This mechanistic step is “productive”: organic chemistry students' backward-oriented reasoning
I.
Caspari
 a,
M. L.
Weinrich
a,
M. L.
Weinrich
 b,
H.
Sevian
b,
H.
Sevian
 b and
N.
Graulich
b and
N.
Graulich
 *a
*a
aJustus-Liebig-University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: Nicole.Graulich@didaktik.chemie.uni-giessen.de
bDepartment of Chemistry, University of Massachusetts Boston, Boston, MA 02125, USA. E-mail: hannah.sevian@umb.edu
First published on 2nd October 2017
Abstract
If an organic chemistry student explains that she represents a mechanistic step because “it's a productive part of the mechanism,” what meaning could the professor teaching the class attribute to this statement, what is actually communicated, and what does it mean for the student? The professor might think that the explanation is based on knowledge of equilibria of alternative steps. The professor might also assume that the student implies information about how one of the alternatives influences the energetics of subsequent steps or how subsequent steps influence the equilibria of the alternatives. Meanwhile, the student might literally mean that the step is represented simply because it leads to the product. Reasoning about energetic influences has much greater explanatory power than teleological reasoning taking the consequence of mechanistic steps as the reason for their prediction. In both cases, however, the same backward-oriented reasoning is applied. Information about subsequent parts in the mechanism is used to make a decision about prior parts. To qualitatively compare the reasoning patterns and the causality employed by students and expected by their professor, we used a mechanistic approach from philosophy of science that mirrors the directionality of a mechanism and its components: activities, entities, and their properties. Our analysis led to the identification of different reasoning patterns involving backward-oriented reasoning. Participants' use of properties gave additional insight into the students' reasoning and their professor's expectations, which supports the necessity for clear expectations in mechanistic reasoning in organic chemistry classrooms. We present a framework that offers a lens to clarify these expectations and discuss implications of the framework for improving student mechanistic reasoning in organic chemistry.
Introduction
Mechanistic reasoning is used by scientists in all natural sciences and is a linchpin of organic chemistry. At the same time, solving mechanism problems is very complex, causing difficulties for organic chemistry students. To predict potential outcomes of reactions with the help of the electron-pushing formalism—the major tool of mechanistic reasoning (Bhattacharyya, 2013)—students have to consider multiple properties of molecules and, in many cases, various reversible pathways and their likelihoods simultaneously (Grove and Bretz, 2010; Kraft et al., 2010). Contrary to these demands, students are used to thinking about a sequence of events, rather than parallel pathways, and often do not consider properties (Bhattacharyya and Bodner, 2005; Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Kraft et al., 2010; Weinrich and Sevian, 2017). When proposing what happens in a mechanistic step, professors teaching organic chemistry classes might expect that the electron-pushing formalism expresses physical and chemical concepts for the students, whereas students might not connect structures and arrows drawn on paper with the underlying concepts (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Anzovino and Bretz, 2015). In this way, students have a tendency to focus on the representation of a mechanism rather than on the underlying meaning and, consequently, to rely on rote-memorization (Kraft et al., 2010; Strickland et al., 2010; Grove et al., 2012a, 2012b).Many students start their mechanistic problem-solving process by mapping the reactant onto the product in order to recall mechanistic steps or fill in steps just to solve the problem (Bhattacharyya and Bodner, 2005; Bhattacharyya, 2014). When asked, why did you propose this mechanistic step?, in many cases, students answer, because it gets me to the product (Bhattacharyya and Bodner, 2005). Trying to reach this goal, some students ignore steps or reaction pathways that do not bring them directly closer to the structure of the product. Students also propose unlikely steps because the intermediates generated build a bridge of structural similarity between the reactant and the product. For example, Marion (pseudonym) in Bhattacharyya and Bodner's study (2005) proposed the formation of a highly unstable phenyl cation to create a sequence of events that leads from anisole to phenol (Fig. 1). In order to propose the formation of the phenyl cation, she must have used the fact that the methoxy group in the reactant does not appear as part of the product, and instead a hydroxyl group is the final substituent of benzene. Compared to the direction of the reaction pathway, the problem-solving strategy she employed involves not only a forward but also a backward process.
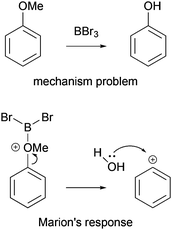 | ||
| Fig. 1 Marion's (pseudonym) response to a mechanism problem while using the it gets me to the product strategy. Reprinted with permission from Bhattacharyya and Bodner (2005). Copyright 2005 American Chemical Society. | ||
In findings from Galloway et al. (2017), a backward tendency in student reasoning can also be observed. In their study, students were asked to draw the resulting product given the electron-pushing arrows of a concerted epoxide forming step (Fig. 2). Although the students were asked to draw solely the product, some of the students proposed a stepwise process in which bromide leaves the carbon first to create a “positive charge as a means to have something for the negatively charged oxygen to bond with” (Galloway et al., 2017, p. 368). Identifying and combining charges could be a stepping stone for students to propose mechanistic steps (Galloway et al., 2017)—although, in this case, it is chemically invalid to create charges to enable ring closure in a following step. Independent of whether this reasoning is valid, it demonstrates an interesting pattern: students use their assumption about a subsequent step in the mechanism (a negatively charged oxygen is able to attack a positively charged carbon) to make a decision about a prior step (bromide leaves creating a positive charge). The reasoning pattern describes which known or assumed parts in a mechanism are used to reason about unknown parts (Darden, 2002; Darden and Craver, 2002; Russ et al., 2008). Thus, it reveals the students' direction of decision making compared to the direction of the sequence of steps in the mechanism. In the aforementioned example, assumed information about a subsequent part is used to make a decision about a prior part. As shown in the following example, if such a reasoning pattern stems from underlying chemical concepts, it is necessary for making a decision about which of several alternative reaction pathways to depict—a key task in solving mechanism problems.
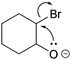 | ||
| Fig. 2 Draw products task. Reproduced from Galloway et al. (2017) with permission from the Royal Society of Chemistry. | ||
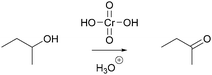
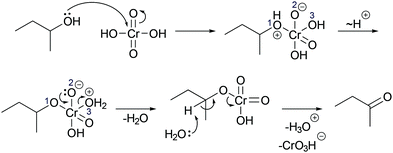
In a reaction mixture of 4-hydroxy-4-methyl-2-pentanone with hydroiodic acid in water, protonation of the carbonyl group (step 1a, Fig. 3) and the hydroxyl group (step 1b, Fig. 3) both occur in rapid equilibria due to low energetic barriers of the proton transfers. Since the representation of a mechanism usually does not show all changes occurring in a reaction mixture, but only changes that are part of the lowest energy path from the reactant to the product (Goodwin, 2012), not all proton transfers are represented. To decide whether the protonation of the carbonyl group (step 1a, Fig. 3) or of the hydroxyl group (step 1b, Fig. 3) should be represented, one has to consider information about subsequent mechanistic steps. Only the protonation of the hydroxyl group (step 1b, Fig. 3) enables the rate-determining elimination of water (step 2b, Fig. 3). While the activation energy of the elimination of hydroxide is not attainable, the activation energy of the elimination after the protonation of the hydroxyl group is attainable. Finally, the elimination of water (step 2b, Fig. 3) enables the nucleophilic addition of iodide (step 3b, Fig. 3), which significantly lowers the potential energy of the reacting molecules. This information about the subsequent steps in the SN1 reaction of an alcohol provides the basis for the decision to depict the prior protonation of the hydroxyl group (step 1b, Fig. 3).
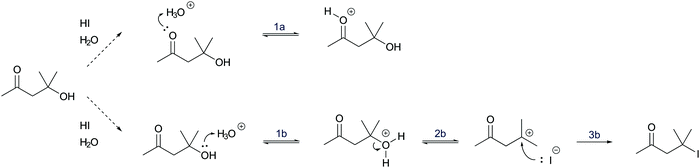 | ||
| Fig. 3 Two alternative reaction pathways in a reaction mixture of 4-hydroxy-4-methyl-2-pentanone with hydroiodic acid in water. | ||
The reasoning pattern of the students in Galloway et al. (2017) and the reasoning pattern in the example of the SN1 reaction are the same. The pattern is to consider information about subsequent steps in a mechanism to make a decision about prior steps. The students in Galloway et al. (2017) considered the ability of the negatively charged oxygen to form a bonding interaction with the positively charged carbon in the subsequent step to decide that bromide is eliminated prior to the nucleophilic addition. In the example of the SN1 reaction, the activation energy of the elimination in the subsequent step (step 2b, Fig. 3) is considered to decide that the hydroxyl group has to be protonated (step 1b, Fig. 3) prior to the elimination. In both cases, the reasoning pattern is reversed relative to the chronological order of the proposed reaction pathway.
The former example of the students in Galloway et al. (2017) demonstrates teleological reasoning (Wright, 1976; Talanquer, 2013) that uses the consequence of a mechanistic step for the prediction of this step. This is because the positive charge enabling the ring closure, which is a consequence of the elimination of bromide, is taken as the explanatory basis for the prediction of the elimination. While the latter example of the SN1 reaction of an alcohol has the same backward-oriented reasoning pattern, it is not teleological. The lower activation energy of the elimination, which is a consequence of the protonation, is not taken as the explanatory basis for the prediction of the protonation. Instead, the occurrence of protonation in prior rapid equilibrium and its influence on the elimination are explained by the underlying energetic causality within the system.
