Microfluidic chips for plasma flow chemistry: application to controlled oxidative processes†
Abstract
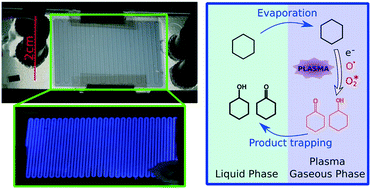
The present paper reports the integration of nonthermal plasma into a biphasic gas–liquid microfluidic chip. It evaluates the potential of plasma activation to become a synthetic tool in organic chemistry, operating under mild conditions (room temperature, atmospheric pressure) and without a catalyst. Few preceding works on plasma chemistry involved a liquid phase and none of them was able to handle the high reactivity of plasma to achieve both high conversion rates and selective reactions. We fabricated a glass-polymer microfluidic chip comprising a one metre long serpentine channel, in which a parallel gas–liquid flow was stabilized thanks to a specific step-like cross-sectional shape. Transparent ITO electrodes, deposited on both sides of the chip and linked to an AC high voltage source, produced a dielectric barrier discharge along the channel. We assessed the behaviour of the flow through optical observations and characterized the discharge through electrical measurements and real time intensified-CCD monitoring. We report the successful treatment of liquid cyclohexane with oxygen plasma inside our chip. The GC analysis of the outflowing liquid revealed only a partial oxidation of cyclohexane into a mixture of cyclohexanol and cyclohexanone (industrially known as “KA oil”), and cyclohexyl hydroperoxide, with a total selectivity above 70% and conversion up to 30%. This indicates that alkanes can be activated and functionalized by means of plasma discharges, in a controlled way. In that respect, we claim to have successfully overcome some of the barriers to industrially relevant plasma chemistry. We believe that the combined use of plasma and microfluidic technologies is essential to the development of this new field of research.


 Please wait while we load your content...
Please wait while we load your content...