Educ., 22(12), 1221–1246.
- Taagepera M. and Noori S., (2000), Mapping students’ thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229.
- Taber K. S., (2008), Conceptual resources for learning science: issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30(8), 1027–1053.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S. and Tan K. C. D., (2011), The insidious nature of ‘hard-core’ alternative conceptions: implications for the constructivist research programme of patterns in high school students’ and pre-service teachers’ thinking about ionisation energy, Int. J. Sci. Educ., 33(2), 259–297.
- Taştan Ö., Yalçinkaya E. and Boz Y., (2010), Pre-service chemistry teachers’ ideas about reaction mechanism, J. Turk. Sci. Educ., 7(1), 47–60.
- Teddlie C. and Tashakkori A., (2009), Foundations of mixed methods research: integrating quantitative and qualitative approaches in the social and behavioral sciences, Thousand Oaks, CA: Sage Publications.
- Thompson T. L. and Mintzes J. J., (2002), Cognitive structure and the affective domain: on knowing and feeling in biology, Int. J. Sci. Educ., 24(6), 645–660.
- Tsai C. C. and Huang C. M., (2002), Exploring students' cognitive structures in learning science: a review of relevant methods, J. Biol. Educ., 36(4), 163–169.
- Vachliotis T., Salta K., Vasiliou P. and Tzougraki C., (2011), Exploring novel tools for assessing high school students' meaningful understanding of organic reactions, J. Chem. Educ., 88(3), 337–345.
- Vachliotis T., Salta K. and Tzougraki C., (2014), Meaningful understanding and systems thinking in organic chemistry: validating measurement and exploring relationships, Res. Sci. Educ., 44(2), 239–266.
- Van Merriënboer J. J. G. and Sweller J., (2005), Cognitive load theory and complex learning: recent developments and future directions, Educ. Psychol. Rev., 17(2), 147–178.
- Vladušić R., Bucat R. B. and Ožić M. (2016), Understanding ionic bonding – a scan across the Croatian education system, Chem. Educ. Res. Pract., 17(4), 685–693.
- Wolf P., (2001), Brain matters: translating research into classroom practice, Alexandria, Virginia: Association for Supervision and Curriculum Development.
- Wren D. and Barbera J. (2014), Psychometric analysis of the thermochemistry concept inventory, Chem. Educ. Res. Pract., 15(3), 380–390.
- Wu Y. T. and Tsai C. C., (2005), Development of elementary school students’ cognitive structures and information processing strategies under long-term constructivist oriented science instruction, Sci. Educ., 89(5), 822–846.
- Zhou Q., Wang T. and Zheng Q., (2015), Probing high school students’ cognitive structures and key areas of learning difficulties on ethanoic acid using the flow map method, Chem. Educ. Res. Pract., 16(3), 589–602.
- Zoller U., (1990), Students’ misunderstandings and misconceptions in college freshman chemistry (general and organic), J. Res. Sci. Teach., 27(10), 1053–1065.
| This journal is © The Royal Society of Chemistry 2018 |