Research findings document that (1) students often do not incorporate explanations about underlying causality given in class into their thinking (Anderson and Bodner, 2008), (2) have fundamental misunderstandings regarding reactivity of reversible reactions (Kraft et al., 2010), and (3) sometimes intuitively explain events teleologically (Kelemen and Rosset, 2009; Talanquer, 2013). Extending these findings, if students are taught mechanisms like the SN1 reaction of an alcohol—even if explained causally—it might be the case that they only adopt the reasoning pattern (considering information about subsequent steps to reason about prior steps) and miss the causal explanation, thereby leading to teleological arguments. For example, a student might think that increasing the leaving group ability for the elimination step (information about a subsequent step) is the reason to predict the protonation in the first step (reasoning about a prior step). In contrast to the explanation of the SN1 reaction given before, this reasoning would not incorporate considerations about energetics, and, instead, the consequence of the event (increased leaving group ability) would provide the reason for the prediction of the event (protonation of the hydroxyl group). Employing the aforementioned reasoning pattern teleologically could be a “didactical transposition” (Chevallard, 1991) in which the complex reason for a necessary decision is communicated in a convenient way. However, if used with unawareness of the underlying causes, it might also be used for implausible mechanistic predictions. If the reasoning pattern is required in some cases, then students must be taught to appropriately differentiate between cases where it is a valid basis for a decision and those where it is not.
In light of this, it is valuable to compare reasoning with this pattern in student explanations and educator expectations. We made one such comparison based on interviews of Organic Chemistry II students solving mechanism problems and the explanation their professor expected from proficient students.
Theoretical framework
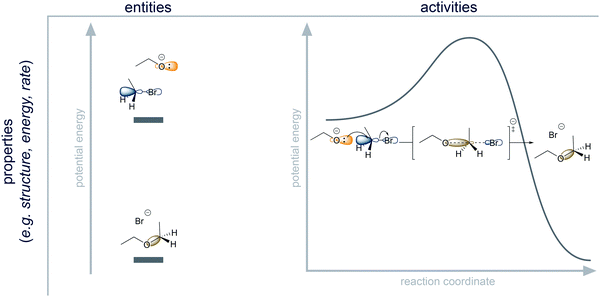
To investigate the reasoning pattern with regard to the direction of the reaction pathway, we used a mechanistic approach from philosophy of science that mirrors the mechanism itself. This approach helped us to analyze the structure of reasoning about mechanisms. Research in philosophy of science has investigated what constitutes a mechanism in the physical and life sciences (Machamer et al., 2000; Glennan, 2002; Bechtel and Abrahamsen, 2005; Illari and Williamson, 2011). From that work, we can describe a mechanism as a process involving entities, the activities they undergo, and the properties of these entities and activities.This mechanistic approach has successfully been used in science education research in molecular biology and physics (Russ et al., 2008; Bolger et al., 2012; van Mil et al., 2013, 2016; Southard et al., 2016) but has not been applied in organic chemistry. Taking this approach to the realm of organic chemistry, we have to consider that mechanisms in organic chemistry are (to a greater extent than mechanisms in biology) underwritten by electronic theory (Ramsey, 2008) and energetic considerations (Goodwin, 2003, 2012). Therefore, we clarify how the mechanistic approach includes electronic theory and energetic considerations in the definitions of components of a mechanism (i.e. entities and activities) before taking a closer look at reasoning patterns.
Components of a mechanism
Entities are physical systems like molecules and atoms (e.g. ethanol). They are the things of a mechanism (Machamer et al., 2000). Entities are characterized by their properties (Machamer et al., 2000), which are all kinds of information about entities (e.g. ethanol contains a hydroxyl group with a partial negative charge on the oxygen). Entities can be thought of as sets of properties. In mechanistic representations in organic chemistry, entities are usually depicted by Lewis structures.Properties of entities are information about physical systems. Information about atom types, connectivity (bonding), and, at times, orientation in space can be considered as properties of molecules that can be explicitly communicated using Lewis structures. For example, the Lewis structure provides the information that the bromoethane in Fig. 4 consists of carbon, bromine and hydrogen atoms organized in a specific way. Positive and negative charges are also properties that are represented in Lewis structures. Further electronic properties are not directly shown in most mechanistic representations. The partial positive and partial negative charge in bromoethane and the shape of the lowest unoccupied molecular orbital are two of many electronic properties of this entity (Fig. 4). The potential energy of an entity in a given system is an example of an energetic property (Fig. 4).
Activities describe the dynamic part of a mechanism (e.g. nucleophilic addition) (Machamer et al., 2000). They are transformations of entities and of their sets of properties (e.g. change in bonding, change in potential energy). In mechanistic representations, activities are mostly depicted by curved arrows. Organic chemists also occasionally include other representations of activities alongside electron pushing, e.g. a proton transfer, ∼H+. But the electron-pushing formalism is the most important tool that organic chemists use to represent activities in a reaction mechanism. According to Machamer et al. (2000), “activities are identified and individuated in much the same way as are entities” (p. 5). To be consistent in wording, we therefore consider these individuation conditions as properties of activities.
Properties of activities are information about change in physical systems. Some properties of activities describe which properties of entities are changed in a process. Bond formation and bond cleavage in the SN2 reaction of ethoxide and bromoethane (Fig. 4) include changes in the structure of these entities and changes of the charges on the oxygen and bromine atoms. Further changes of electronic properties are not directly shown in most mechanistic representations. The change in orbital configuration from the highest occupied molecular orbital of ethoxide and the empty σ* orbital of the C–Br bond in bromoethane to the new σ bond is one of many electronic properties of the activity (Fig. 4). The change of potential energy is also a property of the activity (Fig. 4). Additionally, some properties of activities go beyond describing which properties of entities are changed in a process. They individuate the activities on the basis of how the activities take place. For example, activities can be fast or slow, and they can be reversible or irreversible.
Organic chemistry is a strongly entity-centered discipline because the actual activities of chemical processes are typically inferred indirectly through analysis of the products or experimental observation of entities, e.g. from the kinetic detection of intermediates (Huisgen, 1970). The model of the physical mechanism that organic chemists use is thus more like “snap shots” (Goodwin, 2012, p. 310) of a continuous process than a dynamic film. Moreover, similar structural features of a molecule could indicate similar activities; however, activities of a molecule also depend on the environment (e.g. other reactants and reaction conditions). Due to the fact that organic chemistry is entity-centered, but entities can react differently depending on the environment, the concept of capacities of entities in their environment plays an important role in mechanistic reasoning.
Capacities are the properties of entities comprising information about the ability of entities to engage in activities under certain conditions (Ramsey, 2008). For example, nucleophilicity is a capacity of ethoxide, and leaving group ability is a capacity of bromide in bromoethane (Fig. 4). According to philosophy of science, it would be consistent to describe capacities as a type of property of entities. From our educational point of view, it is more useful to ascribe capacities to the dynamic part of mechanisms (i.e. activities) because thinking about the capacity of an entity implies consideration of a probable process rather than consideration of static structure.
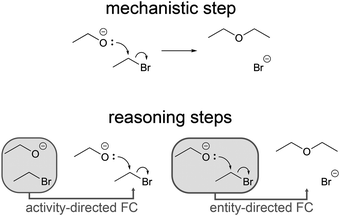
Reasoning patterns
Darden and Craver (2002) extended the account of mechanisms by linking important mechanistic phenomena in molecular biology and biochemistry to the reasoning strategies leading to their elucidation. Alongside other reasoning strategies, they describe chaining as “reasoning about one part of a mechanism on the basis of what is known or conjectured about other parts in the mechanism” (Darden, 2002, p. 362). Russ et al. (2008) used chaining as a code indicating evidence of mechanistic reasoning in first-grade students' discourse about physical phenomena. In our study, rather than having focused on multiple reasoning strategies, we focused on chaining in finer detail. Chaining utilizes connections between different parts of a mechanism. When one part is used as a basis to reason about another part, different reasoning patterns can emerge depending on which parts are used. Therefore, we refer to several distinct reasoning patterns (chaining types) that all fall in the category of the reasoning strategy called chaining.Chaining mirrors the directionality of the steps involved in the mechanistic phenomena it describes and is therefore capable of describing forward and backward reasoning patterns. Using earlier parts of the forward reaction of a physical mechanism (i.e. entities or activities) as a basis to reason about later parts (i.e. other entities or activities) is called forward chaining (Darden, 2002; Darden and Craver, 2002). Forward chaining can lead to the representation of subsequent parts of the mechanism using prior parts, which are already represented. Reasoning in the opposite direction, using later parts of the forward reaction of a physical mechanism as a basis to reason about earlier parts, is called backward chaining† (Darden, 2002; Darden and Craver, 2002). Backward chaining can lead to the representation of prior parts of the mechanism using information about subsequent parts, which are not represented yet and are either known, assumed or derived by several steps of forward chaining without representation. Using information about subsequent steps in a mechanism to reason about prior steps, as in the example of the SN1 reaction of an alcohol given in the introduction, falls in the category of backward chaining. Integrating Darden and Craver's description of different ways of chaining in life sciences (Darden, 2002; Darden and Craver, 2002) with our own theoretical assumptions about chaining in organic chemistry provides a lens to analyze reasoning patterns in organic chemistry in terms of chaining types.
Forward chaining (FC) refers to situations where the reasoning process is in the direction of the mechanism. Darden and Craver (2002) differentiate two types of FC, which we name based on whether the reasoning process leads to proposed activities or entities.
Activity-directed FC means reasoning from the entities at the beginning of a mechanistic step (e.g. ethoxide and bromoethane) to activities in the mechanistic step (e.g. nucleophilic addition). Given the entities, one might ask, which activity could occur among these entities in the mechanistic step in question? Activity-directed FC in the example starts with the structures of ethoxide and bromoethane and proposes the concerted nucleophilic substitution represented with the electron-pushing formalism (Fig. 4). This can be justified with further properties of entities and activities. For example, the shape and energy of the highest occupied molecular orbital of ethoxide and the σ* orbital of the C–Br bond in bromoethane are crucial properties of the entities that are necessary for changing the orbital configuration with an attainable activation energy.
One mechanistic step of an organic reaction could be divided into two FC steps, activity-directed FC followed by entity-directed FC (Fig. 5).
Entity-directed FC means reasoning from the activities in a step to entities at the end of a step. In organic chemistry, this means following the consequences of application of the electron-pushing formalism when it is given (i.e. the curved arrows are already drawn). There is a strong focus on this in other studies (Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017). Since we wanted to investigate how participants in our study used reasoning about subsequent steps to make a decision about prior steps, and since entity-directed FC was not verbalized in combination with this decision, entity-directed FC is not considered further in this publication. Instead, we now focus on backward chaining, and only FC related to the decision of backward chaining is represented in the following figures.
Backward chaining (BC) refers to situations where the reasoning pattern is reversed with respect to the direction of the mechanism. Darden and Craver (2002) also differentiate two types of BC, which we name based on whether the reasoning process leads to proposed activities or entities.
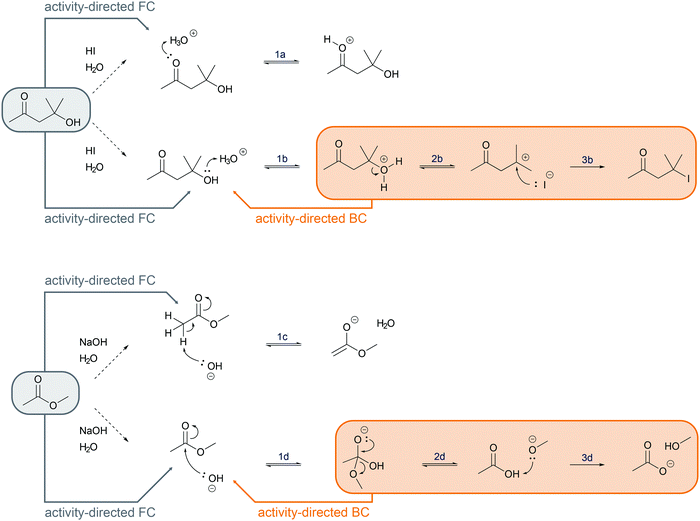
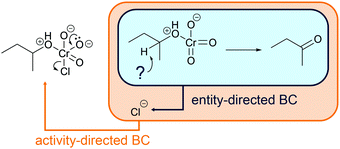
Activity-directed BC (Fig. 6) uses information about activities in subsequent mechanistic steps (e.g. elimination of water in a SN1 reaction) to make a decision about activities of prior steps (e.g. protonation of the hydroxyl group). The capacity of entities for activities in subsequent mechanistic steps is the starting point of the reasoning process. One might ask, which activity in the current mechanistic step brings about entities capable of or best suited for engaging in the activities of subsequent steps? In the example SN1 reaction of an alcohol in the introduction, the leaving group ability (step 2b, Fig. 6) could be the starting point of activity-directed BC to make a decision about the prior step: protonation of the hydroxyl group (step 1b, Fig. 6) increases the leaving group ability. The protonation of the hydroxyl group suggested by this activity-directed BC is the first step to represent in the mechanism.
More information is needed to employ activity-directed BC causally. Activity-directed BC can be employed causally in situations where a decision between alternative activities cannot be based on activity-directed FC alone but can be based on the interdependence of one of the alternative activities with subsequent steps. In the case of the reaction mixture of 4-hydroxy-4-methyl-2-pentanone with hydroiodic acid in water, activity-directed FC could predict protonation of the carbonyl group (step 1a, Fig. 6) or protonation of the hydroxyl group (step 1b, Fig. 6) occurring in prior rapid equilibria to subsequent steps. Following the convention to represent only the lowest energy path from the reactants to the products (Goodwin, 2012), one is in a situation of relative uncertainty concerning which protonation to represent. Therefore, activity-directed BC is necessary. Only the protonation of the hydroxyl group (step 1b, Fig. 6) enables the leaving group activity in the subsequent step (step 2b, Fig. 6). The leaving group activity (step 2b, Fig. 6) is part of the lowest energy path to the product because it enables the following nucleophilic addition (step 3b, Fig. 6) leading to a significant decrease in potential energy. These subsequent steps (step 2b and 3b, Fig. 6) cannot occur without protonation of the hydroxyl group (step 1b, Fig. 6) because the activation energy of the elimination of hydroxide is not attainable. While the rate of the protonation of the hydroxyl group (step 1b, Fig. 6) does not influence the rate of the overall reaction, the enabled elimination is the rate-determining step (step 2b, Fig. 6). Therefore, the protonation of the hydroxyl group (step 1b, Fig. 6) is the first step to represent in the mechanism. The interdependence of the prior (step 1b, Fig. 6) and the subsequent step (step 2b, Fig. 6) can be described by the prior step's (step 1b, Fig. 6) influence on the energetic barrier of the subsequent step (step 2b, Fig. 6).
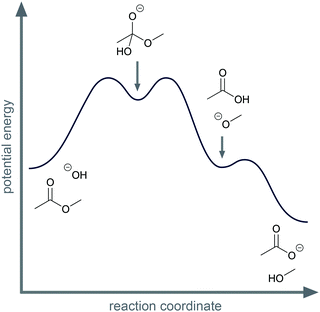
Activity-directed BC is not limited to use in decisions about proton transfers. For example, for the first step of the alkaline ester hydrolysis, activity-directed FC leads also to uncertainty about which activity to represent (Fig. 6). In the reaction mixture, the hydroxide reacts as a nucleophile forming the tetrahedral intermediate (step 1d, Fig. 6), but hydroxide also reacts as a base deprotonating the ester in the α-position (step 1c, Fig. 6). Both alternatives are reversible. Subsequent steps after the nucleophilic addition (step 2d and 3d, Fig. 6) are the starting point to decide which alternative to represent. After the nucleophilic addition (step 1d, Fig. 6), elimination of methoxide in the second step (step 2d, Fig. 6) and the acid–base reaction in the third step (step 3d, Fig. 6) finally lead to a significant decrease in potential energy (Fig. 7). Therefore, activity-directed BC predicts that the equilibrium of the rate-determining nucleophilic addition (step 1d, Fig. 6) is continually shifted toward the tetrahedral intermediate. Hence, the nucleophilic addition (step 1d, Fig. 6) is the activity to represent in the mechanism. The interdependence of the prior step (step 1d, Fig. 6) and the subsequent steps (step 2d and 3d, Fig. 6) can be described by the subsequent steps' (step 2d and 3d, Fig. 6) influence on the equilibrium of the prior step (step 1d, Fig. 6).
When comparing the examples of the SN1 reaction of an alcohol and the alkaline ester hydrolysis, it seems as if there are two distinct possibilities of interdependence between the prior and the subsequent steps; however, this is not the case. While in the SN1 reaction the protonation of the hydroxyl group (step 1b, Fig. 6) influences the energetic barrier of the elimination (step 2b, Fig. 6), the elimination and the nucleophilic addition (step 2b and 3b, Fig. 6) also continually shift the equilibrium of the protonation (step 1b, Fig. 6). In the alkaline ester hydrolysis, the nucleophilic addition in the first step (step 1d, Fig. 6) also influences the rate of the subsequent step (step 2d, Fig. 6) drastically: the elimination of methanol (step 2d, Fig. 6) could not occur without the prior addition of hydroxide (step 1d, Fig. 6). In both examples prior steps influence the rate of subsequent steps and subsequent steps shift the equilibria of prior steps. Causal activity-directed BC is based on this interdependence and leads to the representation of the lowest energy path to a product even if the product is energetically disfavored. For example, in the case of an acidic acetal formation, protonation of the hydroxyl group enables the leaving group activity of water, while removing water shifts the equilibria of prior steps. Consequently, the protonation of the hydroxyl group is part of the lowest energy path to the acetal and is represented in the mechanism.
Activity-directed BC cannot be employed causally in every case. It cannot be employed causally if activity-directed FC already leads to a final decision concerning which activity to represent. For instance, given the reactant of the epoxide forming step in Galloway et al.'s (2017) study (Fig. 2) activity-directed FC would already lead to the decision that a concerted process is the lowest energy path to the product. Consequently, activity-directed BC is not causally applicable in this case. Furthermore, even if activity-directed BC can be employed causally for a mechanistic step, activity-directed BC cannot use capacities of all entities produced by an activity as the basis of a causal decision to represent this activity. This is because not every entity produced by an activity influences the energetic barrier of subsequent steps in a mechanism. For example, the proton transfer between an alcohol and hydronium has not only the protonated alcohol as a product (e.g. step 1b, Fig. 6) but also a water molecule (not represented in Fig. 6). In contrast to the structure of the protonated alcohol, the structure of the water molecule does not influence the energetic barrier of subsequent steps in a SN1 reaction of the alcohol. While the capacity of the protonated alcohol for subsequent steps can be used as the basis of causal activity-directed BC resulting in the decision to represent the proton transfer in the SN1 mechanism, capacities of the water molecule cannot be used as the basis for this activity-directed BC.
Due to the reverse chronological order with respect to the proposed reaction pathway, activity-directed BC might easily be incorporated with a teleological instead of a causal conceptual mode (Weinrich and Talanquer, 2015; Yan and Talanquer, 2015). One uses activity-directed BC teleologically if information about a subsequent step is used as the reason to predict an activity in a prior step. One applies it causally if the occurrence and the interdependence of activities in prior and subsequent steps are explained with energetic properties of entities and activities. According to our framework, whether activity-directed BC can be employed causally or only teleologically depends on the mechanistic situation. Activity-directed BC can be employed teleologically in every situation. It can be employed causally in situations (1) where the predicted activity can reasonably be said to be present in the reaction mixture and (2) where the interdependence of this activity with subsequent activities leads to the energetically favored path to a product.
Entity-directed BC (Fig. 8) is reasoning from activities of entities (e.g. protonation of carboxylate in a fourth workup step after the alkaline ester hydrolysis) to additional entities that are required to enable the activity (e.g. adding an acid to the solution). Aiming at a certain activity, e.g. in a workup step, one might ask, which further entity could be engaged to enable the activity? For example, if after the alkaline ester hydrolysis (Fig. 6) the carboxylic acid instead of the carboxylate anion is the required product, protonation of the carboxylate anion could be the starting point of entity-directed BC. Entity-directed BC in this case predicts an acid capable of protonating the carboxylate anion (Fig. 8). In practice, this acid can then be added to the reaction mixture.
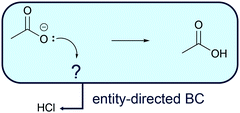 | ||
| Fig. 8 Entity-directed backward chaining (BC) starts at the activities in a mechanistic step. The result of entity-directed BC is additional entities that are required to enable the activities. | ||
Methods
Goals and research questions
We observed evidence in the present organic chemistry education literature (Bhattacharyya and Bodner, 2005; Galloway et al., 2017) that students sometimes use assumptions about subsequent steps in a mechanism to make decisions about prior steps. According to our framework, this reasoning pattern could be identified as activity-directed BC and can be applied causally in some cases (we explained in detail mechanistic contexts in which it can be applied causally using an SN1 reaction of an alcohol and the alkaline ester hydrolysis as examples). Students can only differentiate between cases where activity-directed BC is an appropriate basis for a decision and those where it is not if their reasoning reflects the actual nature of the mechanism (e.g. alternative reversible steps occur, energetic properties play a crucial role). Teaching can only support this if educators expect the same in their students' reasoning. Therefore, it is valuable to compare different aspects of the use of activity-directed BC in students' reasoning and in their professor's expectations.Activity-directed BC might be used in combination with other chaining types to make a decision about a mechanistic step. Investigating the combination of other chaining types used together with activity-directed BC can reveal the extent to which the overall reasoning pattern reflects the actual nature of the mechanism. For example, the combination of activity-directed BC with activity-directed FC to alternative activities would reflect the awareness that other activities are also plausible.
Only causal use of activity-directed BC can reflect the nature of a mechanism because the consequence of an event is never the actual cause of an event. We therefore wanted to know whether activity-directed BC was used teleologically or causally.
Two uses of activity-directed BC were further distinguished depending on whether the context was appropriate or inappropriate. We were interested in this because, in an appropriate context, a teleological use of activity-directed BC might not always imply unawareness of underlying causes, it might also be a convenient way of communicating the complex reason for a decision easily. However, expressing the same explanation in an inappropriate context might indicate that activity-directed BC is overused or misapplied in some situations.
Our study was guided by the primary research questions: How did students use activity-directed BC and how does their usage compare to their professor's expectations? The following sub-questions were explored:
• Which further chaining types did students apply when they used activity-directed BC to make a decision about a mechanistic step? In what ways does the combination of chaining types reflect their professor's expectations?
• Did students employ activity-directed BC causally or teleologically? In what ways does this reflect their professor's expectations?
• Was activity-directed BC employed in an appropriate or inappropriate context?
Research context
The students whose data were used were recruited on a voluntary basis from the Organic Chemistry II course at a medium-sized, non-traditional public commuter university in the Northeastern United States. The average age of undergraduate students at this university was 25 years. 72.3% of the undergraduate students were full-time students, and 27.7% were part-time students. For their participation in the study, the students received extra credit, which was 2% of their final course grade. The students are given pseudonyms in this publication. We also included the data of the professor who taught the course. The male professor had two years of teaching experience.This study was conducted as a secondary data analysis using de-identified data obtained through informal data sharing (Heaton, 2008). The data were previously collected to capture students' abstraction in problem solving (Sevian et al., 2015; Weinrich and Sevian, 2017). The university's Institutional Review Board (IRB) determined that the study met the criteria for secondary data analysis and did not require further oversight.
Data sources
The previously collected data (Sevian et al., 2015) used in this study included transcripts of interviews of 20 students and the professor teaching the class, smart pen videos, scans of students' exam problem solutions (names were removed), and slides from the professor's lecture. The interviews were conducted as semi-structured interviews in combination with a LiveScribe pen, which simultaneously records students' writing and verbalized reasoning. The students were interviewed after the first and the third of the three exams of their Organic Chemistry II course. Each interview included two mechanism problems. The first problem was chosen from the exam problems by the professor because he judged the problem to be a good indicator of whether a student understood the material taught in class. The second problem was provided by the professor as a similar problem to the exam problem, with the difference that the students had not seen it before. The problems included in the two interviews are shown in Table 1. For the exam problems, students were asked to explain how they solved the problem and why they proposed each step. For the similar problems, they were asked to think aloud while reasoning about the mechanism. The professor was interviewed before the two exams and was asked to solve the mechanism problems and explain the reasoning he expected from a proficient student.| Exam 1 | Exam 3 |
|---|---|
|
1A: Exam Problem
Please provide the complete mechanism for the following transformation. |
3A: Exam Problem
Please provide the complete mechanism for the following transformation. |
|
1B: Similar Problem
Please provide the complete mechanism for the following transformation. |
3B: Similar Problem
Please provide the complete mechanism for the following transformation. |
As we used existing data, the mechanism problems were not created for this study, but they proved to be very appropriate for answering our research questions. This is because, for three of the four problems (1A, 3A and 3B, Table 1), the mechanisms taught in class included a proton transfer that would not be the only plausible proton transfer predicted solely by FC without activity-directed BC (Fig. 9 and 10). Although some textbooks (e.g.Clayden et al., 2012; Karty, 2014) show alternative mechanisms for the mechanism problems, for our analysis’ purpose we assume that the mechanisms taught by the professor or the ones shown in the textbook used in class (Klein, 2012) are the most plausible pathways. We do so because student reasoning can only be judged to be valid based on the mechanism the students learned. That means if we want to decide whether the use of activity-directed BC is reasonable for a step predicted as taught in class or shown in the textbook, we do not discuss if the predicted step is most plausible but rather if the use of activity-directed BC is valid for the given step.
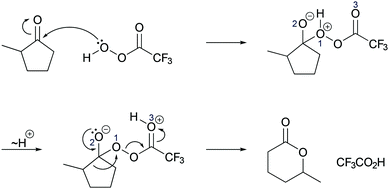 | ||
| Fig. 10 Mechanism of the Baeyer–Villiger oxidation as taught by the professor exemplarily shown for problem 3A, also applicable for problem 3B. | ||
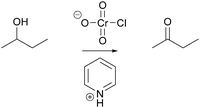
Using problems 1A, 3A and 3B (Table 1), we investigated in two different mechanistic contexts, oxidation (Jones oxidation, Fig. 9) and rearrangement (Baeyer–Villiger oxidation, Fig. 10), whether and how students used activity-directed BC for a mechanistic step that would not be the only solution predicted by FC. Because chloride provides the leaving group on chromium in problem 1B (Table 1), no protonation improving the leaving group ability is needed in the pyridinium chlorochromate (PCC) mechanism. This differs from the Jones oxidation (Fig. 9) so that a plausible mechanism could be provided without using activity-directed BC. It was of special interest to examine whether and how activity-directed BC also occurs to predict activities in problem 1B (Table 1) or activities other than proton transfers in problems 1A, 3A and 3B (Table 1).
Data analysis
Using the existing responses to the four mechanism problems, we wanted to qualitatively address the questions, how did students use activity-directed BC and how does their usage compare to their professor's expectations? Therefore, while listening to the participants' recordings and simultaneously viewing the LiveScribe videos of their drawings, we selected all responses in which participants (20 students and their professor) used activity-directed BC to make a decision about a mechanistic step. The indicator for usage of activity-directed BC was reasoning about activities in subsequent steps in order to make a decision about activities of prior steps. Activities in the subsequent steps can be expressed as actual activities (e.g. water leaves) or capacities of entities (e.g. water as a leaving group), which we ascribe to activities. Parts of transcripts indicating this reasoning pattern were coded with activity-directed BC (Table 2). An absence of the code activity-directed BC in participants' responses to a mechanism problem does not necessarily indicate an absence of this chaining type in the participants' reasoning—merely a lack of evidence because activity-directed BC was not verbalized.| Codes for chaining types | Examples from the participants | Descriptions of the codes using the examples |
|---|---|---|
| Activity-directed backward chaining (BC) | “And then I knew that I wanted this positive charge to end up over here to make a better leaving group, so I just did a hydrogen shift.” | Activity-directed BC uses activities in subsequent mechanistic steps (e.g. elimination of a leaving group) to make a decision about activities of prior steps (e.g. hydrogen shift that improves leaving group ability) |
| Entity-directed backward chaining (BC) | “Knowing that this somehow has to break, so that means the hydrogen here has to be taken off by something in the solution. Whether it's the Cl or whether it's the base.” | Entity-directed BC is reasoning from activities of entities (e.g. abstraction of a proton) to additional entities that are required to enable the activities (e.g. chloride) |
| Activity-directed forward chaining (FC) to alternative activities | “Take this proton here and do a proton transfer, not to this guy here, which would seemingly want it, but it would proton transfer from this oxygen here to this other oxygen right here” | Activity-directed FC means reasoning from the entities at the beginning of a mechanistic step (e.g. a protonated molecule) to alternative activities in the mechanistic step (e.g. two different proton transfers) |
Only responses with this code were further analyzed. These were 2 responses from the professor and 18 student responses. The 18 student responses included examples of each of the 4 problems and came from 12 different students. We were interested in characterizing how activity-directed BC in the students' verbalized reasoning compared to their professor's expectations. Therefore, the next step of the analysis was to collect regions of the responses containing participants' complete reasoning processes about the mechanistic steps for which activity-directed BC had been employed. These excerpts comprised the data of our coding analysis, which uses a closed coding scheme derived from our theoretical framework.
We used codes derived from our framework to describe the combinations with other chaining types in which activity-directed BC was employed. Hence, every combination included activity-directed BC, which was used either as the only chaining type to make a decision about an activity in a mechanistic step or in combination with activity-directed FC to alternative activities or with entity-directed BC (Table 2).
We further coded if students employed activity-directed BC causally or teleologically and which sort of explanation their professor expected. The indicator for teleological activity-directed BC was that the activity addressed was predicted in order to enable subsequent steps. The indicator for causal activity-directed BC was that the decision to represent a particular activity was based on energetic considerations that explain why the activity occurs in the reaction mixture and how the prior and the subsequent steps influence each other.
We additionally coded if activity-directed BC was employed in an appropriate context or an inappropriate context. To give this code, we decided if the entities and activities included in an instance of activity-directed BC have the required properties that make activity-directed BC applicable, even if these properties were not expressed in the participant's excerpt.
In addition to the coding analysis of the aforementioned excerpts, we used other portions of the interviews to address questions that arose during analysis. For interrater reliability, the first and the second author independently coded 20 percent of the responses. Results were discussed until complete agreement was reached before the first author proceeded with the analysis of the remaining responses. The second author completely reviewed and agreed on the final coding done by the first author.
Results
Activity-directed BC was used in 20 cases which included 2 examples from the professor and 18 student examples from 12 different students. The fraction of participants' total verbalized reasoning about a problem that fit in one of the following combinations of chaining types ranged from a single thought to more than half of the total reasoning. On average this fraction comprised about a fifth of the total reasoning about a problem. This corresponded to reasoning leading to a decision about one mechanistic step. Comparing participants’ use of activity-directed BC revealed three combinations of chaining types:(1) Activity-directed BC combined with activity-directed FC to alternative activities was only found in the professor's expectations.
(2) In 10 of the 18 student cases, activity-directed BC was not combined with any other chaining type.
(3) In 8 of the 18 student cases, activity-directed BC was combined with entity-directed BC.
The results section is organized according to the three combinations of chaining types. Each section is given a headline intended to provide an intuitive first impression of the reason for employing activity-directed BC in the combination of chaining types considered therein. Regardless of which combination of chaining types was employed, in all cases, activity-directed BC was teleological, and this includes the professor's expectations. It is not surprising that students did not engage in causal reasoning if instruction focused on teleological explanations. However, the identification of different combinations of chaining types can give insight into how missing causality in instruction probably affected the use of activity-directed BC. Revealing if teleological activity-directed BC was employed in an appropriate or inappropriate context completes the comparison of teleological instruction and students' teleological reasoning. Therefore, we describe in each section whether additional properties (not mentioned by the participants) of the same entities and activities (mentioned by the participants) uncovered an appropriate or an inappropriate context for application of activity-directed BC. Although this was the last step of our analysis, we sometimes point out these properties before explaining why the applied activity-directed BC was coded to be teleological because contrasting a participant's statement with the causal explanation increases the transparency of this coding decision.
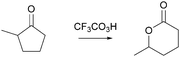
What the professor expected: even though something else could happen in the mechanism, this is the “productive part”
Activity-directed BC combined with activity-directed FC to alternative activities was only found in the professor's expectations. Making his expectations about the second step of the Baeyer–Villiger oxidation (Fig. 10) explicit, the professor stated at the beginning:Now what we normally would do if we were talking about a regular mechanism we would protonate, we would reduce all these separate charges [on O1 and O2, Fig. 10 ], so we would take this negative charge right here [on O2] and add a proton to it which would be very local to it to give everything here a neutral feeling, a neutral charge you know for the entire molecule.
This statement demonstrates activity-directed FC because the professor's explanation started with entities at the beginning of the second mechanistic step (O1 and O2) and suggested an alternative activity (proton transfer between O1 and O2) than that proposed by activity-directed BC:
But we aren't doing that here so the student that would know what they are doing would take this, would take this proton here [on O1, Fig. 10 ] and do a proton transfer, not to this guy here [O2], which would seemingly want it, but it would proton transfer from this oxygen here [O1] to this other oxygen right here [O3] […] the reason why you want to have that is because this protonated species here [in the second intermediate] further spring loads this oxygen–oxygen bond right there, once I have this guy here, this proton up there [on O3], there is further impetus to give some electron density back to this entire system right here.
In this activity-directed BC, the leaving group capacity for the subsequent rearrangement step (“there is further impetus to give some electron density back to this entire system”) was taken as a reason to explain which proton transfer should be represented in the prior mechanistic step (proton transfer from O1 to O3). According to the mechanism taught in class, this is an appropriate context in which to apply activity-directed BC because protonation and deprotonation in both proton transfers are likely to occur and are reversible. The proton transfer from O1 to O3 lowers the energetic barrier of the rate-determining rearrangement step leading to an energy minimum, which can therefore be used to explain the representation of this proton transfer in the mechanism. However, the professor's expectations remained teleological because he did not explicitly incorporate these energetic properties of entities and activities. Instead, the consequence of the proton transfer (“this protonated species here further spring loads this oxygen–oxygen bond”) seemed to be the expected reason that students should use to predict this activity.
It was more important to the professor that students' problem solving included the proton transfer (O1 to O3) suggested by activity-directed BC than that it included the alternative proton transfer (O1 to O2). In the professor's opinion, students who predicted a teleologically derived activity (proton transfer from O1 to O3) would have solved the difficult part of predicting this step and “know what they are doing.” Indeed, reasoning about an activity reached by activity-directed FC using properties of entities (i.e. “charges”) and activities (i.e. “reduce all these separate charges”) explicitly depicted in the mechanistic representation was also expected by the professor. However, he did not stress this reasoning because it is “what we normally would do.”
The same combination of activity-directed BC and activity-directed FC to an alternative activity appeared in the professor's expectations about the second mechanistic step of the Jones oxidation (Fig. 9):
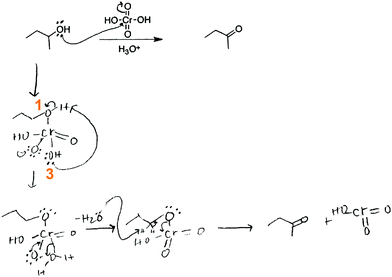
And then there is a proton transfer, so in here there's lots of, and I go over this in class, there's a proton transfer right here […] the reason why this proton [on O1, Fig. 9 ] goes toward this specific alcohol [O3] is because it's a productive part of the mechanism, and it makes the reaction go forward. Normally the proton, I'm speaking to this thing [on O1], does that make sense? The reason why I don't put the proton on this oxide here [O2], is, even though I can do that, it's not a productive part of the mechanism. It just stays there if I do it. So I'm going to do it this way [proton transfer to O3].
In this activity-directed BC, the general capacity for subsequent mechanistic steps (“it makes the reaction go forward”) was taken as a reason to predict an activity for the mechanistic step in question (proton transfer from O1 to O3). The professor made explicit that FC from the entities at the beginning of this mechanistic step could also lead to an alternative activity (proton transfer from O1 to O2). According to the mechanism taught in class, this is an appropriate context in which to apply activity-directed BC because protonation and deprotonation in both proton transfers are separated by small energetic barriers (i.e. they are reversible) and only the protonation of an OH-group on chromium enables the leaving group activity and the following oxidation step, which together lead to a potential energy minimum. However, the professor did not expect a proficient student to include these energetic considerations. The professor mentioned teaching his students that different protonation events could occur but stressed more that the proton transfer from O1 to O3 is represented because it is “a productive part of the mechanism.” Without referring to the underlying causes, this explanation is teleological and is reminiscent of the expression it gets me to the product (Bhattacharyya and Bodner, 2005).
For both mechanisms, we found that the professor expected his students to propose a proton transfer that enables further mechanistic steps even though it may be obvious that something else could happen. It seemed much more important to him that students predict the activity suggested by activity-directed BC than that they consider an alternative or discuss energetic properties of entities and activities. In other words, he expected teleological activity-directed BC and assumed that the occurrence of alternative reaction pathways is obvious.
What students did: I want this to happen because it makes the next step possible
In 10 of the 18 student cases, activity-directed BC was not combined with any other chaining type. This differed from the combination of chaining types in the professor's excerpts; yet, these students still fulfilled the main expectations of their professor.For example, Maddie started her activity-directed BC with the leaving group capacity for the third step of the Baeyer–Villiger oxidation in problem 3A to explain the proton transfer in the second step, which she drew as taught in class (Fig. 10):
And then I knew that I wanted this positive charge to end up over here [on O3, Fig. 10 ] to make a better leaving group, so I just did a hydrogen shift.
Bailey made the same reasoning explicit for problem 3A and repeated it for problem 3B before drawing the proton transfer as taught in class (Fig. 10):
And yeah, like I said before, I just think that this has to leave so the positive charge has to go somewhere here.
These students used activity-directed BC to reason about the mechanistic step for which activity-directed BC was expected by their professor. Alberto proposed protonation of an OH-group on chromium in the Jones oxidation using activity-directed BC:
Then what I did is I added the hydrogen from the acid [hydronium] to make H 2 … I am trying to make because I remember it has to make H 2 O in order for it to be able to push it off the structure.
All three students proposed a proton transfer between an acidic and a basic species in order to increase the leaving group ability in the subsequent mechanistic step. In this context, activity-directed BC can be applied meaningfully; however, the properties of entities and activities included in the students' verbalized reasoning were not sufficient for causal explanation. Energetic properties, which would be required to make activity-directed BC non-teleological, did not appear. This is not surprising because it was not expected by their professor.
Given that none of the students mentioned alternative proton transfers, one can assume that most students did not consider the predicted protonation as one of various protonation and deprotonation events that could be predicted using activity-directed FC. Rather, they considered the requirement to produce a good leaving group as a reason for the prediction of this specific protonation. It is surprising that students did not chain forward to alternative proton transfers because charges and their neutralization seem to be one of the most easily applicable concepts used by students to reason in a forward direction (Anzovino and Bretz, 2015; Galloway et al., 2017), and students in this study frequently used them to justify decisions. For example, Maddie and Bailey used the positive charge that increases leaving group ability as the reason to propose the proton transfer that gives rise to this localization of positive charge, but they did not propose the proton transfer that would neutralize charges. Instead of using properties that can easily be inferred from Lewis structures to chain forward to an alternative activity, they used accessible properties to enable a subsequent mechanistic step. Compared to the instruction the students received, they fulfilled the main expectation of their professor. In their verbalized reasoning, however, they did not consider alternative proton transfers at all, a consideration the professor took for granted.
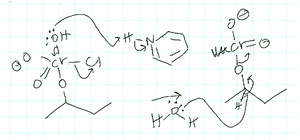
Activity-directed BC was also applied teleologically by Alvina in a completely different and, for the usage of activity-directed BC, inappropriate context when proposing the step shown in Fig. 11 for problem 1B:
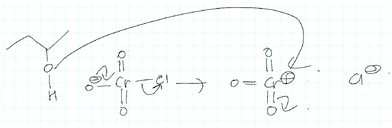 | ||
| Fig. 11 First mechanistic step for problem 1B proposed by Alvina using activity-directed backward chaining (BC). | ||
Alvina: Yes, so if I can move this … these electrons move back to the chromium, then … okay maybe … okay so if this goes here it may lose chlorine … and then …
Interviewer: Is that something that you want to happen? Or, why do you … why would that be helpful … I am asking just to see what is going on in your head, not because I am doubting or anything…
Alvina: Right, because what I am thinking was … if the leaving group leaves the chromium … because there is some way to attach this to O … so in order to connect them together … and then Cr will have a positive charge.
Alvina used the capacity of a positively charged chromium to form a bonding interaction with 2-butanol in the subsequent mechanistic step as a reason for activity-directed BC to the elimination of chloride in the first step shown in Fig. 11. Although Alvina was the only student who applied activity-directed BC in this context, we included it in our results because producing a positive charge in order to enable a nucleophilic addition in the next step has previously been observed by Galloway et al. (2017). Our findings indicate that reasoning potentially originating in instruction and reasoning that seemed to be an intuitive strategy of the students both had the same reasoning pattern, namely activity-directed BC, and were both teleological.
In 10 of the 18 student cases, we found that students used activity-directed BC to make an activity happen because it makes the next step possible. As was expected by their professor, their reasoning was teleological instead of based on consideration of energetic properties. Most of the students employed activity-directed BC in an appropriate context. Therefore, they fulfilled their professor's expectation that distinguishes between a proficient and a non-proficient student. In contrast to their professor's assumption that reasoning about alternatives can be taken for granted, they did not combine activity-directed BC with activity-directed FC to alternative activities. What sounds like a contradiction might be an influence of one on the other. The professor assumed reasoning about alternatives to be trivial and therefore only stressed teleological activity-directed BC. This might have influenced the students to only consider the results of activity-directed BC and not alternatives.
What students also did: I need something to do that in a following step, so we should generate an entity that can do it
In 8 of the 18 student cases, activity-directed BC was combined with entity-directed BC. This contrasted with the professor's expectations and led to the employment of activity-directed BC in an inappropriate context.For example, Tong, when reasoning about the second step of the PCC mechanism (problem 1B), started entity-directed BC with the activity of the oxidation step to propose chloride as the second involved entity. He used this capacity of chloride as a starting point of activity-directed BC to propose the leaving group activity in the mechanistic step in question (Fig. 12):
Let's work, if I don't know it from here I would work backwards. Knowing that this somehow has to break, so that means the hydrogen here has to be taken off by something in the solution. Whether it's the Cl or whether it's the base. But this is already…pyridinium, which already has an H + on it, so the Cl has to have come off, somehow.
Fig. 12 shows the combination of these two BC types. The activity-directed BC used by Tong was teleological because the purpose of the entity, chloride, to act as a base was taken as the reason to predict the leaving group activity that produces this entity. In the case of this combination of chaining types, further properties of entities and activities revealed an inappropriate context. Even if the chloride had the capacity to abstract the proton (a plausible mechanistic step in our analysis since it was taught that way by the professor in class, as discussed below), chloride as a product of the leaving group activity would not influence the energetic barrier of the oxidation step.
In our theoretical framework, we have shown how entity-directed BC and activity-directed BC can be used separately in sound chemical reasoning. As shown in the example of Tong, students combined activity-directed BC with entity-directed BC. We then wanted to know if the professor encouraged use of entity-directed BC by itself. There was evidence of this in his expectations for the last steps of the Jones oxidation and PCC mechanisms. For the last step of the PCC mechanism, the professor explained:
So then what happens there is that then some base, and my students always want to know which base pulls it off, and I can never give them a straight answer because it's, they want to say it's pyridine because pyridine's a good thing, but it's not pyridine, it's pyridinium, pyridinium is not a base at this point, it's still protonated, but you may have some free pyridine but I think it's probably more the chlorine. If you have some floating around, chlorine can grab that proton off of there.
To make a decision about which base is engaged in the oxidation step of the PCC mechanism, the professor expected the same entity-directed BC as shown by Tong. The professor remarked that students often demonstrate confusion about which base is engaged in the oxidation step. Much of this confusion seemed to stem from the instruction the students received. While the professor expected his students to use entity-directed BC to identify the base, he did not expect them to know the necessary causality for this identification (e.g. if he expected causal entity-directed BC, he would not expect his students to propose chloride as the base). Lacking a suitable answer to the question, which entity acts as a base in the oxidation step?, may have led Tong, against the professor's expectations, to combine entity-directed BC about the oxidation step with activity-directed BC to propose the leaving group activity of chloride in a prior mechanistic step, thus providing the base he sought. To find a base to abstract the proton in the oxidation step seemed to be very important to the students and less important to the professor, as evidenced by his explanation regarding the last step of the Jones oxidation (Fig. 9):
So then what happens next is that a proton from next to this oxygen group here, is pulled up by some other local base, the local base could be water, it could be another alcohol for all I know or care, that's not really the most important part, but since they have to be innovative, they'll probably just choose water as the best base to pull it off. Water is not traditionally thought of as a base, but when you're putting it next to a chromic acid, it's basic.
There is a discrepancy in the professor's expectations: On one hand, the identification of the base is not trivial and he expected “innovative” entity-directed BC. On the other hand, he did not provide a basis for causal entity-directed BC (e.g. his explanation belies the entire phenomenon of acidity in water) and it did not seem to be his intention that students causally identify the base. In his opinion, “that's not really the most important part.” In other words, the students were encouraged to employ entity-directed BC but they were not taught a causal basis to solve this problem. This probably contributed to students' inappropriate combination of chaining types.
Nori combined the entity-directed BC expected by the professor for the Jones oxidation with activity-directed BC, taking the capacity of water to abstract the proton in the oxidation step as a reason for proposing the proton transfer that makes water a leaving group (Fig. 13):
Nori: So then I want to generate water so I had this oxygen [O3, Fig. 13 ] attack this hydrogen [on O1] and then that bond goes down to the oxygen [O1] again and then that generates water.
Interviewer: So why do you want to generate water?
Nori: The reason is because I need to attack the carbon that's next to the oxygen to make that double bond with the oxygen so I can make the chromine group leave.
This example shows that, when combining entity-directed BC (about the last step, Fig. 13) with activity-directed BC (to make a decision about step 2, Fig. 13), the two mechanistic steps involved in the reasoning process were not always consecutive. As explained for Tong's reasoning about chloride, Nori's reasoning was also teleological and employed in an inappropriate context for activity-directed BC. This combination of entity-directed BC and activity-directed BC is chemically invalid in all cases and does not reflect the actual nature of mechanisms.
Nori used the same reasoning pattern to propose a mechanistic step for problem 1B (Fig. 14). Entity-directed BC from the oxidation step to water as the second entity involved in this step was followed by activity-directed BC. Starting activity-directed BC with this capacity of water led her to predict the mechanistic step shown in Fig. 14, which seeks to produce the water molecule. Although the proposed proton transfer in the Jones oxidation (Fig. 13) was plausible (it is shown with intramolecular electron movements in the textbook used in class (Klein, 2012)) whereas the mechanistic step depicted for problem 1B looked like a personal creation (Fig. 14), the reasoning pattern and application of a teleological argument were the same in both cases.
 | ||
| Fig. 14 Mechanistic step for problem 1B proposed by Nori using a combination of entity-directed backward chaining (BC) and activity-directed BC. | ||
In 8 of the 18 student responses, we found activity-directed BC employed when students were looking for an additional entity (other than the reactant) to be engaged in a particular activity. Consequently, students generated an entity they thought capable of that activity. This reasoning process started with entity-directed BC about mechanistic steps for which the professor expected it. Students then combined this with activity-directed BC to a prior step. This is a chemically unsound combination of chaining types even when it predicts a step for which the professor expected the use of activity-directed BC on its own. The students' combination of activity-directed BC with entity-directed BC cannot be used in an appropriate context because the reasoning pattern contradicts the properties of the actual reaction system. The professor encouraged entity-directed BC and activity-directed BC separate from each other without providing causal reasoning about properties. Although he did not intend for his students to combine these two chaining types, he taught them no basis to causally decide that this combination of chaining types is invalid. Students' combination of entity-directed BC with activity-directed BC seemed to be influenced by being challenged to employ entity-directed BC without being taught the necessary causality to solve this problem.
Discussion and conclusions
Organic chemistry students' use of a backward-oriented reasoning pattern was qualitatively analyzed with a theoretical framework derived from philosophy of science and biology and physics education (Machamer et al., 2000; Darden, 2002; Darden and Craver, 2002; Russ et al., 2008; van Mil et al., 2013). Reverse to the chronological order of the steps in a mechanism, the students' backward-oriented reasoning pattern utilized information about subsequent mechanistic steps to make a decision about prior steps; this was identified as activity-directed BC. About half of the students employed activity-directed BC in mechanistic contexts where it is necessary to make a decision about alternative reaction pathways (e.g. proposing a proton transfer to improve leaving group ability). The other students used activity-directed BC in inappropriate contexts (e.g. creating a water molecule to act as a base). Independent of the context, students' activity-directed BC was teleological because the subsequent step was taken as the reason to predict the prior step. Even in situations where activity-directed BC is necessary to make a decision between various activities, students used it to make a decision but never mentioned an alternative. While using activity-directed BC, students ignored reversibility and parallel pathways and often focused more on sequence of events than on properties as has been observed in the same data by Weinrich and Sevian (2017) and also in other studies (cf.Graulich, 2015). Causal employment of activity-directed BC necessitates using energetic properties to explain the interdependence of steps; however, students did not incorporate energetic considerations in their explanations. Comparing these characteristics of students' activity-directed BC with the expectations of their professor leads to critical reflection on how the basis of mechanistic decisions is presented to organic chemistry students.While the professor expected the use of activity-directed BC in appropriate contexts, in which it can be applied causally, he expected teleological activity-directed BC. The professor discussed alternative activities to the activity suggested by activity-directed BC; however, he stressed teleological activity-directed BC to a greater extent. While in the professor's expectations the prior and subsequent activities comprising the basis and result of activity-directed BC had the necessary interdependence, he did not expect students to explain the interdependence with energetic properties.
If students want to fulfill these expectations, they have no reason to employ causal instead of teleological reasoning. In this regard, the students did what was expected. When looking only at the cases where students employed teleological activity-directed BC in an appropriate context, students' reasoning was mostly successful. All but one student who employed activity-directed BC in an appropriate context proposed a plausible mechanistic step.
It is not a unique teaching strategy of the professor in our study to communicate the representation of proton transfers and other reversible steps in the context of the influence these steps have on subsequent steps. This way of communication is also found in organic chemistry textbooks. For example, to explain protonation prior to a nucleophilic addition, Kurti and Czakó (2005) state that “in the first step, the carbonyl group is protonated to increase its electrophilicity” (p. 28). Talanquer's (2007) analysis of general and organic chemistry textbooks shows that teleological explanations can play an important role for instructional explanations of some topics in chemistry. Explaining the representation of a mechanistic step based on its consequence for a subsequent step might be an appropriate “didactical transposition” (Chevallard, 1991). However, if students are not aware of the underlying causes of such explanations, they might adopt the reasoning pattern and misuse it in inappropriate contexts.
Our findings do not imply that students' use of activity-directed BC in inappropriate contexts was in all cases a result of the instruction they received. For example, we cannot judge the exact influence of what Alvina was taught on what she did. She proposed elimination of a leaving group to produce a positive charge enabling nucleophilic addition in the subsequent step. This seems like an intuitive use of activity-directed BC. Findings of Galloway et al. (2017) as well as our own findings in an ongoing study reveal similar examples. However, for other cases where students employed activity-directed BC in an inappropriate context, our analysis suggests a direct influence of what students were taught on what they did. Students used activity-directed BC combined with entity-directed BC when they generated a base to abstract a proton in a subsequent step. The professor's teaching probably influenced this chemically invalid use of activity-directed BC due to the discrepancy between challenging the students to identify a base and not focusing on providing them with the necessary causality. The professor did not intend for the students to combine activity-directed BC with entity-directed BC to create the base they sought. Still, it might be a consequence of his unclear teaching goal regarding the identification of a base and of his teleological explanations given in class. Due to the employed teleology, the difference between activity-directed BC in appropriate and inappropriate contexts was not communicated by the professor. Consequently, the students were not provided with a causal basis to decide whether employing activity-directed BC was appropriate. This is also true for cases of activity-directed BC in inappropriate contexts where a direct influence of what students were taught on what they did was not observable, as was the case for Alvina. Not clearly defining teaching goals and not providing students with the causal basis to decide whether a reasoning pattern is applicable in a context might hinder meaningful learning. In more than half of the cases where activity-directed BC was employed in an inappropriate context, the students predicted an implausible mechanistic step. While teleological activity-directed BC in an appropriate context seems to be successful, and teleological activity-directed BC in an inappropriate context seems less successful, only causal activity-directed BC would enable students to differentiate between an appropriate and an inappropriate context.
Moreover, the professor assumed that the occurrence of alternatives is obvious to his students and stressed the result of activity-directed BC to a greater extent. Information that the professor assumed to be obvious did not appear in the students' verbalized reasoning. Students might not realize general chemical principles if educators do not communicate or stress information that is self-evident to the educator. Basic chemical principles like the occurrence of parallel pathways are also not visualized in mechanistic representations. Instead of representing parallel pathways, mechanisms only show final decisions. Alternative activities are not visualized if a mechanistic representation follows the convention to show the lowest energy path from the reactants to the products. To our knowledge, rather than representing alternatives, all mechanisms in organic chemistry textbooks show this “productive continuity” (Machamer et al., 2000, p. 3). Only one of several pathways is the productive pathway because it is the lowest energy path to a product (Goodwin, 2012); however, this information is also not visualized in a typical mechanistic representation. Instead of parallel pathways and the reason why one of them is productive, students see only the result of activity-directed BC. Not representing alternatives could lead to difficulties because students might value written information as more important than oral information. Consider the scenario where a student assumes that the written mechanism is the only process that occurs in a reaction mixture, it might be very confusing if textbooks show different sequences of events for one reaction (e.g. different representations of the Baeyer–Villiger oxidation in Kurti and Czakó, 2005; Clayden et al., 2012; Klein, 2012).
If students do not consider alternative pathways, another difficulty might occur when they try to use properties of a molecule to predict an activity: if this activity-directed FC leads to the prediction of an alternative step compared to the one represented, students might get the impression that using properties to predict which activities occur in a reaction system does not help them to write down the expected mechanism. For students who try to understand processes in a forward direction, experiencing this might lead to the situation where they no longer rely on their abilities or the usefulness of predicting steps based on properties (Anderson and Bodner, 2008). Consequently, they might rely on rote-memorization rather than on conceptual understanding (Graulich, 2015). This is not a desirable result because we know that mechanistic thinking in a forward direction improves student success in solving organic chemistry problems that involve transfer of knowledge (Grove et al., 2012a).
If students try to use properties in their reasoning about mechanisms while educators do not appreciate the prediction of alternative activities, students might use the properties they can identify to explain what is represented instead of actually proposing mechanistic steps. For example, students in our study used the localization of positive charge that increases leaving group ability to justify the representation of a proton transfer that gives rise to this localization. However, they did not use positive and negative charges to predict an alternative proton transfer that would neutralize the charges. Yet, for students' usage of properties in mechanistic reasoning, the aforementioned activity-directed FC could be a stepping stone. Students' use of charges has been previously identified as an intuitive early use of properties to predict activities in single mechanistic steps (Anzovino and Bretz, 2015; Galloway et al., 2017). By not explicitly expecting reasoning about alternative pathways based on properties, educators might sometimes undermine this first step to meaningful mechanistic reasoning.
Communicating backward-oriented reasoning patterns and representing their outcome in mechanisms without communicating or visualizing the underlying causality might lead to multiple misunderstandings in organic chemistry classes. Using expressions of the professor in our study, we want to sum up possible misunderstandings. If an educator explains that a mechanistic step is represented because it is “productive,” the educator might think students understand that it is one of multiple alternative steps and is the only one that is part of the lowest energy path. The educator might expect that students are aware of the step's interdependence with subsequent steps. Instead, a student might understand that the step is represented because it builds a bridge of structural similarity between the reactant and the product. If students explain in an appropriate context that they represent a step because it is “productive,” their educator might think they “know what they are doing.” If students explain the same in an inappropriate context, their educator might recognize that they use the it gets me to the product strategy just to solve the problem (Bhattacharyya and Bodner, 2005). Instead, it might be the case that, in both explanations, students mean that representing the step brings them closer to the product regardless of any properties of the system.
Implications for teaching
In our study, students employed activity-directed BC teleologically without activity-directed FC to alternative activities and referring to the underlying energetics. When applying activity-directed BC in an appropriate context, they fulfilled their professor's main expectations but communicated no basis that could be used to differentiate between appropriate and inappropriate contexts. From these results arise implications for teaching regarding the communication and representation of the basis of mechanistic decisions as well as the definition of teaching goals.When teaching organic reactions for which a decision about alternative reaction pathways necessitates activity-directed BC, it might be helpful for students to represent alternative pathways instead of the single lowest energy path. For instance, given the mechanistic example in Fig. 15, educators could represent both nucleophilic additions. To explicitly communicate the basis of the decision about the alternatives, it could be visualized that the decision cannot be based on the alternative steps alone but must also be based on subsequent steps (Fig. 15). Representing alternative pathways might help students to use applicable properties to employ activity-directed FC more often. In the example given in Fig. 15, students might favor the nucleophilic addition of hydroxide at the ketone group if they consider inductive and resonance effects. Only if alternatives are represented would they find their favored alternative. Representing alternatives might also improve students' recognition and understanding of reversible steps.
 | ||
| Fig. 15 Visualization of alternative reaction pathways and the basis for the decision between the alternatives. | ||
Based on our findings that some students employed activity-directed BC in inappropriate contexts, we also see the necessity to communicate explicitly when activity-directed BC can be used. When and how to use each chaining type should be explicitly taught, something that is important for all reasoning strategies (Cartrette and Bodner, 2009). Teaching when and how to employ activity-directed BC necessitates energetic considerations. To understand that activity-directed BC can be employed causally in the example of the alkaline ester hydrolysis in Fig. 15, one has to consider that the last step of the reaction leads to a significant decrease in potential energy, which shifts the equilibria of the prior steps. To provide visual information that students can use to determine whether activity-directed BC is appropriate, educators could use potential energy diagrams more often.
Our findings also indicate that explicitly defining goals of teaching mechanisms in organic chemistry is important. If the goal is to foster activity-directed FC, reasoning about single steps including alternative possibilities might be more appropriate than teaching the lowest energy path from reactants to products. If the goal is teaching how to employ activity-directed BC, the occurrence of alternatives and energetics underlying the system should be highlighted in explanations and representations. If the teaching goal is that students should learn that a mechanistic representation shows “productive continuity” (Machamer et al., 2000, p. 3), students cannot understand this concept without discussing energetics. Upon explicitly defining teaching goals, it might be best to focus on sets of chemical contexts showcasing use of a single chaining type. In the case of our study, predicting the result of activity-directed BC was a signal for the professor that the students “know what they are doing.” If predicting an activity based on subsequent steps was a teaching goal, it could have been helpful for students not to combine it with a situation where they might apply entity-directed BC later in the mechanism. If entity-directed BC was not the teaching goal, then being allowed to represent an undefined basic species instead of a concrete base might have helped students not to employ teleological thoughts.
According to the complexity of mechanistic decisions, like DeFever et al. (2015), we propose that before being challenged to reason across multiple mechanistic steps, students should be taught how to apply activity-directed FC for a single step. To deal with this complexity, stepping stones toward predicting whole mechanisms are needed. To reduce the complexity and foster single chaining steps, a first stepping stone could be solving exercises like those developed by Flynn and her research group (Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017), which require students to add entities and/or activities to complete single mechanistic steps. As a stepping stone toward complex mechanistic reasoning, we also propose exposing students to learning situations where they are explicitly challenged to employ energetic considerations. In an ongoing study, we are investigating in depth how students decide for which of two different reactants (different in more than one property) a given mechanistic step has lower activation energy. This will yield insight into students' productive resources and difficulties that influence their reasoning about one mechanistic step. We are also testing a teaching instrument that might provide a scaffold for student mechanistic reasoning using multiple properties of entities and activities including energetic properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication represents a component of the first author's doctoral (Dr. rer. nat.) thesis in the Faculty of Chemistry at the Justus-Liebig-University Giessen, Germany. The data used in this study were collected with support of the US National Science Foundation, award 1348722. Further parts of this study including design and analysis were not funded. We thank Andrew Gnann for inspiring discussions about content and wording and for his help with preparing figures.Notes and references
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn't ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9, 93–101.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Bechtel W. and Abrahamsen A., (2005), Explanation: a mechanist alternative, Stud. Hist. Phil. Biol. Biomed. Sci., 36, 421–441.
- Bhattacharyya G., (2013), From source to sink: mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90, 1282–1289.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15, 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: how students propose organic mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bolger M. S., Kobiela M., Weinberg P. J. and Lehrer R., (2012), Children's Mechanistic Reasoning, Cognit. Instruct., 30, 170–206.
- Cartrette D. P. and Bodner G. M., (2009), Non-mathematical problem solving in organic chemistry, J. Res. Sci. Teach., 47, 643–660.
- Chevallard Y., (1991), La transposition didactique. Du savoir savant au savoir enseigné, Grenoble, France: La Pensée Sauvage.
- Clayden J., Greeves N. and Warren S., (2012), Organic Chemistry, Oxford, UK: Oxford University Press.
- Darden L., (2002), Strategies for discovering mechanisms: schema instantiation, modular subassembly, forward/backward chaining, Philos. Sci., 69, S354–S365.
- Darden L. and Craver C. F., (2002), Strategies in the interfield discovery of the mechanism of protein synthesis, Stud. Hist. Philos. Biol. Biomed. Sci., 33, 1–28.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental Rolodexing: Senior Chemistry Majors' Understanding of Chemical and Physical Properties, J. Chem. Educ., 92, 415–426.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students' strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18, 64–77.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before Reactions: A Mechanistic Approach to the Organic Chemistry Curriculum Based on Patterns of Electron Flow, J. Chem. Educ., 92, 803–810.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students' interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18, 353–374.
- Glennan S., (2002), Rethinking mechanistic explanation, Philos. Sci., 69, S342–S353.
- Goodwin W., (2003), Explanation in Organic Chemistry, Ann. NY Acad. Sci., 988, 141–153.
- Goodwin W., (2012), in Woody A. I., Hendry R. F. and Needham P. (ed.), Philosophy of chemistry, pp. 309–327.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16, 9–21.
- Grove N. P. and Bretz S. L., (2010), Perry's Scheme of Intellectual and Epistemological Development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Grove N. P., Cooper M. M. and Cox E. L., (2012a), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89, 850–853.
- Grove N. P., Cooper M. M. and Rush K. M., (2012b), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89, 844–849.
- Heaton J., (2008), Secondary analysis of qualitative data: an overview, Hist. Soc. Res., 33, 33–45.
- Huisgen R., (1970), Zum kinetischen Nachweis reaktiver Zwischenstufen, Angew. Chem., 82, 783–794.
- Illari P. M. and Williamson J., (2011), What is a mechanism? Thinking about mechanisms across the sciences, Eur. J. Phil. Sci., 2, 119–135.
- Karty J., (2014), Organic Chemistry: Principles and Mechanisms, New York: WW Norton & Company.
- Kelemen D. and Rosset E., (2009), The human function compunction: teleological explanation in adults, Cognition, 111, 138–143.
- Klein D., (2012), Organic Chemistry, Hoboken, New Jersey: Wiley.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Kurti L. and Czakó B., (2005), Strategic applications of named reactions in organic synthesis, Amsterdam: Elsevier.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about Mechanisms, Philos. Sci., 67, 1–25.
- Ramsey J. L., (2008), Mechanisms and Their Explanatory Challenges in Organic Chemistry, Philos. Sci., 75, 970–982.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008), Recognizing mechanistic reasoning in student scientific inquiry: a framework for discourse analysis developed from philosophy of science, Sci. Educ., 92, 499–525.
- Sevian H., Bernholt S., Szteinberg G. A., Auguste S. and Pérez L. C., (2015), Use of representation mapping to capture abstraction in problem solving in different courses in chemistry, Chem. Educ. Res. Pract., 16, 429–446.
- Southard K., Wince T., Meddleton S. and Bolger M. S., (2016), Features of Knowledge Building in Biology: Understanding Undergraduate Students' Ideas about Molecular Mechanisms, CBE Life Sci. Educ., 15, 1–16.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Talanquer V., (2007), Explanations and Teleology in Chemistry Education, Int. J. Sci. Educ., 29, 853–870.
- Talanquer V., (2013), When Atoms Want, J. Chem. Educ., 90, 1419–1424.
- van Mil M. H. W., Boerwinkel D. J. and Waarlo A. J., (2013), Modelling Molecular Mechanisms: A Framework of Scientific Reasoning to Construct Molecular-Level Explanations for Cellular Behaviour, Sci. Educ., 22, 93–118.
- van Mil M. H. W., Postma P. A., Boerwinkel D. J., Klaassen K. and Waarlo A. J., (2016), Molecular Mechanistic Reasoning: Toward Bridging the Gap Between the Molecular and Cellular Levels in Life Science Education, Sci. Educ., 100, 517–585.
- Weinrich M. L. and Sevian H., (2017), Capturing students' abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Pract., 18, 169–190.
- Weinrich M. L. and Talanquer V., (2015), Mapping students' conceptual modes when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 16, 561–577.
- Wright L., (1976), Teleological explanations, Berkeley, CA: University of California Press.
- Yan F. and Talanquer V., (2015), Students' Ideas about How and Why Chemical Reactions Happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37, 3066–3092.
Footnote |
| † While Darden (2002) uses the term backward chaining, Darden and Craver (2002) use the term backtracking. In terms of implications for this publication, we see no difference between the reasoning described by Darden and Craver (2002) with backtracking and the reasoning described by Darden (2002) with backward chaining. Therefore, we included Darden and Craver (2002) as a literature source although they used different terminology. |
| This journal is © The Royal Society of Chemistry 2018 |